American Journal of Analytical Chemistry
Vol. 3 No. 6 (2012) , Article ID: 20045 , 6 pages DOI:10.4236/ajac.2012.36057
Synthesis and Determination of Stability Constants of a New Bis-1,2,4-triazole Ligand for Complexation with Zinc(II), Copper(II) and Nickel(II) in Acetonitrile
Department of Chemistry, Faculty of Science, Urmia University, Urmia, Iran
Email: *m.bahram@urmia.ac.ir
Received April 6, 2012; revised May 7, 2012; accepted May 17, 2012
Keywords: Zn(II), Cu(II) and Ni(II) Complexes; Triazole Ligand; Spectrophotometry
ABSTRACT
In this work, the synthesis and complexation properties of a new compound, 1,3-bis[5-(2-hydroxyphenyl)-4-phenyl- 1,2,4-triazole-3-yl-thio]propane (BTP), towards certain transi-tion metal ions, (M(II) where M = Zn, Cu, Ni) in acetonitrile is reported. A hard-modeling strategy was applied to UV-Visible spectroscopy data obtained from monitoring the reaction between BTP and the selected metal ions to determine the concentration profiles of each species and the corresponding stability constant(s) of the complex(es). The stability constants of complexes are always defined in terms of their free metal, free ligand and complexed forms. These constants are influenced by parameters such as the type of metal, ligand, counterion or solvent. In this study, the formation constants of the complexes were determined for the synthesized ligand with several metallic cations in acetonitrile solvent by UV-Vis spectrophotometry.
1. Introduction
The synthesis and metal complex structures of substituted 1,2,4-triazole ligands have gained considerable attention in recent years [1-5]. 1,2,4-triazole and its derivatives are very interesting ligands because they combine the coordination geometry of both pyrazole and imidazole with regard to the arrangement of their three heteroatoms. Many transition metal complexes of 1,2,4-triazoles derivatives can be synthesized [6]. Triazoles are nitrogencontaining organic compounds, and their metal complexes display a broad range of biological activity, acting as antitumor, antibacterial, antifungal and antiviral agents [7]. Substituted 1,2,4-triazoles have been actively studied as bridging ligands between transition metal(II) ions coordinating through their N1 and N2 atoms. The design and construction of coordination ligands using the 1,2,4-triazole moiety as a part of a ligand system has gained great attention in recent years [8]. The complexes of 1,2,4-triazole and its derivatives are also good candidates for the construction of various metal coordination polymers [9-13].
Traditionally, in order to study the complaxation of new synthesized ligand with metallic cations, potentiometry, paper electrophoresis, membrane permeation, affinity capillary electrophoresis, UV-vis and fluorescence spectroscopy, and mass spectrometry can be used [14-17]. In this study, UV-vis spectroscopy was chosen because of its simplicity, low cost and availability of the UV-vis spectrophotometer in most laboratories.
The development of chelating reagents specific for bivalent metal ions (Zn(II), Cu(II) and Ni(II)) is becoming increasingly important in a wide variety of applications in the medical, pharmaceutical and related fields. In this work the complexation behavior of a newly synthesized BTP ligand with selected metallic cations has been studied using a model-based analysis. The formation constants of the complexes of the synthesized ligand, BTP, with several metallic cations in acetonitrile solvent were determined by UV-vis spectrophotometry and hard model analysis.
2. Experimental
2.1. Materials and Methods
All reagents were of analytical grade, and all solvents were commercially available (from Merck) and used without further purification. 3-(2-Hydroxyphenyl)-4-phenyl-1H-1,2,4-triazole-5(4H)-thione was prepared according to the literature [18].
The CHNS elemental analyses were performed on a Leco CHNS 932 elemental analyzer. IR spectra were obtained using a Nexus 670 FT-IR Thermo Nicolet spectrometer using a KBr disk. 1H NMR and 13C-NMR spectra were recorded on a Bruker 300 MHz Ultrashield spectrometer using TMS as an internal standard in CDCl3. The melting point was determined on an electrothermal digital melting point apparatus and was uncorrected.
Syntheses and characterization of 1,3-bis[5-(2-hydroxyphenyl)-4-phenyl-1,2,4-triazole-3-yl-thio]propane (BTP).
A mixture of 3-(2-Hydroxyphenyl)-4-phenyl-1H-1, 2,4-triazole-5(4H)-thione 5.66 gr (0.021 mol) and 0.84 gr (0.021 mol) NaOH was dissolved in 100 ml 20% aqueous DMF, then 2.02 gr (0.01 mol) of 1,3-dibromopropane was added. The reaction mixture was stirred 12 h; the solution was poured on to crushed ice (100 gr). The precipitate was filtered and crystallized from EtOH. (Scheme 1).
