Surgical Science
Vol.5 No.1(2014), Article ID:41759,5 pages DOI:10.4236/ss.2014.51005
Can pH Monitoring Predict Gastric Emptying Measured by 13C-Acetate Breath Test in Gastroesophageal Reflux with Neurological Impairment?
Department of Gastroenterological Surgery I, Hokkaido University Graduate School of Medicine, Sapporo, Japan
Email: *okadata@med.hokudai.ac.jp
Received October 21, 2013; revised November 20, 2013; accepted November 28, 2013
ABSTRACT
Introduction: Delayed gastric emptying (DGE) often occurs in patients with gastroesophageal reflux (GER) due to neurological impairment (NI). 13C has been used as an alternative tool for measuring the gastric emptying rates. The aim of this study was to predict gastric emptying in children with GER using 13C-acetate breath test (ABT) by 24-hour pH monitoring. Methods: Nineteen patients were divided into 2 groups: a DGE group with NI (14 patients), and normal-emptying group without NI (5 patients). The liquid test meal consisted of RacolTM (5 ml/kg) mixed with 13C-acetate (50 mg for infants, 100 mg for children, and 150 mg for adolescents). 13CO2 was measured using a gas chromatograph-isotope ratio mass spectrometer. The results are expressed as the % of 13C expired per hour and cumulative 13C excretion over a 3-hour periods including the parameters of half excretion and lag time. Results: The mean half excretion time was 1.762 hour in the DGE group and 1.095 hour in the normal-emptying group (P = 0.0196). The mean lag time was 0.971 hour in the DGE group and 0.666 hour in the normal-emptying group (P = 0.0196). Therefore, DGE was significantly more prevalent in the DGE compared to the normal-emptying group. The percentage of the time when the pH was less than 4 on 24-hour esophageal pH monitoring was 21.6% ± 9.2% in the DGE group and 28.5% ± 11.6% in the normal-emptying group (P = 0.4634). Conclusion: The percentage of time when the pH is less than 4 on 24-hour pH monitoring cannot predict DGE measured by the 13C-ABT in GER.
Keywords:Delayed Gastric Emptying; Gastroesophageal Reflux; Neurological Impairment; 13C-Acetate Breath Test; 24-Hour pH Monitoring
1. Introduction
Delayed gastric emptying (DGE) is often observed in children with gastroesophageal reflux (GER) [1,2]. The etiology of DGE is related to motility disorder of the antrum and occasionally of the entire stomach [1]. The incidence of DGE may be higher than 50% in children with neurological impairment (NI) [3]. Radionuclide scintigraphy has been reported to provide reliable estimates of gastric emptying rates of both solids and liquids [4]. However, there are some disadvantages regarding the use of radionuclides, including the risk of radiation exposure of the patient [1]. In addition, these methods require expensive and complex equipment and may also lead to the false detection of abnormalities.
Recently, 13C has been used as an alternative tool for measuring the gastric emptying rates of both solids and liquids [4-6]. 13C is a stable, naturally occurring, nonradioactive isotope that can easily be detected by mass spectrometry [4]. An ingested 13C-containing test meal is converted to 13CO2, which is absorbed quantitatively from the intestinal tract and then exhaled rapidly by the lungs [4,5]. 13C-octanoate is hydrophobic and has been used to evaluate the gastric emptying of solids in test meals [6-8]. More recently, based on its hydrophilicity, 13C-acetate was developed for use in measuring the gastric emptying of liquids in test meals used in clinical diagnosis. The rate of 13CO2 expiration following 13C-acetate ingestion is an indirect indicator of gastric emptying that can be used as an alternative to radio isotope scintigraphy [1,5].
We previously showed that children with NI exhibited DGE differing from that found in those without NI [9]. Based on these data, our hypothesis in this study was that DGE increases esophageal acid exposure and that esophageal pH monitoring can be used to predict DGE presence by the 13C-acetate breath test (ABT) in children with symptomatic GER with and without NI. The aim of this study was to evaluate the effectiveness of the identified esophageal 24-hour pH monitoring parameters to diagnose DGE by the 13C-ABT among children with and without NI.
