International Journal of Clinical Medicine
Vol.07 No.05(2016), Article ID:66852,8 pages
10.4236/ijcm.2016.75035
Pro-Oxidant Antioxidant Balance in Inflammatory Bowel Disease
Abbas Esmaelzadeh1, Hassan Vosooghinia2, Mohammad Reza Sheikhian3, Hadi Bagheri Hosseini3, Daryoush Hamidi Alamdari4, Fatemeh Ahmadi5, Maryam Emadzadeh5, Seyed Mahdi Pakdaman Shahri6*
1Department of Internal Medicine, Imam Reza Hospital, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
2Department of Internal Medicine, Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran
3Department of Internal Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
4Stem Cell & Regenerative Medicine Research Group, Stem Cell Laboratory, Department of Clinical Biochemistry, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
5School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
6Neyshabur School of Medical Sciences, Neyshabur, Iran

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 18 April 2016; accepted 24 May 2016; published 27 May 2016
ABSTRACT
Background: Inflammatory bowel disease (IBD) is associated with increased morbidity and incident of colon cancer; however its etiology is still unclear. Currently, one of the most probable pathogenesis patterns is increased permeability of bowel membrane and it seems that oxidative stress plays an important role in this pathway. This study was done to assess the pro-oxidant antioxidant balance (PAB) in these patients. Materials: This was a cross sectional study of 2 groups including 50 patients with diagnosed IBD and 50 healthy controls. Patients were selected purposively from those referring to adult gastroenterology clinic in 2013. SPSS (ver11.5) has been. A P value < 0.05 was regarded as statistically significant. Results: Mean PAB in patients and controls group was 119.98 ± 38.98 HK unite and 52.67 ± 22.80 HK unite, respectively, (P value < 0.001). PAB mean in ulcerative colitis patients was 120.60 ± 33.90 HK unite and in CD patients was 118.20 ± 45.99 HK unite, and showed no significant difference (P value = 0.85). PAB and MDA could detect healthy subjects from IBD patients with sensitivity more that 90% and specificity more than 84%. Conclusion: PAB shifted to pro-oxidants in IBD patients; moreover this shift was unrelated to disease type. These tests could use as screening test.
Keywords:
Inflammatory Bowel Disease, Pro-Oxidant Antioxidant Balance, Oxidative Stress, Ulcerative Colitis, Crohn’s Disease

1. Introduction
Inflammatory bowel diseases (IBD) are a group of debilitating and chronic diseases consisting of two different phenotypic forms, ulcerative colitis (UC) and Crohn’s disease (CD) [1] .
Several epidemiological studies have evaluated prevalence and incidence of IBD in different geographical areas. In North America, the incidence of ulcerative colitis and Crohn’s disease were 2.2 - 19.2 and 3.1 - 20.2 in 100,000 persons per year, respectively. Also the prevalence of ulcerative colitis and Crohn’s disease in the adult population of the United States were 238 and 201 in 100,000 individuals [2] .
Despite lots of research done to unravel the pathogenesis and pathophysiology of IBD, but it still has unknown etiology. Researchers have shown that genetic predisposition and inappropriate immune response to intestinal microorganisms and their products are the possible causes of this disease. Increased membrane permeability of the gastrointestinal (GI) tractto bacterial products may also play an important role in progression and initiation of IBD [3] - [9] .
On the other hand, this increase in membrane permeability is due to oxidative stress and pro-inflammatory cytokines such as TNF-α [9] . Although oxygen is necessary for life in aerobes and it is not possible to live without it, but it can also produce life-threatening toxic compounds and reactive oxygen species, which named oxidants. Several human defense mechanisms have evolved to minimize the toxic effects of these compounds. Unfortunately, in some cases these mechanisms are insufficient and cannot fully eliminate the toxic effects and leads to initiation and progression of many diseases such as IBD [10] - [12] .
Generally, an increase in oxidant production or a decrease in antioxidant defense mechanisms is known as oxidative stress [13] - [15] .
Accordingly by the given role of oxidative stress in the pathophysiology of IBD, many studies have done to investigate the pro-oxidant antioxidant imbalance in IBD patients [11] .