Yield: 63%; Mp: 193-195˚C. IR (KBr, cm-1): 3053.7, 2929.2, 2844.3, 2708.9, 2627.3, 1619.3, 1585.2, 1487.2, 1431.9, 1401.3, 1295.7, 1254.1, 1164.3, 752.2, 694.6, 606.7; 1H-NMR (300 MHz, CDCl3): 2.33 (2H, q, CH2, J = 6.9 and 6.6), 3.37 (4H, t, SCH2, J = 6.9 and 6.6), 6.5 (2H, t, ArH, J = 7.5), 6.57 (2H, d, ArH, J = 7.8), 7.03 (2H, d, ArH, J = 8.1), 7.18 (2H, t, ArH, J = 7.5), 7.32 (4H, d, ArH, J = 6.3), 7.56 (2H, s, ArH), 7.6 (4H, s, ArH)11.32 (2H, broad s, OH); 13C-NMR (300 MHz, CDCl3): 28.66, 30.75, 110.15, 117.90, 118.53, 125.47, 127.62130.53, 130.79, 131.35, 134.20, 152.93, 153.41, 157.95; Anal. Calcd for C31H26N6O2S2: 64.34 C, 4.53 H, 14.52 N and 11.08% S. Found: 64.39 C, 4.41 H, 14.67 N and 11.16 % S.
2.2. Complex Formation
All solutions were prepared using acetonitrile. Small volumes of concentrated metal cation solutions (e.g. 0- 150 µL from stock solutions with a concentration of 10–3 M) were added to ligand solutions (2 mL of a 10–4 M stock solution). The solutions were thermostated at 25˚C. An example of the change in absorbance due to complex formation is given in Figure 1. This figure shows the complexation of Cu2+ by the BTP ligand. Scheme 2 represents the possible structure of the M2+ in complex with the BTP ligand.
An intramolecular O-H…N hydrogen bonds in ligand exists between the hydroxyphenyl group and the triazole N atom, resulting in a nearly planar six-membered ring in the central part of the molecule. Also these hydrogen bonds in complex was exist that indicate with FT-IR spectra of ligand and complex (not shown hear).
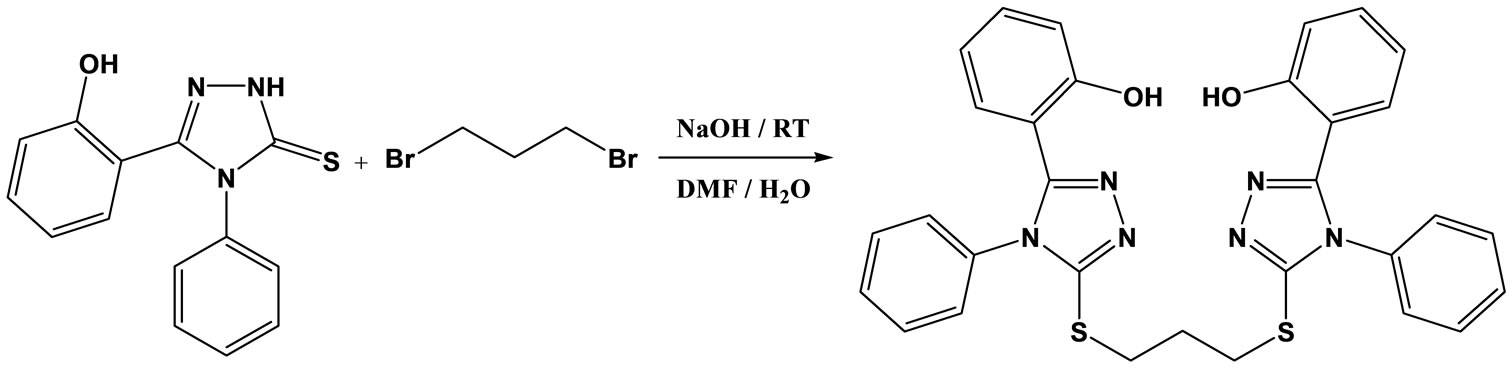
Scheme 1. Synthesis rout of BTP.
