Open Journal of Medical Microbiology
Vol.4 No.2(2014), Article
ID:46907,8
pages
DOI:10.4236/ojmm.2014.42015
Diagnostic Evaluation of Ehrlichia canis Human Infections
Bogdanka Andrić
Clinic for Infectious Diseases, Clinic Center of Montenegro, Medical School, University of Montenegro, Podgorica, Montenegro
Email: bogdankaandric0@gmail.com
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 2 May 2014; revised 2 June 2014; accepted 9 June 2014
ABSTRACT
Ehrlichiosis is an infection of the group of Vector Borne Diseases (VBD), caused by different echrlichia species (spp). Ehrlichiae are primarily recognized as causers of animal diseases, later as human diseases—Humane Monocytic (HME) and Humane Granulocytic (HGE) ehrlichiosis. The dogs, cats and rodents have natural reservoirs of ehrlichial agents. Ehcrlichiae are spread to human’s trough, the bites of infected ticks, sometimes resulting from contact with animals, possible to the transfusion. In period from 2008 to 2013, we analyzed 250 patients suspect of VBD. On examination, there were included clinical, laboratory and epidemiological methods. The confirmation of Ehrlichia canis was in serum of 64 patients, thanks to Indirect Immune fluorescent Assay (IIF). The other laboratory and serological methods (Elisa, IIF, and Western blood) are utilized for differential diagnosis, and detection of co-infective forms of diseases. Ehrlichia canis infection has been reported in humans as a cause of serious illnesses characterized by non specific symptoms: fever, headache, musculoskeletal pain, enlarged liver, neurological, hematological dysfunction. Co-infections with E. canis due to causers of different VBD have been documented in humans. In 53 cases, we detected co-infective participation of E. canis with Rickettsia conorii in 24 cases, Coxiella burneti in 6, and Borrelia burgdorferi in 23 cases. The preferred drug for both human ehrlichiosis is doxycycline, except children to 8 years, pregnant females. Ehrlichiosis is a difficult infectious disease to diagnose and prognose because of dominant non specific symptoms and co-infections.
Keywords:Ehrlichia canis, Diagnosis, Importance

1. Introduction
Ehrlichioses has ticks borne bacterial diseases, caused by different ehrlichia/anaplasma species. The dogs, cats, rodents, and ixodes vectors are the natural reservoirs of ehrlichia agents. The different mammals have sensitive for infection [1] .
The causers of disease are small gram-negative pleomorphic, obligate intracellular (intracytoplasmic) coocy that infected mononuclear cells and granulocytes. Clusters of ehrlichia multiply in host cell, vacuoles to form large mulberry-shaped aggregates so called morulae (Table 1).
Ehrlichia canis, their natural life cycle realized in domestic dogs and primary vectors ticks: Riphicephalus sangvineus (in Americas) and Ixodes ricinus (in Europe), black dog tick, dermacentor variabilis and the other [2] .
Ticks are most commonly infected through blood meal of the infected reservoirs, and were not able to inoculate these agents a new host before 24 - 48 hours. Direct transmission from infected to naïve animals is rare. Ehrlichia agents can be transmitted through blood transfusion, if they are infected donors [3] .
Infections with Ehrlichia and Anaplasma spp. are distributed worldwide. Infection is most common in regions where infestations of ticks with different vector borne agents are the highest and very often associated. Infection of dogs with E. canis, often is accompanied with E. evingii. In common endemic areas, the geographical distribution of ehrlichia agents often coincides with different agents of arthropod borne complex [4] [5] .
In Europe, Ehrlichia canis, Rickettsia conorii and Borrelia burgdorferi are agents as co-infective infectious agents. Because these agents are tick-borne, they are limited in their geographical and host distribution [6] [7] (Table 2).
After tick bite, the ehrlichial agents invaded circulating leukocytes and disseminated trough, and the blood to peripheral tissues, maintains a predilection zone.