2. Materials and Methods
2.1. Patients
Between April 2002 and March 2011, 19 patients with symptomatic GER were admitted to our institution and diagnosed by upper gastrointestinal studies and 24-hour esophageal pH monitoring. Patients did not undergo operations such as the antireflux procedure. We previously showed that children with NI exhibited DGE differing from that found in those without NI [9]. Therefore, they were divided according to gastric emptying by the 13CABT into 2 groups: a DGE group with NI (14 patients aged 2 to 16 years old, mean: 10.0 years old), and a normal-emptying group without NI (5 patients aged 3 months to 8 years old, mean: 4.2 years old). The esophageal 24-hour pH monitoring parameters of the two groups were compared with respect to individual variation in gastric emptying parameters by the 13C-ABT.
None of the patients in the DGE or normal-emptying group received any medication with a potential influence on gastrointestinal motility during the study, excluding anticonvulsants. Drugs for gastrointestinal motility and H2 blockers stopped for 3 days. Informed consent to perform 24-hour esophageal pH monitoring and the 13CABT was obtained from the parents.
2.2. Diagnostic Methods
2.2.1. Twenty Four-Hour Esophageal pH Monitoring
The 24-hour esophageal pH monitoring was performed with a Digitrapper Mk Ⅲ (Medtronic, Copenhagen, Denmark) device. The correct positioning of the probe above the esophagogastric junction was verified by fluoroscopy. On pH monitoring, the signal was sampled every 4 seconds, and a drop in pH below 4 lasting at least 20 seconds was considered a reflux episode. The 24-hour esophageal pH monitoring was considered pathologic if the total recorded time for which the pH was <4 was more than 4% (percentage of time the pH was less than 4) [10].
2.2.2. Test Meal for 13C-Acetate Breath Test
All children fasted 2 hours for liquids and 6 hours for solids prior to 13C-ABT. RacolTM (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) was used as the test meal and was administered at 5 ml/kg (maximum dose: 200 ml). The nutrient composition of 100 ml of RacolTM (100 kcal) is 4.4 g of protein, 15.6 g of carbohydrates and 2.2 g of fat. 13C was used to label acetate (99%; Cambridge Isotope Laboratories, Woburn, MA, USA), which is absorbed in the duodenum but not in the stomach. RacolTM was mixed with 13C-acetate (50 mg for infants, 100 mg for children, and 150 mg for adolescents) [1]. In all children, the meal was consumed within a few minutes or administered by nasogastric tube.
2.2.3. 13C-Acetate Breath Test
Breath samples were collected for 13CO2 measurement before the intake of the meal, every 15 minutes during the first 2 hours after the meal, and every 30 minutes thereafter for ingestion of 13C-acetate and RacolTM [9]. 13C is absorbed in the duodenum but not in the stomach. An ingested 13C-containing test meal is converted to 13CO2, which is absorbed quantitatively from the intestinal tract and then exhaled rapidly by the lungs.
During each expiration phase, exhaled air was collected into a bag using a modified face mask or tracheostomy tube 11 times in all. It took about 1 minute to collect the exhaled air into one bag. 13CO2was measured using a gas chromatograph-isotope ratio mass spectrometer (UBiT-IR300, Ootsuka Electronic Corp). For the 13C-ABT, the concentration of 13CO2 (the final product in exhaled air) was measured. The results are expressed as % of 13C expired per hour and %13CO2 cumulative excretion over a 3-hour period.
2.2.4. Mathematical Analysis
The %13CO2 cumulative excretion in the breath was assessed using a nonlinear regression formula: 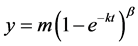 to fit the curve of the cumulative 13C recovery (Figure 1) [1,11]. The %13CO2 excretion per hour was fitted to the formula:
to fit the curve of the cumulative 13C recovery (Figure 1) [1,11]. The %13CO2 excretion per hour was fitted to the formula: 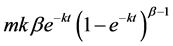 [1, 11]. T is time and m, k, and β are constants. The value of m represents the total cumulative 13CO2 recovery when the time is infinite. The half excretion time of the 13CABT
[1, 11]. T is time and m, k, and β are constants. The value of m represents the total cumulative 13CO2 recovery when the time is infinite. The half excretion time of the 13CABT 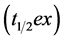 was calculated using the formula:
was calculated using the formula: 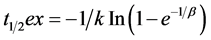 [1,11]. The lag time (t lag), which reflects the initial delay in gastric emptying, was expressed as
[1,11]. The lag time (t lag), which reflects the initial delay in gastric emptying, was expressed as 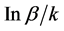 [1,11]. We compared the half excretion andlag times of the 13C-ABT between DGE and normal-emptying groups.