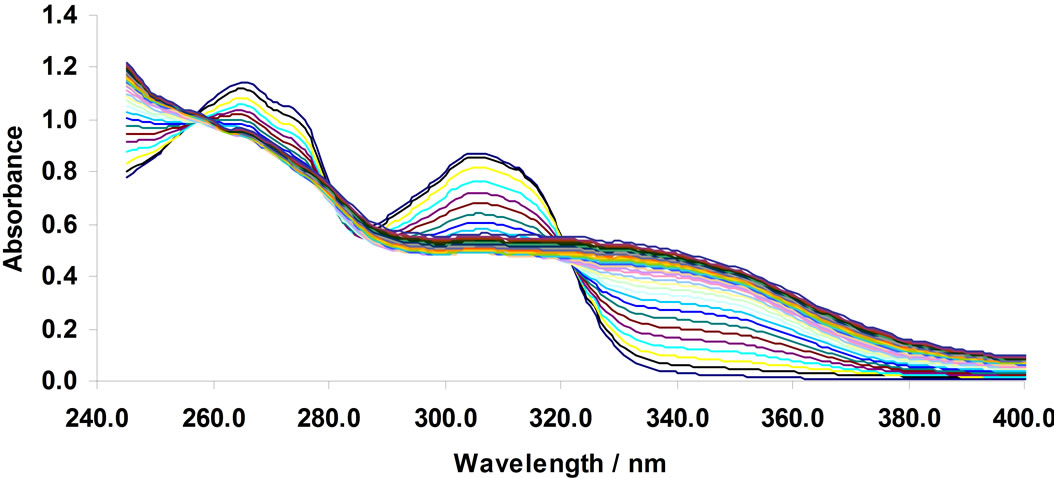
Figure 1. changes in UV-Vis absorption spectra of 1,3-bis [5-(2-hydroxyphenyl)-4-phenyl-4H-1,2,4-triazole-3-yl-thio] propane in acetonit-rile (4.76 × 10–5 mol/L); arrows indicate a change in the absorbance with increase in the concentration of Cu2+. [Cu2+] mol/L: 0, 4.74 × 10–6, 9.43 × 10–6, 1.41 × 10–5, 1.87 × 10–5, 2.33 × 10–5, 2.78 × 10–5, 3.23 × 10–5, 3.67 × 10–5, 4.11 × 10–5, 4.55 × 10–5, 4.98 × 10–5, 5.41 × 10–5, 5.83 × 10–5, 6.25 × 10–5, 6.67 ×10–5, 7.08 × 10–5, 7.49 × 10–5, 7.89 × 10–5, 8.3 × 10–5, 8.7 × 10–5, 9.09 × 10–5, 9.48 × 10–5, 9.87 × 10–5, 1.03 × 10–4, 1.06 × 10–4, 1.1 × 10–4, 1.14 × 10–4, 1.18 × 10–4, 1.21 × 10–4, and 1.25 × 10–4.
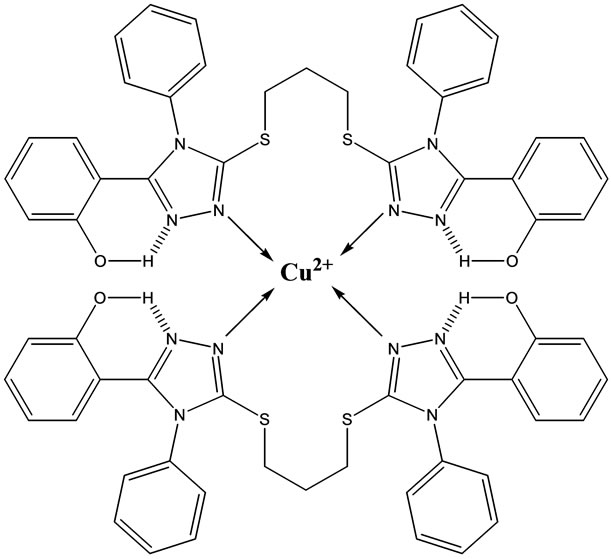
Scheme 2. Possible structure of Cu2+ complexed with BTP [Cu(BTP)2].
3. Results and Discussion
3.1. Treatment of the Experimental Data
For the case of a one-step ML2 complex formation, the following equation could be derived using the ligand concentration [19]:
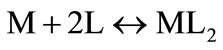 (1)
(1)
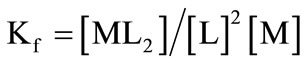 (2)
(2)
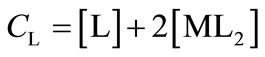 (3)
(3)
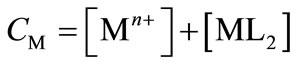 (4)
(4)
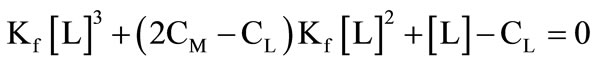 (5)
(5)
3.2. Theory of Model-Based Analysis
The theory of model based analysis (hard model analysis) has been presented in the litrtures [20].
Absorption data are governed by Beer-Lambert’s law and the measurements are well-described by a matrix equation:
 (8)
(8)
where D is a matrix with rows formed by the absorption spectra measured as a function of the progress of the process. The columns of D are the absorption traces measured at different wavelengths. According to Beer-Lambert’s law, this matrix can be decomposed into the product of a matrix C containing, column-wise, the concentration profiles of the absorbing species and a matrix S containing, row-wise, their molar absorptivities. Matrix E is a collection of the residuals, the difference between the measurement D and its calculated representation CST.
Hard model analysis consists of finding a set of parameters for which the sum over all the squares, ssq, over all the elements of the error matrix, E, is minimal [20].