After incubating period of 8 - 20 days, starting acute phase of infection takes the following in the next 2 - 3 months. During this time, microorganisms can multiply in circulating monocytes and mononuclear phagocytes. In the cytoplasm of infected cells, a sea generates, which can be visualized by Giemsa-i staining and the white blood cell linage: monocytes (E. canis, E. chaphensis, E. Evingii, E. ruminatus), granulocytes (E. phagocytophila, E. evingii) [8] [9] and platelets (Anaplasma platus) [10] [11] . Microorganisms are very rare in the peripheral blood and can except their findings only in the acute phase of infection. Inflammatory process induced by infection, resulting in the emergence of hematological, liver, and large specter of the other discorders. Cells infected with ehrlichia adhere to the vascular endothelium, inducing infection of sub-endothelial tissue. These changes can be affected by the platelets, causing their adherence, destruction, sequestration, with consequent thrombocytopenia still in the acute phase of infection, with leucopenia and anemia [12] [13] . Thrombocytopenia associated with infection may be consistent or cyclical course, associated with immune mediated destruction of
Table 2. Frequent potentially participant in cotransmision andcoinfections with b.burgdorferi by ixodes ticks in Europe.
platelets or abnormal platelet destruction in chronic phase. Decrease in platelet count, is clinically detectable on basis of the petechial rash or ecchymosed hemorrhages to the development of hemorrhagic diathesis.
Experimental study of the immune-competent dogs showed that after 6 - 9 weeks, ehrlichiae can be eliminated by immune system of the host, or occur bacteriemia with varying in severity and duration of thrombocytopenia, leucopenia, anemia. Dogs, without an effective immune response, develop chronic infection [14] [15] .
2. Methodology
In our study, we have shown the experience in the diagnosis of Ehrlichia canis (E. canis) infection in humans, and examined the prognostic value of disease.
On the basis of data the frequency of participation E. canis in co-infective forms of VBD, were also considered aspects of prognostic value of disease.
In period from 2008-2013, we have analyzed 250 patients suspect of VBD. The examination there were included clinical, laboratory and epidemiological methods.
2.1. Clinical Diagnosis
The largest number of human ehrlichial infections passed asymptomatically /silent disease/ and discovered only by accident. Clinical manifest infection with ehrlichia agents, passed through three stages:
In acute stage, the bacteria reproduce in white blood cells. Non specific symptoms are dominated. Classically manifested ehrlichiosis present as a rather non specific multisystem diseases. Within 1 - 2 weeks following exposure to an infecting tick, patients experience non specific symptoms, including fever, headache, myalgia and general weakness, but also enlarged spleen and liver, adenopaty. Hematological discarders most commonly associated with ehrlichiosis included non regenerative anemia and thrombocytopenia. Serum chemistry commonly reveals hyperglobulinaemia/monoclonal and polyclonal gammopaty, hypoalbuminaemia and low albumin― globulin ratio, high level of serum transaminases, leucopenia, anemia, and possible exanthemas. During the course of the illness, the manifestations of multisystemic disease develop in approximately 10% to 40% of patients, including cough, pharyngitis, lymphadenopathy, diarrhea, vomiting, abdominal pain and changes in mental status. Less frequently reports manifestations include conjunctivitis, disuria. Human patients may present uveitis and/or retinal petechie, polymiositis, polyarthritis and central nervous system disturbances.
2.2. Laboratory Diagnosis
Serologic tests may provide negative results for the majoryti of patients during the first week of illness.
Indirect Immunofluorescent assay (IIF): Most patients with HME have been diagnosed by IIF. The original IIF format for detecting antibodies reactive with E. chaphensis used a surrogate antigen E. canis, as substrate. Paired sera during a 3 - 6 week interval represent the preferred specimen for serologic evaluation of HME. Both immunoglobulin IgM and IgG antibodies can be measured using the IIF. It is important to obtain a convalescent phase serum specimen since most 80% patient’s diagnostic IIF titers by six weeks post infections.
Western blotting (WB): The use of WB has permitted the identification of antigenic variability among isolates of E.chaffensis and identified variability in the reactivity of patient’s sera to a number of E. chaffensis antigens.
Visualization of morulae and staining methods: Microscopy diagnosis of ehrlichia spp. founded of the Giemsa-i strain and identification of the morulae in circulating monocytes, neutrophils and platelets in peripheral blood and bone marrow aspirates by using various eosin-azure (Romanovski) type stains, including Wrights, Diff-Quik, Giemsa-i. Although this technique offers the most rapid method of diagnosis, it is considered relatively insensitive and is seldom confirmatory in clinical practice, In this context, morula positive smears are characteristically seen a minority of patients even in patients from whom the organism has been isolated.
PCR amplification: Polymerase Chain Reaction (PCR) identifies DNA from ehrlichia species in whole blood, cerebrospinal fluid (CFS) and serum are becoming standard complements to serologic assay. Frequently positive results can be obtained by PCR using an acute phase whole blood sample from E. chaphensis patients at the time when testing is still negative.