So far, these studies evaluated the oxidative stress in IBD patients based on some components of oxidative system such as Malondialdehyde (MDA) or some components of anti-oxidative system such as Myeloperoxidase (MPO) [16] - [21] . But the use of TMB-TMB cation (3,3’,5,5’-tetramethylbenzidine and its cation) in Pro- oxidant antioxidant balance (PAB) assessment [22] seems to has more accuracy than previous methods. So this study is designed to evaluate PAB in IBD patients compared with the control group, and its usefulness in the treatment and management of these patients.
2. Materials and Methods
2.1. Study Settings and Sample Selection
This was a cross sectional two groups study. The case group was chosen using purposive selection from adult patients with IBD that came to Gastroenterology department of Ghaem (POH) and Imam Reza (POH) hospitals Mashhad, Iran in 2013 and control group was selected from patients’ non-family escorts, whom were healthy and somewhat similar in terms of according to age and sex.
This study was done for the first time and no similar studies were conducted before and nothing was known about the probable results, therefore these studies was considered as a pilot study, and with regards to all aspects 50 patients and same number of controls that were similar in terms of age and gender were selected.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria was having a diagnosed IBD for patients group and consenting to participate in the study for both groups.
Exclusion criteria for both groups were being pregnant, having infectious disease, diabetes, severe cardiovascular disease, renal failure or proctitis, and taking antioxidants or smoking.
2.3. Categorization & Disease Classification
A careful history was taken from all patients and illness records and medication histories were also recorded. Vital signs and symptoms were checked and required tests such as, fasting blood glucose, liver function tests, ESR and CRP were requested.
Based on current colonoscopy and pathology results, patients were further sub grouped into 2 groups; ulcerative colitis (UC) and crohn’s disease (CD).
UC patients were further divided into three mild, moderate, and severe groups based on severity of disease based on Truelove and Witts classification. These patients were also classified depending on the extent of involvement into two groups; those that had are involvement before splenic flexure and involvement beyond it. These patients were categorized as severe in terms of the course of disease if they were corticosteroid dependent, had two or more relapses per year, needed intravascular corticosteroid for remission, used thiopurines or anti- TNFs or had severe illness.
Crohn patients were divided into three groups considering the extent of involvement; these are involvement of large intestine, involvement of small intestine alone, or involvement of both. In case of normal terminal ileum in colonoscopy, computed tomography (CT) enterography, or small bowel follow-through was used to carefully evaluate small intestine. Crohn patients were categorized severe in terms of the course of disease if they were corticosteroid dependent, had two or more relapses per year, needed intravascular corticosteroid for remission, used thiopurines or anti-TNFs or developed complications of disease such as fistula or obstruction.
2.4. Lab Tests
Pro-Oxidant Antioxidant Balance and Serum Malondialdehyde Measurement
In order to evaluate pro-oxidant antioxidant balance (PAB) and serum malondialdehyde (MDA), 5 cc of blood was drawn and necessary measures were taken to prevent the lysis. After being centrifuged for 15 minutes, serum was separated and freezed in −20˚C.
After collecting all samples in order to minimize lab errors, samples were transferred to biochemistry department of Mashhad Buali research institute to evaluate PAB and sent to biochemistry department of Mashhad University of medical sciences to measure serum MDA.
Pro-oxidant antioxidant balance (PAB) was assessed by Tetramethylbenzidine (TMB) in two different reactions; one was enzymatic reaction (in which chromogen was oxidized to cationated TMB by a peroxidase [H2O2]), the other was cation reaction (in which uric acid was reduced by anti-oxidants). PAB was assessed according to the procedure previously used in a study by Alamdari et al. [22] .
2.5. Statistical Analysis
In order to compare serum levels of pro-oxidant antioxidant balance (PAB), dependent T test or Mann-Whitney U test were used as needed. To analyze statistical information version 16 of SPSS was used. In all calculations, P value < 0.05 was considered as significant.