This crucial sum is a function of the measurement, D, the pre-defined model and the parameters:
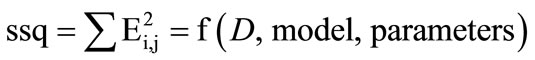 (9)
(9)
Starting with initial guesses of the equilibrium constants (Kf1 and Kf2), the concentration profiles based on the model proposed are constructed. These equilibrium constants are refined in an iterative approach using the NGL/M algorithm so as to minimize the ssq. If the improvement in ssq in two successive iterations is below a certain threshold, i.e. the shift in the parameters resulted in no further improvement of the ssq value, then the process is terminated, and the final values of the parameters leading to the last ssq is reported. It must be mentioned that in each iteration, the matrix of the linear parameters (ST) is calculated explicitly as ST = C+D.
To summarize, given the model and the measurement D, Equation (8) can be written as
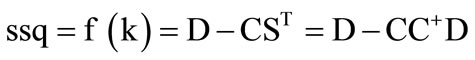 (10)
(10)
Also it should be noted that the most difficult aspect of the model-based approach is to define the correct chemical model. A soft model process (e.g. multivariate curve resolution-alternative least squares or MCR-ALS) [21-23] can be used prior to applying any equation during any hard model approach.
3.3. Analysis of Experimental Data
The complexometric spectra for the complexation of Cu2+ with the ligand BTP was represented in Figure 1. The number of spectrophotometrically active components was specified by singular value decomposition (SVD) of data. The results indicated that there are three major components (Figure 2). The results of the MCR analysis for this dataset (not shown) support the assumption that the system obeys Equations (1)-(5), therefore the absorbing components in this system could be assumed to be L, CuL2 and Cu2+ (solvated cation).
Hard model analysis was then applied for determination of Kf as well as resolving the system and acquiring the pure spectra. The results are shown in Figure 3. The equilibrium constant of the CuL2 complex was calculated to be 106.01 - 106.10 using different initial estimates.
The same analysis was done for the complexation of Ni2+ with BTP in acetonitrile. Figure 4 represents the complexometric spectra of BTP with Ni2+. The number of significant components was estimated using the SVD algorithm. Figure 5 shows that there are 2 significant components in this matrix. Because the investigated wavelength range for this data was from 240 nm to 400 nm and also because the solvated form of Ni2+ had no absorbance in this range, it can be concluded that the active spectroscopic components were the ligand itself and the resulting complex. Model-based analysis of this dataset as a 2:1 complex formed in one step is shown in Figure 6. The stability constant of this complex was calculated to be 106.
The complexometric spectra for the complexation of Zn2+ by the ligand BTP was presented in Figures 7-8.
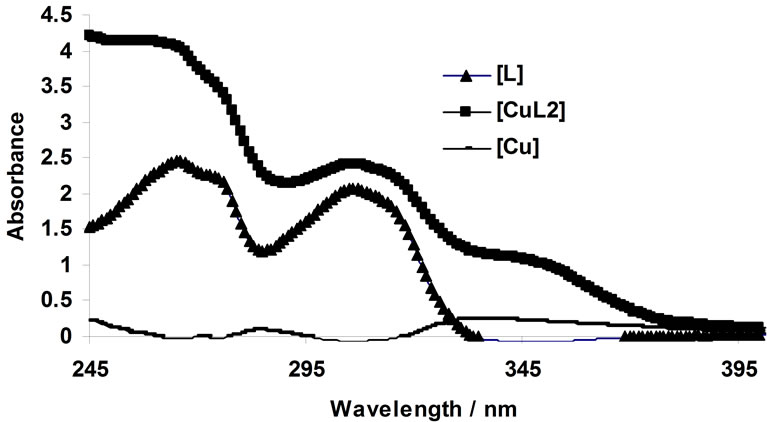
Figure 2. consecutive singular value of singular value decomposition of Cu2+-BTP a function of components.
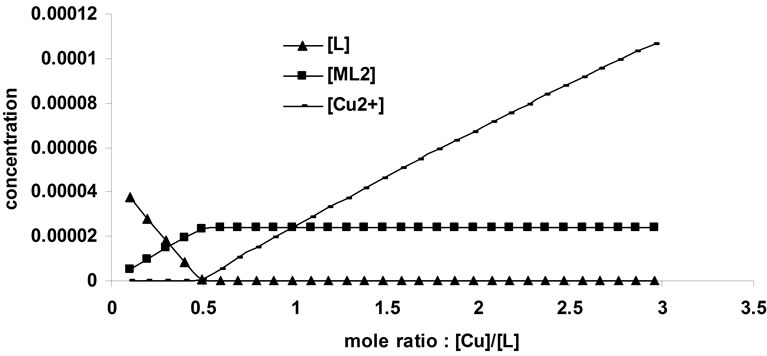
Figure 3. Results of model-based analysis of complexation of Cu2+ with 1,3-bis [5-(2-hydroxyphenyl)-4-phenyl-4H-1,2, 4-triazole-3-yl-thio] pro-pane in acetonitrile.