3. Results
In period from 2008-2013, we have analyzed 250 patients suspect of VBD. The confirmation of Ehrlichia canis was in serum of 64 patients, thanks to IIF. The other laboratory and serological methods (Elisa, Western blood) are used for differential diagnosis and detection of co-infective forms of diseases. In 53 (82.81%) cases we detected co-infective participation of E. canis with Rickettsia conorii in 24 (45.28%) cases (by IFA and Elisa method), Coxiella burneti in 6 (11.32%) cases (by IIF and Elisa method), and Borrelia burgdorferi in 23 (43.39%) cases (by Elisa, WB and PCR method).
3.1. Results of Epidemiological Studies
Ehrlichial agents are speed to human’s trough the bite of infected ticks, sometimes resulting from contact with animals. Our study showed that 67% of cases there was a tick bite, and in 33% there was no tick bite anamnesis. Maximal frequency of tick bites and the number of patients with HGE caused by E. canis, has registered in the suburban settlement which are endemic areas for tick borne infections. Incidence of ehrlichiosis increases with age. The highest incidence reported among persons aged 60+ years.
3.2. Results of Clinical Diagnosis
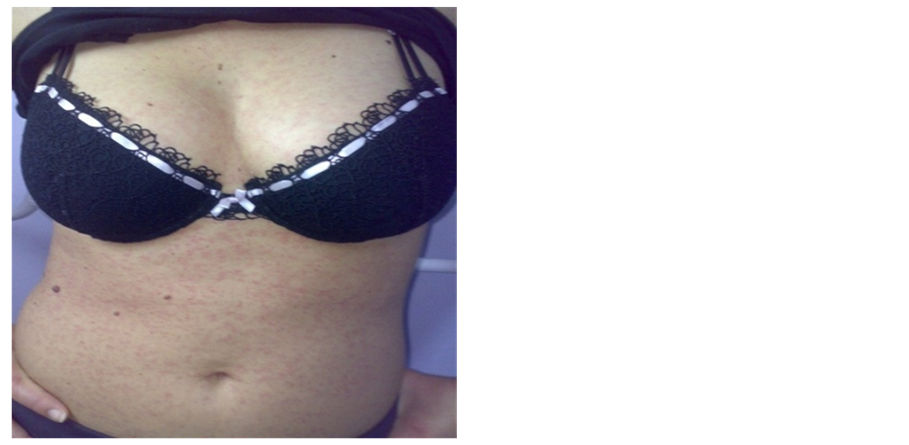
Physical findings due to ehrlichiosis are minimal, except co-infections. The highest manifest exanthemas are increase in cases with co infective participation of Ehrlichia canis and Ricketsia conorii (Figure 1(a) and Figure 1(b)).
In our investigate group, dominant non specific symptoms has presenting in Table3
3.3. Results of Serological Studies
In our series IIF method showed participation of E. canis in 64 patients. In 53 cases we detected co-infective participation of E. canis with other vector borne agents: Rickettsia conorii in 24 cases, Coxiella burneti in 6 cases, and Borrelia burgdorferi in 23 cases.
3.4. Results of Therapeutic Treatment
Tetracycline appears to be an effective therapy for HME. More active than tetracycline against are many pathogens, especially pathogens of the vector borne agents group. Different adverse effect profile and pharmacokinetics compare to tetracycline. By blocking dissotiation of peptide t-RNA from ribosome’s, inhibits bacterial growth, causing RNA-dependent protein synthesis to arrest.
 (a)
(a)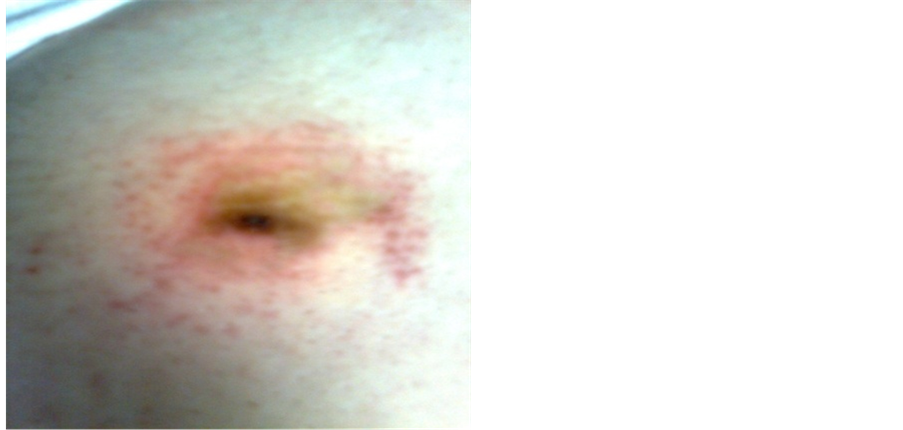 (b)
(b)
Figure 1. (a) Maculopapular rash in co infection between Ehrlichia canis and Rickettsia conory agents; (b) Primo affect in site of tick bite (Original material of Prof. dr Bogdanka Andrić).