[1,11]. We compared the half excretion andlag times of the 13C-ABT between DGE and normal-emptying groups.
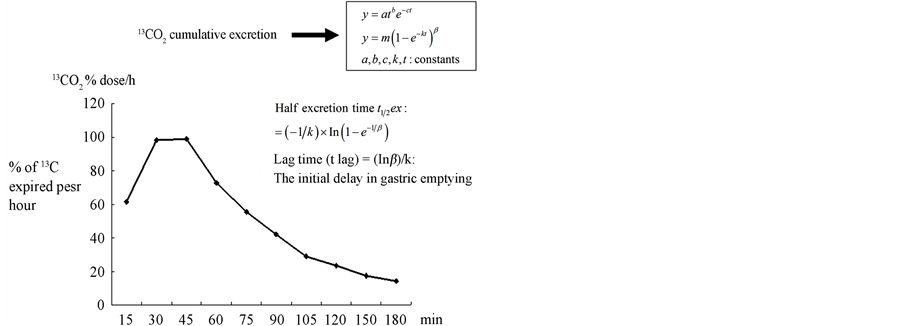
Figure 1. The %13CO2 cumulative excretion curve using by mathematical analysis.
2.2.5. Statistical Analysis
The Mann-Whitney U test was used to evaluate the significance of differences of each parameter of the 13CABT and percentage of time when the pH was less than 4 of 24-hour esophageal pH monitoring between DGE and normal-emptying groups.
3. Results
3.1. Findings of 13C-ABT in DGE and Normal-Emptying Groups
The half excretion and lag times of individual patients are shown in Figure 2. The mean half excretion time was 1.762 hour in the DGE group and 1.095 hour in the normal-emptying group (P = 0.0196). The mean lag time was 0.971 hour in the DGE group and 0.666 hour in the normal-emptying group (P = 0.0196). Therefore, DGE was significantly more prevalent in the DGE compared to the normal-emptying group.
3.2. Findings Regarding the Percentage of Time the pH Was Less Than 4 in DGE and Normal-Emptying Groups
Figure 3 shows the relationship between the percentage of time the pH was less than 4 in the DGE and normalemptying groups. The percentage of time the pH was less than 4 on 24-hour esophageal pH monitoring was 21.6% ± 9.2% in the DGE and 28.5% ± 11.6% in the normalemptying groups (P = 0.4634). Due to the findings that patients with DGE showed no significant difference in total acid exposure than those with normal gastric emptying, the percentage of time the pH was less than 4 on esophageal 24-hour monitoring was not useful to predict DGE presence.
4. Discussion
Patients with NI have a high incidence (up to 15%) of
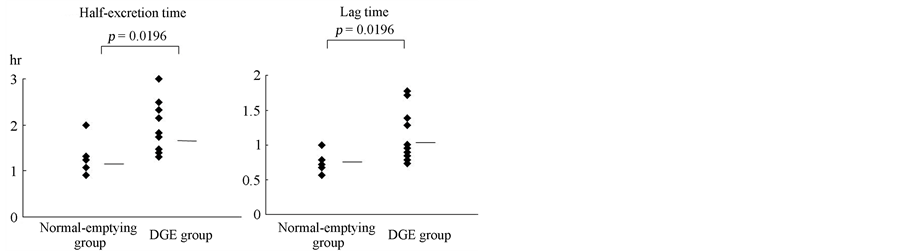
Figure 2. Half excretion and lag times on the 13C-ABT in patients in DGE and normal-emptying groups. DGE was significantly more prevalent in the DGE compared to the normal-emptying group (13C-ABT, 13C-acetate breath test; DGE, delayed gastric emptying).