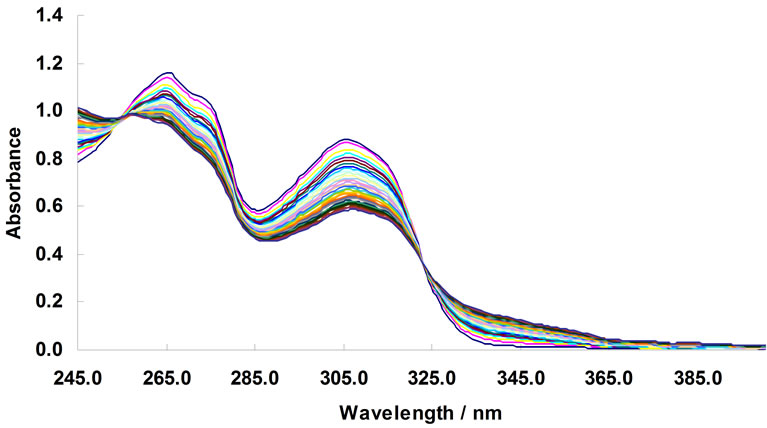
Figure 4. changes in UV-Vis absorption spectra of 1,3-bis [5-(2-hydroxyphenyl)-4-phenyl-4H-1,2,4-triazole-3-yl-thio] propane in acetonit-rile (4.76 × 10–5 mol/L); arrows indicate a change in the absorbance with increase in the concentration of Ni2+. [Ni2+] mol/L: 0, 4.74 × 10–6, 9.43 × 10–6, 1.41 × 10–5, 1.87 × 10–5, 2.33 × 10–5, 2.78 × 10–5, 3.23 × 10–5, 3.67 × 10–5, 4.11 × 10–5, 4.55 × 10–5, 4.98 × 10–5, 5.41 × 10–5, 5.83 × 10–5, 6.25 × 10–5, 6.67 ×10–5, 7.08 × 10–5, 7.49 × 10–5, 7.89 × 10–5, 8.3 × 10–5, 8.7 × 10–5, 9.09 × 10–5, 9.48 × 10–5, 9.87 × 10–5, 1.03 × 10–4, 1.06 × 10–4, 1.1 × 10–4, 1.14 × 10–4, 1.18 × 10–4, 1.21 × 10–4, and 1.25 × 10–4.
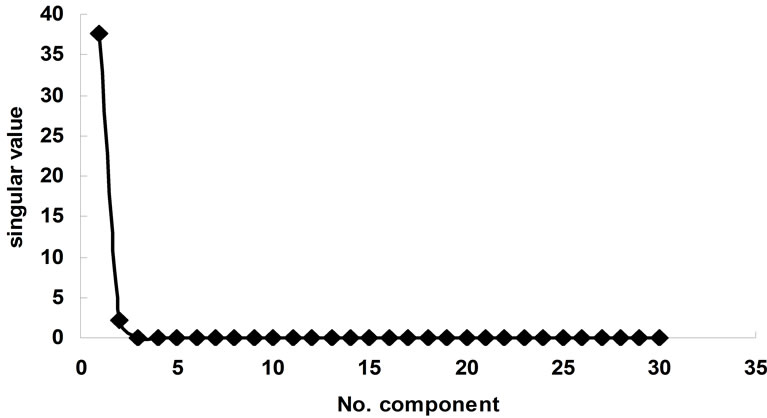
Figure 5. Consecutive singular value of singular value decomposition of Ni2+-BTP a function of components.
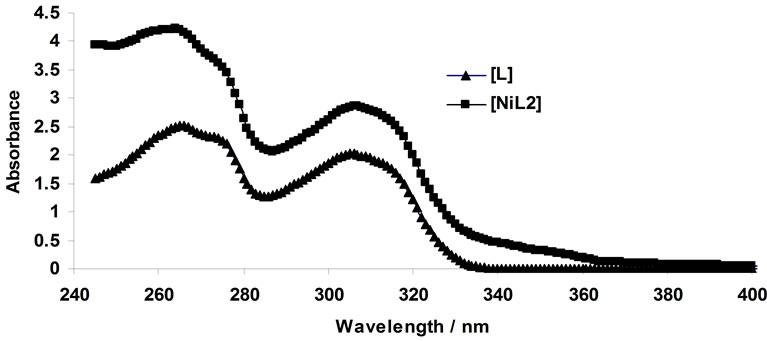
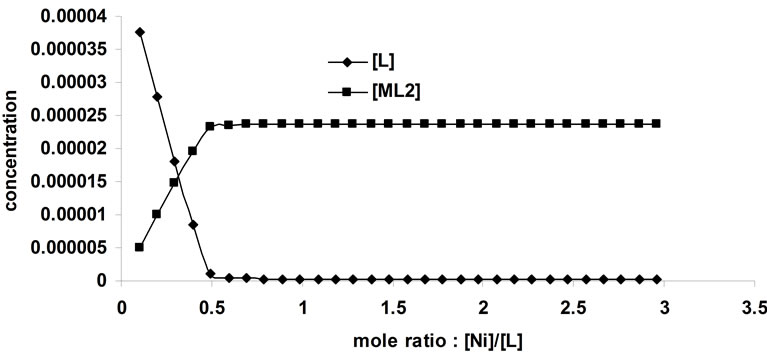
Figure 6. Results of model-based analysis of complexation of Ni2+ with 1,3-bis [5-(2-hydroxyphenyl)-4-phenyl-4H-1, 2,4-triazole-3-yl-thio] propane in acetonitrile.