In our studies Doxiciclyne are administrate in all 64 adult patients with good therapeutically effect. The therapeutic dose 100 - 200 mg into the 12 hours. In our investigated group, is not are the patients who are unable to take doxicycline (children to the 8 ages and pregnant females). With proper treatment the prognosis is good. Most pets respond within 1 - 4 days and will make a complete recovery. We have occasionally seen humans that have developed immune system problems after an infection with ehrlichiosis. In these cases the humans require anti-inflammatory medications in addition to antibiotics.
Fluoroquinolones may be useful against ehrlichia, but experience is limited.
4. Discussion
Ehrlichia canis was first discovered in Algeria in 1935. Interest for this agent increased when it was determined to cause a fatal hemorrhagic disease in dogs. In 1986 E. canis was implicated serologically and morphologically as the cause of HME in humans. The primary vectors transmit of ehrlichia agents are the different ticks. In Europe the different ixodes ticks. In America, tick Amblioma americanum (Lone Star Tick) [1] .
Ehrlichiosis is an infection of white blood cells that affects various mammals, and humans.
During 1990 Bakken [4] and Buller [8] identified additional ehrlichia spp. Anaplasma (formerly ehrlichia) phagocytophila, the agent of HME and E. evingii (causative agent a HME in dogs) as human pathogens, and these reports greatly expanded the geographic regions and the size of the human population at risk for acquiring one of these potentially lethal infections.
Ehrlichioses is a difficult diagnostic problem, usually in co-infections with various tick-borne pathogens transmitted by the same vector [9] -[14] .
In Montenegro the first etiological confirmation of ehrlichiosis in human and animals was in 2008. We present our experience in context of global perspectives of the disease.
Classical microscopy diagnosis of E. canis entails identifying the morulae of circulating monocytes, neutrophils or platelets [1] , we not identified. Fact that their findings cannot expected only in the acute phase of infection, and what their findings in the peripheral blood is small, present big diagnostic problem.
For the detection of specific antibodies and proteins in serum are used IIF, WB and PCR [15] -[17] .
In experimentally infected dogs with E. canis, detection of specific antibodies with IIF method earlier than 7 days after infection, but there are statements about the development of seropositive 28 days after infection. The finding of positive titers of specific antibodies, it is not a marker for the estimation of age or activity of infection. For these reasons, if ehrlichiosis is suspect in seronegative patients, diagnosis should be repeated after 2 - 3 weeks. In the dogs specific IgG antibodies is greater than 1:80, confirm the diagnosis, but also the titer of less than 1:80 repeat for 2 - 3 weeks, or PCR or WB test [18] [19] .
In known endemic foci, where the dogs are chronically infected, and with positive titers of specific antibodies to E. canis likelihood of false positives is small.
However, positive serological findings without clinical symptoms of the disease may represent an indicator of past infection, and no evidence of active infection with ehrlichia. Titers of antibodies to E. canis increase in the stage of active infection. May be increased when co-infection, with multiple infections caused by different agents of TAZ complex (with other types of ehrlichia, rickettsia, bartonella, borrelia, babesia and other). Diseases caused to some of these agents can be clinical, serological resemble each other [20] .
As an example, it is stated that finding immunodominant protein E. canis, shows serological cross-reactivity with E. chaphensis (causer of HME). The studies have shown that serological IIF tests can not exactly make a distinction between these two species was observed [21] .
Therefore, the interpretation of E. canis serology should include: clinical course of infection [21] , cross-reactivity with other ehrlihia species, features causing of the multiple tick-borne infections and persistent antibody after therapeutic treatment.
Titers of antibodies it is also important to avoid mistakes in the therapeutic treatment. The treatment must be based on the clinical management of remission of symptoms and signs of disease, changes of the serological response to E. canis and competitive increasing concentrations of gama globulin [7] . PCR is a sensitive method for detection of acute phase of infection with E. canis, but it has its limitations, and is indicated as a complement to serological methods [20] .