2.6. Ethical Considerations
The study protocol was approved by the ethics committee of Mashhad University of Medical Sciences. Informed consents were obtained from both patient and control groups. This study was adherent to declaration of Helsinki and clinical good practice (CGP).
3. Results
Fifty IBD subjects, 37 with ulcerative colitis and 13 with crohn’s disease, were in case group.
Control group consisted of 50 healthy people. Patient group included 34 females (68%) and 16 males (32%), whereas 31 females (62%) and 19 males (38%) were in control group (P value = 0.603). UC group consisted of 24 females (65%) and 13 males (35%) whereas CD patients included 10 females (77%) and 3 males (23%).
Mean age in UC group was 31.62 ± 11.68 and in CD group was 31.76 ± 13.82 and 30.46 ± 8.11 in control group and there was no statistically significant difference among the three groups (P value = 0.07) (Table 1).
Mean PAB in patients group was 119.98 ± 38.98 HK unite and in control group was 52.67 ± 22.80 HK unite, considering normal distribution of PAB in both patient and control group, T test was used to compare mean PAB in these groups and showed a significant difference (P value < 0.001). In other words, mean PAB was significantly higher in patient group than controls. MDA in patients and controls, their comparison showed significantly higher mean MDA in patients (2.34 ± 0.76 μM/L) than controls (0.94 ± 0.39 μM/L) (P value < 0.001) (Table 1).
Mean PAB in UC patients were 120.60 ± 33.90 HK unite and in CD patients were 118.20 ± 45.99 HK unite, which showed no statistical significance (P value = 0.85).
This comparison was also done about MDA in both UC and CD patients; Mean MDA in UC group was 2.34 ± 0.79 and in CD group was 2.35 ± 0.67, has no significant difference (P value = 0.74) (Table 1).
In UC patients 28 people (75%) had involvement before splenic flexure, 9 people (25%) had involvement beyond that. PAB in patients with pre splenic flexure involvement (left coloitis) was 116.36 ± 38.61 HK unite and in other group was 133.82 ± 28.96 HK unite, with no significant difference (P value = 0.22).
MDA was 2.48 ± 0.88 μM/L in pre splenic flexure involved (left coloitis) group and 2.06 ± 0.36 μM/L in the other group withno significant difference (P value = 0.34) (Table 1).
UC patients were categorized into three groups based on severity of their disease. 20 (54%) patients were in mild group, 6 (16%) patients were in moderate group and 11 (30%) patients were in severe group.
PAB was measured in these groups and the results were 111/60 ± 37/40 HK unite, 121/60 ± 30/98 HK unite and 136/43 ± 36/43 HK unite, respectively. PAB in these groups showed no significant difference (P value =0.2). MDA were 2/54 ± 0/87 μM/L, 2/13 ± 0/75 μM/L, and 2/10 ± 0/63 μM/L respectively in these groups and MDA had no significant difference between these groupsalso (P value = 0.3) (Table 1).
UC patients were categorized in two mild and severe groups based on disease progression. 25 patients (67.6%) were in mild group and 12 patients (32.4%) were in severe group. PAB results were 113.67 ± 36.40 HK unite in mild group and 135.05 ± 35.07 HK unite in severe group no significant difference comparing PAB in two groups (P value = 0.1). Moreover, MDA was measured in these two groups and was 2.45 ± 0.86 μM/L in mild
Table 1. General characteristics, PAB and MDA of the studied population.
P value < 0.05, Post Hoc was done by Tukey test: P1 = Control Vs. UC, P2 = Control Vs. CD, P3 = UC Vs. CD.
group and 2.10 ± 0.60 μM/L in severe group with no significant difference (P value = 0.39) (Table 1).
CD patients were categorized into three groups based on extent of involvement, 3 cases (23%) had involvement of small intestine, 3 cases (23%) had involvement of large intestine and 7 cases (54%) had involvement of both organs. PAB was measured in these patients and results were 116.42 ± 35.24, 110.05 ± 38.83 and 122.46 ± 57.07 HK unite, respectively no significant difference (P value = 0.93).