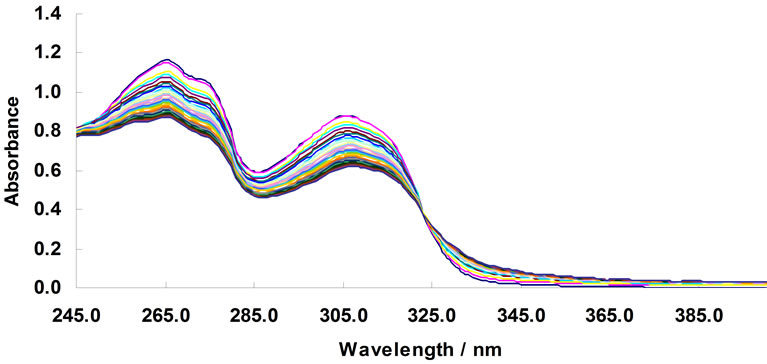
Figure 7. changes in UV-Vis absorption spectra of 1,3-bis [5-(2-hydroxyphenyl)-4-phenyl-4H-1,2,4-triazole-3-yl-thio] propane in acetonit-rile (4.76 × 10–5 mol/L); arrows indicate a change in the absorbance with increase in the concentration of Zn2+. [Zn2+] mol/L: 0, 4.74 × 10–6, 9.43 × 10–6, 1.41 × 10–5, 1.87 × 10–5, 2.33 × 10–5, 2.78 × 10–5, 3.23 × 10–5, 3.67 × 10–5, 4.11 × 10–5, 4.55 × 10–5, 4.98 × 10–5, 5.41 × 10–5, 5.83 × 10–5, 6.25 × 10–5, 6.67 ×10–5, 7.08 × 10–5, 7.49 × 10–5, 7.89 × 10–5, 8.3 × 10–5, 8.7 × 10–5, 9.09 × 10–5, 9.48 × 10–5, 9.87 × 10–5, 1.03 × 10–4, 1.06 × 10–4, 1.1 × 10–4, 1.14 × 10–4, 1.18 × 10–4, 1.21 × 10–4, and 1.25 × 10–4.
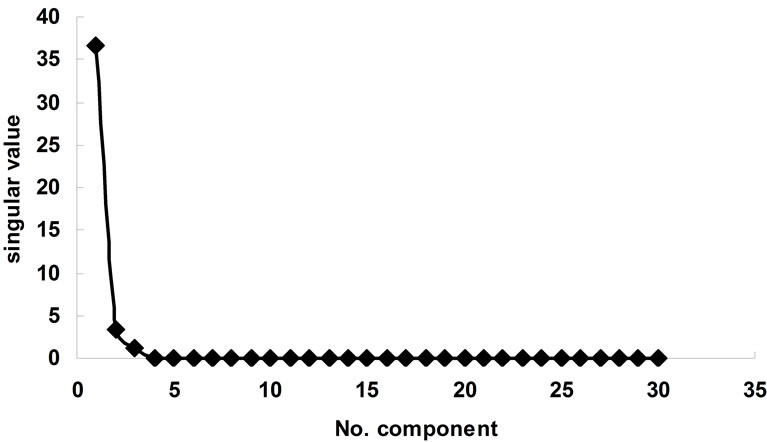
Figure 8. consecutive singular value of singular value decomposition of Zn2+-BTP a function of components.

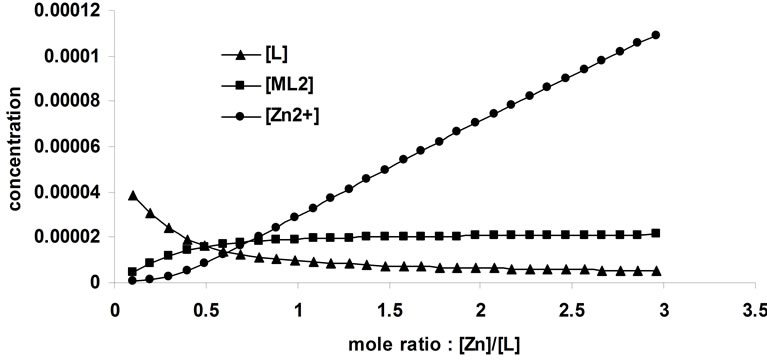
Figure 9. Results of model-based analysis of complexation of Zn2+ with 1,3-bis [5-(2-hydroxyphenyl)-4-phenyl-4H-1,2, 4-triazole-3-yl-thio] propane in acetonitrile.