The high capacity of adaptation of ehrlichial agents is associated with the ability to survive in many and various hosts. It is important for the development of antibiotics resistance and survival in the cerebrospinal fluid (CSF), as well as to change the morphological forms of microorganisms, including their spirochete, cystic forms, oval, granular, L-forms (no cell wall). This allows the microorganism long persistence, to antibiotic therapy treatment and immune activity and the immune based disease.
Targeted studies of surface protein MSP2 of ehrlichia, it was found that its antigenic variation correlated with patogeniti and persistence of causer. This antigen is significant as a primary focus in the pathogenesis of the disease and research related to vaccines.
In the acute stage, in clinical presentation, dominant non specific symptoms including fever, musculoskeletal pain, fatigue, weakness etc. When the bacteria has reproduce in white blood cells and reaches the lymph nodes, spleen, liver, bone marrow, sub clinical remains in the body for years without symptoms, except for anemia [21] . Chronic stage is a difficult diagnostic problem, because make direct symptoms which include neurological, rheumatologic manifestations.
During our investigations, the dominant non-specific clinical symptoms impeded the clinical reccognisation of the disease. Presence of the symptoms of vector borne diseases: primo affect, erythema migrans at the site of a tick bite, exanthemas, manifestation of signs of mononucleosis syndrome, acute respiratory and hematological disorders, masked the clinical picture [21] .
5. Conclusions
Based on studies conducted in Europe, ehrlichiae are classified among the most frequent tick-borne co-transmits agents with borrelia burgdorferi and other agents of VBD complex, as confirmed by our investigations. Endemic distribution of these agents defines the participant in co-infective vector transmission, and co-infective participation in common diseases.
The series is small for making exacting conclusions, but enough to hint numerous diagnostic, differential diagnostic, therapeutic problems, related to the prognostic assessment of disease, which is classified among the “emerging zoonoses”.
Ehrlichiosis is a difficult infectious disease to diagnose. Co-infections of various tick-borne pathogens transmitted by the same vectors additionally complicated the diagnosis.
The preferred drugs for both human ehrlichiosis are doxiciclyne. Rifampyn may be useful in patients who are unable to take doxicycline (children, pregnant women).
Ehrlichiosis is also present in our country, so this disease should be considered every day, especially in the infectology practice. The mainstay of prevention of ehrlichiosis is tick control.
The drug of choice for treatment for all forms of ehrlichiosis is doxicycline for at least one month.
References
- Harkess, J.R. (1991) Ehrlichioses. Infectious Disease Clinics of North America, 5, 37-51.
- Dumler, J.S., Barbet, A.F., Bakkerr, C.P.J., et al. (2001) Reorganisation of Genera in the Familles Rickettsiace and Anaplasmatace in the Order Rickettsiales: Unification of Some Species of Ehrlichia with Anaplasma, Cowdra with Ehrlichia and Ehrlichia with Neoricettsia, Description of Six New Species Combinations and Designation of Ehrlichia equi and HE Agent as Subjective Synonyms of Ehrlichia phagocytophila. International Journal of Systematic and Evolutionary Microbiology, 51, 2145-2165. http://dx.doi.org/10.1099/00207713-51-6-2145
- Childs, J.E. and Paddock, D.C. (2003) The Ascendanct of Amblioma Americanum as a Vector of Pathogens Affecting Humans in the United States. Annual Review of Entomology, 48, 307-337. http://dx.doi.org/10.1146/annurev.ento.48.091801.112728
- Mc Call, C.L., Curns, A.T., Rotz, L.D., et al. (2001) Fort Chaffee Revised: The Epidemiology of Tick-Borne Rickettsial and Ehrlichial Diseases at a Natural Focy. Vector Borne Zoonotic Diseases, 1, 119-127. http://dx.doi.org/10.1089/153036601316977723
- Neer, T.M., Breitschwerdt, E.B., Greene, R.T. and Lappin, M.R. (2002) Consensus Steatment on Ehrlichial Disease of Small Animals from Infectious Disease Study Group of the ACVIM. Journal of Veterinary Internal Medicine, 16, 309- 315.
- Ettinger, S.J. and Feldman, E.C. (2000) Textbook of Veterinary Internal Medicine―Diseases of the Dog and Cat. 2nd Edition, WB Saunders Co., Philadelphia, 402-406.
- Neer, T.M. (1998) Canine Monocytic and Granulocytic Ehrlichiosis. In: C.E. Greene, Ed., Infectious Diseases of the Dog and Cat, 2nd Edition, WB Saunders Co., Philadelphia, 139-147.