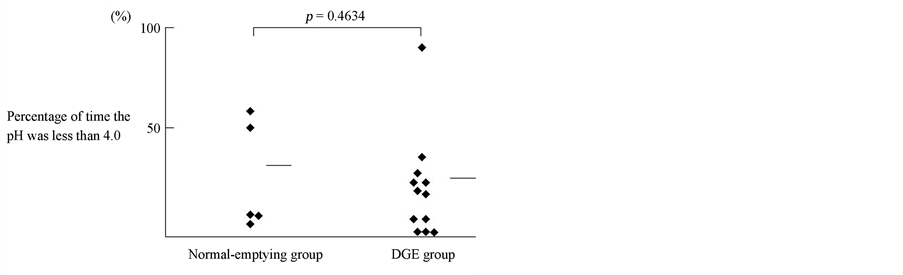
Figure 3. Relationship between the percentage of time the pH was less than 4 by esophageal 24-hour pH monitoring in the DGE and normal-emptying groups. The percentage of time the pH was less than 4 on esophageal 24-hour monitoring was not useful to predict DGE presence (DGE, delayed gastric emptying).
GER [12]. It is known that the probability and rate of GER episodes are related to DGE [12].The likelihood of this has been reinforced by the apparent success of medications which improve gastric emptying in patients with GER and DGE. In children with severe NI, the incidence of DGE is higher than 50% [1]. It is unknown if the gastric emptying time influences the severity of GER. We showed that the gastric emptying rate could be measured by 13C-ABT in patients with GER associated with NI, and that DGE often occurs in such patients [9]. Therapeutic enhancement of gastric emptying has been applied with apparent success in GER with DGE.
The various diagnostic modalities available for GER include barium swallow, radionuclide scintigraphy, 24-h esophageal pH monitoring, and endoscopy. In the present study, pH-metry was used as a prognostic tool to detect clinically significant GER. Bergmeijer et al. [13] defined GER as pathologic if pH-metry showed that the percentage of time the pH was below 4 was over 4%. We hypothesized that esophageal pH monitoring could be used to predict DGE in patients with GER under this threshold value of 4%. Spiroglou et al. and our data showed that there was also no relationship between the emptying time and GER severity [14].
13C-ABT parameters such as the half excretion and lag times were significantly different in all children with GER associated with NI in this study. Furthermore, the percentage of time the pH was less than 4% on 24-hour esophageal pH monitoring was 21.6% ± 9.2% in the DGE group and 28.5% ± 11.6% in the normal-emptying group, with no significant difference. Therefore, this study verified that the parameter of the percentage of time the pH is less than 4 (esophageal acid exposure time) was not useful to predict DGE. Our findings suggest that DGE measured by 13C-ABT parameters such as the half excretion and lag times was not related to esophageal acid exposure on 24-hour esophageal pH monitoring, which was significantly different in all children with GER associated with NI. The reasons for these results were speculated to be due to good esophageal clearance, neutralization of gastric acid by bile due to duodenogastric reflux, or the influence of the post prandial state and fasting [15,16]. Although unexpected, this correlates with the findings of José Estevão-Costa et al., where by the percentage of time the pH was less than 4% in the DGE group (12.3%) was not significantly different from that in the normal-gastric emptying group (23.7%). Similar to our findings, the percentage of time the pH is less than 4% one esophageal 24-hour pH monitoring cannot be used to predict DGE measured by the 13C-ABT in GER patients with NI (DGE group). Therefore, these series suggest that DGE is not a marker of severe GER.
Our study involving esophageal 24-hour pH monitoring was performed without distinguishing the postprandial state from the fasting one. The stomach may indirectly contribute to GER [15]. The stomach may induce episodes of reflux if the intraluminal pressure exceeds the LES pressure; moreover, gastric distension can also lead to valve dysfunction by shortening the LES length or inducing transient LES relaxation. The process of gastric emptying is affected by a number of factors, such as gastrointestinal electrical activity, neural regulation, hormones, and meal composition [17]. In patients with severe NI, several mechanisms combine to determine the gastric emptying process depending on the balance between propulsive forces and resistance to outflow: these mechanisms include gastric accommodation and tone, antral contractivity, antroduodenal coordination, pyloric function, and entero-enteric reflux [1]. The gastric pacemaker is located in the fundus; therefore, gastric emptying may be delayed as a result of abnormalities of the cardiohiatal section [18]. In newborns, gastric peristaltic waves do not become normal until several weeks after birth. During this early phase, GER can easily occur [12]. This transient immaturity may also involve the gastric pacemaker.