The results of model-based analysis of the complexation of Zn2+ by the ligand BTP was presented in Figure 9. The stability constant of the ZnL2 complex was calculated to be 109.11 - 109.12 using different initial estimates.
4. Conclusion
In this work, the synthesis and complexation properties of a new compound, 1,3-bis[5-(2-hydroxyphenyl)-4- phenyl-1,2,4-triazole-3-yl-thio]propane (BTP), towards certain transition metal ions, (M(II) where M= Zn, Cu, Ni) in acetonitrile is reported. A hard-modeling strategy was applied to UV-Visible spectroscopy data obtained from monitoring the reaction between BTP and the selected metal ions to determine the concentration profiles of each species and the corresponding stability constant(s) of the complex(es). Among the tested metallic cations Cu(II), Zn(II) and Ni(II). Were formed the most stable complexes with this BTP. Based on the results of this study the new synthesized ligand is introduced as a new active emissive probe to detect Cu(II), Zn(II) and Ni(II) by absorption spectroscopy either directly or after preconcentration.
REFERENCES
- X. He, J. J. Liu, H. M. Guo, M. Shao and M. X. Li, “Syntheses, Topological Networks and Properties of Four Complexes Based on 4-Amino-3,5-bis(3-pyridyl)-1,2,4- triazole Ligand,” Polyhedron, Vol. 29, No. 3, 2010, pp. 1062-1068. doi:10.1016/j.poly.2009.11.011
- K. Liu, X. Zhu, J. Wang, B. Li and Y. Zhang, “Four Coordination Polymers Derived from 4-amino-3,5-bis(3- pyridyl)-1,2,4-triazole and Copper Sulfate,” Inorganic Chemistry Communications, Vol. 13, No. 8, 2010, pp. 976-980. doi:10.1016/j.inoche.2010.05.011
- Q. G. Zhai, M.C. Hu, Y. Wang, W. J. Ji, S. N. Li and Y. C. Jiang, “Synthesis and Characterizations of a Novel dia-type Ag-BPT Coordination Polymer with Tetranuclear Motifs as Jointing Points (BPT=3,5-bis(3-pyridyl)- 1,2,4-triazole),” Inorganic Chemistry Communications, Vol. 12, No. 4, 2009, pp. 286-289. doi:10.1016/j.inoche.2009.01.011
- J. C. Chen, A. J. Zhou, S. Hu, M. L. Tong and Y. X. Tong, “Synthesis, Structure and Magnetic Property of a New Mixed-Valence Copper(I/II) Complex Derived from 3,5- bis(pyridin-2-yl)-1,2,4-triazole,” Journal of Molecular Structure, Vol. 794, 2006, pp. 225-229.
- M. H. Klingele and S. Brooker, “From N-Substituted Thioamides to Symmetrical and Unsymmetrical 3,4,5- Trisubstituted 4H-1,2,4-Triazoles: Synthesis and Characterisation of New Chelating Ligands,” European Journal of Organic Chemistry, Vol. 2004, No. 16, 2004, pp. 3422- 3434. doi:10.1002/ejoc.200400184
- J. J. Liu, X. He, M. Shao and M. X. Li, “Syntheses, Structures and Thermal Stabilities of Four Complexes with 4-Amino-3,5-bis(3-pyridyl)-triazole Ligand,” Journal of Molecular Structure, Vol. 891, No. 1-3, 2008, pp. 50-57. doi:10.1016/j.molstruc.2008.03.011
- T. W. Kajdan, P. J. Squattrito and S. N. Dubey, “Coordination Geometries of Bis(4-amino-3-ethyl-1,2,4-triazole- 5-thione) Complexes of Mn, Fe, Co, Ni, Cu and Zn: Relationship to the 3-Methyl Analogs,” Inorganica Chimica Acta, Vol. 1082, 2000, pp. 300-302.
- A. L. Matesanz, C. Pastor and P. Souza, “Synthesis and Structural Characterization of a Disulphide-Bridged Tetranuclear Palladium(II) Complex Derived from 3,5-Diacetyl 1,2,4-Triazole Bis(4-ethylthiosemicarbazone),” Inorganic Chemistry Communications, Vol. 10, 2007, pp. 97-100. doi:10.1016/j.inoche.2006.09.016
- X. F. Xie, S. P. Chen, Z. Q. Xia and S. L. Gao, “Construction of Metal-Organic Frameworks with Transitional Metals Based on the 3,5-Bis(4-pyridyl)-1H-1,2,4-triazole Ligand,” Polyhedron, Vol. 28, No. 4, 2009, pp. 679-688. doi:10.1016/j.poly.2008.12.046
- M. S. Dýaz-Cruz, J. M. Dýaz-Cruz, J. Mendieta, R. Tauler and M. Esteban, “Softand Hard-Modeling Approaches for the Determination of Stability Constants of Metal-Peptide Systems by Voltammetry,” Analytical Biochemistry, Vol. 279, No. 2, 2000, pp. 189-201.