- Brouque, P., Bacellar, F., Baranton, G., et al. (2004) Gidelines for Diagnosis of Tick-Borne Bacterial Diseases in Europe. Clinical Microbiology and Infectious Diseases, 10, 1108-1132. http://dx.doi.org/10.1111/j.1469-0691.2004.01019.x
- Uver, A., Felek, S., Paddock, D.C., Yhi, N., Horowiity, H.W., Wormeser, G.P. and Culman, L.C. (2001) Western Blood Analysis of Sera Reactive to Human Monocytic Ehrlichiosis and Human Granulocytic Ehrlichiosis. Clinical Microbiology, 39, 3982-3986. http://dx.doi.org/10.1128/JCM.39.11.3982-3986.2001
- Olano, J.P., Masters, E., Hogrefe, W. and Walker, D.H. (2003) Human Monocytotropic Ehrlichiosis, Missouri. Emerging Infectious Diseases, 9, 1579-1586. http://dx.doi.org/10.3201/eid0912.020733
- Nedelman, R.B., Horowitz, H.W., Hsieh, T.-C., et al. (1997) Simultaneous Human Granulocytic Ehrlichiosis and Lyme borreliosis. New England Journal of Medicine, 337, 27-30. http://dx.doi.org/10.1056/NEJM199707033370105
- Des Vignes, F., Piesman, J., Heffernan, R., Schulze, T.L., Stafford III, K.C. and Fich, D. (2001) Effect of Tick Removal in Transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes Scapularis Nimpphs. Journal of Infectious Disease, 183, 773-778. http://dx.doi.org/10.1086/318818
- Meada, K., Markowitz, N., Hawley, R.C., Ristic, M., Cox, D. and Mc Dade, J.E. (1987) Human Infection with Ehrlichia canis, a Leukocytic Rickettsia. New England Journal of Medicine, 316, 853-856. http://dx.doi.org/10.1056/NEJM198704023161406
- Waner, T., Harrus, S., Jongejan, F., Bark, H., Keysary, A. and Cornelissen, A. (2001) Significance of Serological Testing for Ehrlichial Disease in Dog with Special Emphasis on the Diagnosis of Canine Monocytic Ehrlichiosis Caused by Echrlichia Canis. Veterinary Parasitology, 95, 1-15. http://dx.doi.org/10.1016/S0304-4017(00)00407-6
- Goldman, E.E., Breitschwerdt, E.B., Grindem, C.B., Hegarty, B.C., Walls, J.J. and Dumler, J.S. (1998) Granulocytic Ehrlichiosis in Dog from Nort Carolina and Virginia. Journal of Veterinary Internal Medicine, 12, 61-70. http://dx.doi.org/10.1111/j.1939-1676.1998.tb02096.x
- Rikihisa, Y.S.A., Ewing, J.C., Fox, A.G., Sirenger, F.H., Pasaribu, M.B. and Malole, J.C. (1992) Analyses of Ehrlichia canis and Canine Granulocytic Ehrlichiosis Infection. Journal of Clinical Microbiology, 30, 143-148.
- Varela, F., Font, X., Valladares, J.E. and Alberola, J. (1997) Trombocytopenia and Hight-Chain Proteinuria on a Dog Naturally Infected with Ehrlichia canis. Journal of Veterinary Internal Medicine, 11, 309-311.
- Vinasco, J., Li, O., Alvarado, A., Diaz, D., Houos, L., Tabachi, L., Diringireddy, K. and Ferguson, C. (2006) Molecular Evidence of a New Strain of Ehrlichia canis from Sout America. Journal of Clinical Microbiology, 45, 711-712.
- Mc Bride, J.W., Doyle, C.K., Yhang, X., Cardenas, A.M., Popov, V.L., Nethery, K.A. and Woods, X. (2008) Identifications of Glycosylated Ehrlichia canis 19—Kilodalton Major Immunoactive Protein with Serine—Rich Glycopeptide Epitope. Infection and Immunity, 75, 74-82.
- Everett, E.D., Evans, K.A., Henry, R.B. and Mc Donald, G. (1994) Human Ehrlichiosis in Adult after Tick Exposure: Diagnosis Using Polymerase Chain Reaction. Annals of Internal Medicine, 120, 730-735.
- Horowitz, H.W., Aguero-Rosenfeld, M.E., Mc Kenna, D.F., et al. (1998) Clinical and Laboratory Spectrum of Culture Proven Human Granulocytic Ehrlichiosis Comparison with Culture Negative Cases. Clinical Infectious Diseases, 27, 1314-1317.