MDA results were 1.94 ± 0.14, 2.01 ± 0.20 and 2.68 ± 0.78 μM/L, respectivelyno significant difference (P value = 0.17) (Table 1).
CD patients were sorted in two mild and severe groups based on disease progression; 8 people (61%) were in mild group and 5 people (39%) were in severe group.
PAB were 128.61 ± 47.64 and 101.55 ± 42.54 HK unite, respectivelyno significant difference was shown (P value = 0.32). MDA results for these two groups were 2.39 ± 0.81 and 2.30 ± 0.42 μM/L, respectively no significant difference (P value = 0.72) (Table 1).
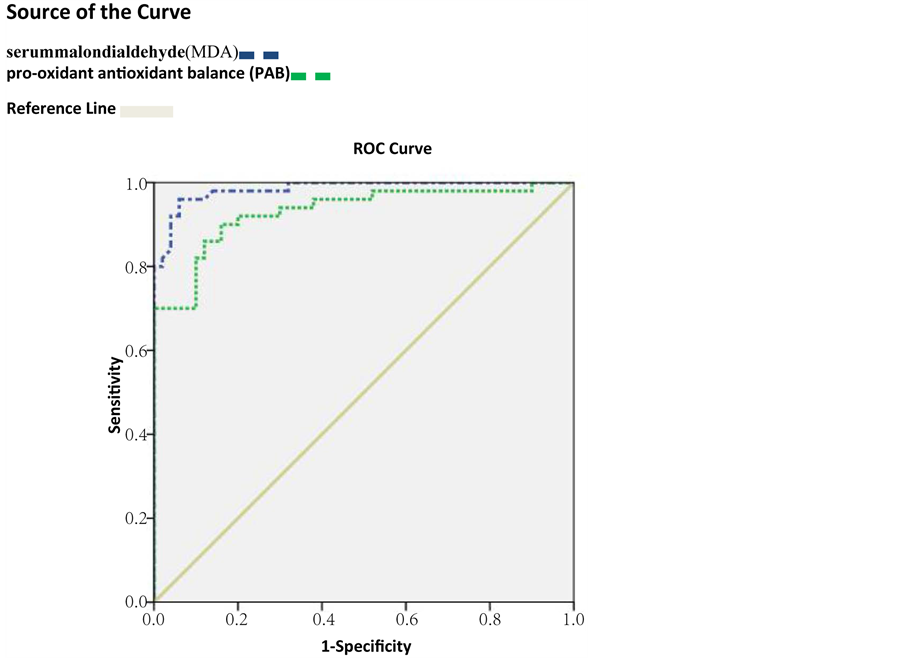
According to the Table 2 and Figure 1, PAB and MDA sensitivity and specificity for detecting healthy subjects from IBD patients are more that 84%.
Table 2. Sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and area under curve of Pro-oxidant Antioxidant Balance (PAB) and serum Malondialdehyde (MDA) for IBD detection from healthy subject.
MDA: serum malondialdehyde, PAB: pro-oxidant antioxidant balance.
Figure 1. ROC Curve for of pro-oxidant antioxidant balance (PAB) and serum malondialdehyde (MDA) for IBD detection from healthy subject.
4. Discussion
In this case-control study we assessed 50 patients with IBD, including 37 patients with ulcerative colitis and 13 with Crohn’s disease, as well as 50 healthy subjects as the control group. In the control and case group, 62% and 68% of patients were women respectively. There was no significant difference between the two groups in terms of gender. The mean age of patients in the control and case group was 30.46 ± 8.11 and 31.66 ± 12.13 years respectively with no significant difference in between (P = 0.07). In addition, the mean age of patients with ulcerative colitis and patients with Crohn’s disease did not differ statistically (P = 0.68).
Mean PAB was 119.98 ± 38.98 HK unite in the case group and 52.67 ± 22.80 HK unite in the control group and because of the PAB normal distribution in both groups, we used T test to compare mean PAB that showed a significant differences between two groups (P < 0.001). In other words, mean PAB in IBD patients was significantly higher than the control group, which means IBD patients had greater levels of oxidants compared with healthy individuals. This result confirms the key role of oxidative stress in IBD pathogenesis.