- A. Abbaspour and M. A. Kamyabi, “Characterization and Determination of Stability Constants of Copper(II)-l-histidine Complexation System by Using Multivariate Curve Resolution Method of Visible Spectra and Two Hard Modeling Methods in Aqueous Solutions,” Analytica Chimica Acta, Vol. 512, No. 2, 2004, pp. 257-269. doi:10.1016/j.aca.2004.02.056
- S. K. Sahoo, M. Baral and B. K. Kanungo, “Potentiometric, Spectrophotometric, Theoretical Studies and Binding Properties of a Novel Tripodal Polycatechol-Amine Ligand with Lanthanide(III) Ions,” Polyhedron, Vol. 25, No. 3, 2006, pp. 722-736. doi:10.1016/j.poly.2005.07.039
- B. Pedras, H. M. Santos, L. Fernandesa, B. Covelo, A. Tamayo, E. Bertolo, J. L. Capelo, T. Aviles and C. Lodeiro, “Sensing Metal Ions with two New AzomethineThiophene Pincer Ligands (NSN): Fluorescence and MALDI-TOF-MS Applications,” Inorganic Chemistry Communications, Vol. 10, No. 8, 2007, pp. 925-929. doi:10.1016/j.inoche.2007.05.001
- J. Petit, V. Geertsen, C. Beaucaire and M. Stambouli, “Metal Complexes Stability Constant Determination by Hyphenation of Capillary Electrophoresis with Inductively Coupled Plasma Mass Spectrometry: The Case of 1:1 Metal-to-Ligand Stoichiometry,” Journal of Chromatography A, Vol. 1216, No. 18, 2009, pp. 4113-4120. doi:10.1016/j.chroma.2009.02.094
- B. B. Tewari, “Paper Electrophoretic Determination of the Stability Constants of Binary and Ternary Complexes of Copper(II) and Cobalt(II) with Nitrilotriacetate and Cysteine,” Journal of Chromatography A, Vol. 1103, No. 1, 2006, pp. 139-144. doi:10.1016/j.chroma.2005.11.002
- M. Cirri, F. Maestrelli, S. Orlandini, S. Furlanetto, S. Pinzauti and P. Mura, “Determination of Stability Constant Values of Flurbiprofen-Cyclodextrin Complexes Using Different Techniques,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 37, No. 5, 2005, pp. 995- 1002. doi:10.1016/j.jpba.2004.09.044
- R. Tauler, A. Smilde and B. R. Kowalski, “Multivariate Curve Resolution Applied to Second Order Data,” Journal of Chemometrics, Vol. 9, No. 1, 1995, pp. 31-58. doi:10.1002/cem.1180090105
- S. GencË, N. Dege, A. CËetin, A. Cansõz, M. SËekerci and M. DincËer, “3-(2-Hydroxyphenyl)-4-phenyl-1H- 1,2,4-triazole-5(4H)-thione,” Acta Crystallographica, Vol. E60, 2004, pp. o1580-o1582.
- P. R. Bevington and D. K. Robinson, “Data Reduction and Error Analysis for the Physical Sciences,” McGrawHill, New York, 2002.
- W. H. Press, W. T. Vetterling, S. A. Teukolsky and B. P. Flannery, “Numerical Recipes in C,” 2nd Edition, Cambridge University Press, Cambridge, 1995.
- A. de Juan and R. Tauler, “Multivariate Curve Resolution (MCR) from 2000: Progress in Concept and Applications,” Critical Reviews in Analytical Chemistry, Vol. 36, 2006, pp. 163-176.
- J. M. Diaz-Cruz, R. Tauler, B. S. Grabaric, M. Esteban and E. Casassas, “Application of Multivariate Curve Resolution to the Voltammetric Data. Part 1. Study of Zn(II) Complexation with Some Polyelectrolytes,” Journal of Electroanalytical Chemistry, Vol. 393, No. 1-2, 1995, pp. 7-16. doi:10.1016/0022-0728(95)04015-G
- J. Mendieta, M. S. Dı´az-Cruz, R. Tauler and M. Esteban, “Application of Multivariate Curve Resolution to Voltammetric Data. Part 2: Study of Metal-Binding Properties of the Peptides,” Analytical Biochemistry, Vol. 240, No. 1, 1996, pp. 134-141. doi:10.1006/abio.1996.0340
NOTES
*Corresponding author.