One limitation of this study was that the mean age in DGE and normal-emptying groups was 4.2 and 10.0 years, respectively. This wide age variation between DGE and normal-emptying groups might have influenced our results concerning gastric emptying. The choice of test meals also warrants some discussion, including the fact that the use of the 13C-ABT may limit the choice to liquids.
Although the number of cases in this study is too small to draw any conclusion, a tendency that the percentage of time when the pH is less than 4 on 24-hour pH monitoring cannot predict DGE measured by the 13C-ABT in GER was recognized. Larger studies are required to determine the accuracy of the esophageal 24-hour pH monitoring which cannot predict the delayed GET.
5. Conclusion
We showed that the rate of gastric emptying could be measured by the 13C-ABT in patients with GER associated with and without NI, and that the percentage of time when the pH is less than 4% on esophageal 24-hour pH monitoring cannot be used to predict DGE measured by the 13C-acetate breath test in GER.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgements
The authors thank Drs. Manabu Nakagawa, Mototsugu Kato, Masahiro Asaka and Yumiko Tsuda for her excellent technical assistance.
- [2] C. Gatti, F. D. Abrola, L. Dall’Ogalio, M. Villa, F. Franchini and S. Amarri, “Is the 13C-Accetate Breath Test a Valid Procedure to Analyse Gastric Emptying in Children?” Journal of Pediatric Surgery, Vol. 35, No. 1, 2000, pp. 62-65. http://dx.doi.org/10.1016/S0022-3468(00)80015-9
- [3] P. R. Rosen and S. Treves, “The Relationship of Gastroesophageal Reflux and Gastric Emptying in Infants and Children: Concise Communication,” Journal of Nuclear Medicine, Vol. 25, No. 5, 1984, pp. 571-574.
- [4] J. G. Papaila, D. Wilmot, J. L. Grosfeld, F. J. Rescorla, K. W. West and D. W. Vane, “Increased Incidence of Delayed Gastric Emptying in Children with Gastroesophageal Reflux,” Archives of Surgery, Vol. 124, No. 8, 1989, pp. 933-936. http://dx.doi.org/10.1001/archsurg.1989.01410080065010
- [5] D. J. Bjorkman, J. G. Moore, P. D. Klein and D. Y. Graham, “13C-Bicarbonate Breath Test as a Measure of Gastric Emptying,” American Journal of Gastroenterology, Vol. 86, No. 7, 1991, pp. 821-823.
- [6] B. Braden, S. Adams, L. Duan, K. H. Orth, F. D. Maul, B. Lembcke, G. Hor and W. F. Caspary, “The [13C] Acetate Breath Test Accurately Reflects Gastric Emptying of Liquids in Both Liquid and Semisolid Test Meals,” Gastroenterology, Vol. 108, No. 4, 1995, pp. 1048-1055. http://dx.doi.org/10.1016/0016-5085(95)90202-3
- [7] M. Choi, M. Camilleri, D. Burton, A. R. Zinsmeister, L. A. Forstrom and K. S. Nair, “13C Octanoic Acid Breath Test for Gastric Emptying of Solids: Accuracy, Reproducibility, and Comparison with Scintigraphy,” Gastroenterology, Vol. 112, No. 4, 1997, pp. 1155-1162. http://dx.doi.org/10.1016/S0016-5085(97)70126-4
- [8] G. Veereman-Wauters, Y. F. Ghoos, S. Schoor, B. Maes, N. Hebbalkar, H. Devlieger, et al., “The 13C-Octanoic Acid Breath Test: A Non Invasive Technique to Assess Gastric Emptying in Preterm Infants,” Journal of Pediatric Gastroenterology and Nutrition, Vol. 26, No. 2, 1996, pp. 111-117. http://dx.doi.org/10.1097/00005176-199608000-00003
- [9] G. Veereman-Wauters, “Measuring Gastric Emptying with the 13C Octanoic Acid Breath Test in Infants and Children,” Gut, Vol. 43, Supplement 3, 1998, pp. S29- S30.