Since no evidence is reported so far about the pro-oxidant antioxidant balance assessment in IBD patients by means of PAB, so it is not possible to compare the results of this study with others. However, in previous studies, evaluation of other antioxidant markers in IBD patients revealed similar results; an increase in antioxidant markers was seen in IBD patients [15] - [20] [23] .
In addition, we further evaluated the role of oxidative stress in IBD by simultaneously measuring MDA as a marker of oxidative stress to confirm the results obtained from PAB measurement. The mean MDA in control and case groups was 0.94 ± 0.39 and 2.34 ± 0.76 μM/L respectively and there was a statistically significant difference (P < 0.001), which means that the average MDA in patients with IBD was significantly higher than the healthy people were. Achitei et al. in their study also concluded that MDA levels were significantly increased in IBD patients [20] .
In this study, we attempted to examine the relationship between oxidative stress and ulcerative colitis (UC) or Crohn’s disease (CD) in IBD patients by comparison of PAB and MDA variables in both groups. Mean PAB in ulcerative colitis and Crohn’s disease patients were 120.60 ± 33.9 and 118.20 ± 45.99 HK unite respectively with no significant difference between them (P = 0.85). In addition, comparison of MDA in both UC and CD patients did not show a significant difference (P = 0.74). In other words, according to this study, however, oxidative stress maybe has been linked to the pathogenesis of IBD, but not associated with ulcerative colitis or Crohn’s disease.
In order to evaluate the possible association between the extent of inflammatory lesions in UC patients and oxidative stress, these patients were divided into two subgroups; UC patients with inflammatory lesions before and after splenic flexure. 28 patients (75%) had inflammatory lesions limited to the splenic flexure and 9 patients (25%) after the splenic flexure. Mean PAB in UC patients that having inflammatory lesions before the splenic flexure was 116.36 ± 38.61 HK unite and 133.82 ± 28.96 HK unite in the other subgroup. No significant difference was observed (P = 0.22). MDA measurement in these two subgroups also showed no significant difference (p = 0.34). Therefore, by considering these results, no significant correlation was observed between oxidative stress and the extent of inflammatory lesions in UC.
To evaluate the relationship between oxidative stress and the severity of ulcerative colitis, UC patients were classified into three different subgroups: mild (54%), moderate (16%), and severe (30%) according to the Truelove and Witts classification. We did not use the Mayo Score to determine the severity of UC, because the Physician Global Assessment criteria are subjective evaluation criteria. Mean PAB in mild, moderate, and severe subgroups was 116.60 ± 37.40; 121.60 ± 30.98 and 136.43 ± 36.43 HK unite respectively. No significant difference observed between these subgroups by using ANOVA test (P = 0.2). In addition, MDA measurement in these subgroups revealed no difference (P = 0.3).
So, according to the result of our study, no significant correlation was observed between oxidative stress and the severity of ulcerative colitis. Ionnis and his colleagues also showed no significant association between severity of ulcerative colitis and the amount of antioxidants reduction in their study [17] .
In order to assess the association between oxidative stress and progression of ulcerative colitis, UC patients were divided into two different subgroups: slightly progressive (67.6%) and rapidly progressive (32.4%). PAB and MDA measurement showed no significant relationship between these two variables (P = 0.1 and P = 0.39 respectively). Therefore, based on our study, there is no correlation between oxidative stress and progression of ulcerative colitis.
We also decided to investigate the relationship between oxidative stress and the extent of inflammatory lesions in patients with Crohn’s disease (CD). We divided them into three subgroups: lesions limited to small intestine (23%), lesions limited to colon (23%) and lesions in both small intestine and colon (54%). Again, PAB and MDA measurement revealed no significant association between these two variables (P = 0.93 and P = 0.17 respectively). Therefore, we conclude that oxidative stress does not associate with extent of inflammatory lesions in CD patients.
CD patients also divided into two different subgroups according to the disease progression: slightly progressive (61%) and rapidly progressive (39%). PAB and MDA measurement showed no association (P = 0.32 and P = 0.72 respectively).
MDA and PAB are good screening tests for detecting suspected patients from healthy subjects, when other differential diagnosis is considered.
5. Conclusion
We conclude that, in our study, oxidative stress was associated with the pathogenesis of IBD, but with the type of IBD, extent of inflammatory lesions and severity of the disease was not.
Acknowledgements
This paper is the subject thesis of Dr. Mahdi Pakdaman, a subspecialist Candidate of the Gastroenterology in Mashhad University of medical sciences.
Conflict of Interest Statement
The authors indicate no potential conflicts of interest.
Cite this paper
Abbas Esmaelzadeh,Hassan Vosooghinia,Mohammad Reza Sheikhian,Hadi Bagheri Hosseini,Daryoush Hamidi Alamdari,Fatemeh Ahmadi,Maryam Emadzadeh,Seyed Mahdi Pakdaman Shahri, (2016) Pro-Oxidant Antioxidant Balance in Inflammatory Bowel Disease. International Journal of Clinical Medicine,07,334-341. doi: 10.4236/ijcm.2016.75035
References
- 1. Longo, D., Fauci, A., Kasper, D., Hauser, S., Jameson, J. and Loscalzo, J. (2011) Harrison’s Principles of Internal Medicine. McGraw Hill Professional.
- 2. Molodecky, N.A., Soon, I.S., Rabi, D.M., Ghali, W.A., Ferris, M., Chernoff, G., et al. (2012) Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology, 142, 46-54.
- 3. Scarpa, M. and Stylianou, E. (2012) Epigenetics: Concepts and Relevance to IBD Pathogenesis. Inflammatory Bowel Diseases, 18, 1982-1996.
http://dx.doi.org/10.1002/ibd.22934 - 4. Glocker, E. and Grimbacher, B. (2012) Inflammatory Bowel Disease: Is It a Primary Immunodeficiency? Cellular and Molecular Life Sciences, 69, 41-48.
http://dx.doi.org/10.1007/s00018-011-0837-9 - 5. Hendrickson, B.A., Gokhale, R. and Cho, J.H. (2002) Clinical Aspects and Pathophysiology of Inflammatory Bowel Disease. Clinical Microbiology Reviews, 15, 79-94.
http://dx.doi.org/10.1128/CMR.15.1.79-94.2002 - 6. Khor, B., Gardet, A. and Xavier, R.J. (2011) Genetics and Pathogenesis of Inflammatory Bowel Disease. Nature, 474, 307-317.
http://dx.doi.org/10.1038/nature10209 - 7. Hollander, D., Vadheim, C.M., Brettholz, E., Petersen, G.M., Delahunty, T. and Rotter, J.I. (1986) Increased Intestinal Permeability in Patients with Crohn’s Disease and Their Relatives: A Possible Etiologic Factor. Annals of Internal Medicine, 105, 883-885.
http://dx.doi.org/10.7326/0003-4819-105-6-883 - 8. Ukabam, S., Clamp, J. and Cooper, B. (1983) Abnormal Small Intestinal Permeability to Sugars in Patients with Crohn’s Disease of the Terminal Ileum and Colon. Digestion, 27, 70-74.
http://dx.doi.org/10.1159/000198932 - 9. Hollander, D. (1988) Crohn’s Disease—A Permeability Disorder of the Tight Junction? Gut, 29, 1621-1624.
http://dx.doi.org/10.1136/gut.29.12.1621 - 10. Halliwell, B. and Gutteridge, J. (1999) Oxygen Is a Toxic Gas: An Introduction to Oxygen Toxicity and Reactive Oxygen Species. Free Radicals in Biology and Medicine, 3, 1-35.
- 11. Aruoma, O.I., Kaur, H. and Halliwell, B. (1991) Oxygen Free Radicals and Human Diseases. The Journal of the Royal Society for the Promotion of Health, 111, 172-177.
http://dx.doi.org/10.1177/146642409111100506 - 12. Halliwell, B. and Gutteridge, J. (1985) Free Radicals in Biology and Medicine. Pergamon.