- [10] T. Okada, F. Sasaki, M. Asaka, M. Kato, M. Nakawawa and S. Todo, “Delay of Gastric Emptying Measured by 13C-Acetate Breath Test in Neurologically Impaired Children with Gastroesophageal Reflux,” European Journal of Pediatric Surgery, Vol. 15, No. 2, 2005, pp. 77-81. http://dx.doi.org/10.1055/s-2004-830357
- [11] T. I. Omari and G. Davidson, “Multipoint Measurement of Intragastric pH in Healthy Preterm Infants,” Archives of Disease in Childhood. Fetal and Neonatal Edition, Vol. 88, No. 6, 2003, pp. F517-F520. http://dx.doi.org/10.1136/fn.88.6.F517
- [12] A. González, C. Mugueta, D. Parra, I. Labayen, A. Martinez, N. Varo, I. Monkeal, et al., “Characterisation with Stable Isotopes of the Presence of a Lag Phase in the Gastric Emptying of Liquids,” European Journal of Nutrition, Vol. 39, No. 5, 2000, pp. 224-228. http://dx.doi.org/10.1007/s003940070015
- [13] J. Boix-Ochoa and M. I. Rowe, “Gastroesophageal Reflux,” In: J. A. O’Neill, M. I. Rowe, J. L. Grosfeld, E. W. Fonkalsrud and A. G. Coran, Eds., Pediatric Surgery, Mosby-Year Book, Missouri, 1998, pp. 1007-1028.
- [14] J. Bergmeijer, T. Tibboel and F. Hazebroek, “Nissen Fundoplication of Gastroesophageal Reflux Occurring after Repair of Esophageal Atresia,” Journal of Pediatric Surgery, Vol. 35, No. 4, 2000, pp. 573-576. http://dx.doi.org/10.1053/jpsu.2000.0350573
- [15] K. Spiroglou, I. Xinias, N. Karatzas, G. Arsos and C. Panteliadis, “Gastric Emptying in Children with Cerebral Palsy and Gastroesophageal Reflux,” Pediatric Neurology, Vol. 31, No. 3, 2004, pp. 177-182. http://dx.doi.org/10.1016/j.pediatrneurol.2004.02.007
- [16] J. Estevão-Costa, M. Campos, J. A. Dias, E. Trindade, A. Teixerira-Pinto and J. L. Carvalho, “Delayed Gastric Emptying and Gastroesophageal Reflux: A Pathological Relationship,” Journal of Pediatric and Gastroenterology and Nutrition, Vol. 32, No. 4, 2001, pp. 471-474. http://dx.doi.org/10.1097/00005176-200104000-00015
- [17] D. O. Castell, J. A. Murray, R. Tutuian, R. C. Orlando and R. Arnold, “Review Article: The Pathophysiology of Gastro-Oesophageal Reflux Disease—Oesophageal Manifestations,” Alimentary Pharmacology & Therapeutics, Vol. 20, Supplement 9, 2004, pp. 14-25. http://dx.doi.org/10.1111/j.1365-2036.2004.02238.x
- [18] M. Montgomery, R. Escobar-Billing, P. M. Hellström, K. A. Karlsson and B. Frenckner, “Impaired Gastric Emptying in Children with Repaired Esophageal Atresia: A Controlled Study,” Journal of Pediatric Surgery, Vol. 33, No. 3, 1988, pp. 476-480. http://dx.doi.org/10.1016/S0022-3468(98)90091-4
- [19] R. R. Dozois, K. A. Kelly and C. F. Code, “Effects of Distal Antrectomy on Gastric Emptying of Liquids and Solids,” Gastroenterology, Vol. 61, No. 5, 1971, pp. 675- 681.
NOTES
*Corresponding author.