- 13. Harris, M.L., Schiller, H.J., Reilly, P.M., Donowitz, M., Grisham, M.B. and Bulkley, G.B. (1992) Free Radicals and Other Reactive Oxygen Metabolites in Inflammatory Bowel Disease: Cause, Consequence or Epiphenomenon? Pharmacology & Therapeutics, 53, 375-408.
http://dx.doi.org/10.1016/0163-7258(92)90057-7 - 14. Rezaie, A., Parker, R.D. and Abdollahi, M. (2007) Oxidative Stress and Pathogenesis of Inflammatory Bowel Disease: An Epiphenomenon or the Cause? Digestive Diseases and Sciences, 52, 2015-2021.
http://dx.doi.org/10.1007/s10620-006-9622-2 - 15. Lih-Brody, L., Powell, S.R., Collier, K.P., Reddy, G.M., Cerchia, R., Kahn, E., et al. (1996) Increased Oxidative Stress and Decreased Antioxidant Defenses in Mucosa of Inflammatory Bowel Disease. Digestive Diseases and Sciences, 41, 2078-2086.
http://dx.doi.org/10.1007/BF02093613 - 16. Hoffenberg, E.J., Deutsch, J., Smith, S. and Sokol, R.J. (1997) Circulating Antioxidant Concentrations in Children with Inflammatory Bowel Disease. The American Journal of Clinical Nutrition, 65, 1482-1488.
- 17. Koutroubakis, I.E., Malliaraki, N., Dimoulios, P.D., Karmiris, K., Castanas, E. and Kouroumalis, E.A. (2004) Decreased Total and Corrected Antioxidant Capacity in Patients with Inflammatory Bowel Disease. Digestive Diseases and Sciences, 49, 1433-1437.
http://dx.doi.org/10.1023/B:DDAS.0000042242.22898.d9 - 18. Ioannidis, O., Varnalidis, I., Paraskevas, G. and Botsios, D. (2011) Nutritional Modulation of the Inflammatory Bowel Response. Digestion, 84, 89-101.
http://dx.doi.org/10.1159/000323456 - 19. D’Odorico, A., Bortolan, S., Cardin, R., D’Inca’, R., Martines, D., Ferronato, A. and Sturniolo, G.C. (2001) Reduced Plasma Antioxidant Concentrations and Increased Oxidative DNA Damage in Inflammatory Bowel Disease. Scandinavian Journal of Gastroenterology, 36, 1289-1294.
http://dx.doi.org/10.1080/003655201317097146 - 20. Achitei, D., Ciobica, A., Balan, G., Gologan, E., Stanciu, C. and Stefanescu, G. (2013) Different Profile of Peripheral Antioxidant Enzymes and Lipid Peroxidation in Active and Non-Active Inflammatory Bowel Disease Patients. Digestive Diseases and Sciences, 58, 1244-1249.
http://dx.doi.org/10.1007/s10620-012-2510-z - 21. Hengstermann, S., Valentini, L., Schaper, L., Buning, C., Koernicke, T., Maritschnegg, M., et al. (2008) Altered Status of Antioxidant Vitamins and Fatty Acids in Patients with Inactive Inflammatory Bowel Disease. Clinical Nutrition, 27, 571-578.
http://dx.doi.org/10.1016/j.clnu.2008.01.007 - 22. Alamdari, D.H., Paletas, K., Pegiou, T., Sarigianni, M., Befani, C. and Koliakos, G. (2007) A Novel Assay for the Evaluation of the Prooxidant-Antioxidant Balance, before and after Antioxidant Vitamin Administration in Type II Diabetes Patients. Clinical Biochemistry, 40, 248-254.
http://dx.doi.org/10.1016/j.clinbiochem.2006.10.017 - 23. Kruidenier, L., Kuiper, I., van Duijn, W., Mieremet-Ooms, M.A., van Hogezand, R.A., Lamers, C.B., et al. (2003) Imbalanced Secondary Mucosal Antioxidant Response in Inflammatory Bowel Disease. The Journal of Pathology, 201, 17-27.
http://dx.doi.org/10.1002/path.1408
NOTES

*Corresponding author.