International Journal of Clinical Medicine
Vol.05 No.19(2014), Article ID:50373,8 pages
10.4236/ijcm.2014.519151
Correlation of Endoscopic Findings with Various Helicobacter pylori Tests among Dyspeptic Patients
Mohammed O. Mohammed
Department of Internal Medicine, School of Medicine, Faculty of Medical Sciences, University of Sulaimani, Sulaimani, Iraq
Email: dr_m_omer@yahoo.com
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 31 August 2014; revised 22 September 2014; accepted 6 October 2014
ABSTRACT
Background: Helicobacter pylori is the most common chronic bacterial infection, and a significant etiological factor in acid peptic diseases and gastric cancer. Dyspepsia is a common gastrointestinal disorder, and the most common indication for gastroscopy. Detection of H. pylori during endoscopy has become standard clinical practice. Elevated levels of inflammatory markers such as C-reactive protein (CRP), are associated with pathological changes, and hence give useful information for exact diagnosis and therapy. Objectives: To determine the relationship between endoscopic findings, highly sensitive C-reactive protein level (hs-CRP) and H. pylori infection among dyspeptic patients using serological tests, stool antigen for H. pylori and antral histology. Methods: This was a prospective study; patients with dyspepsia, who referred to Kurdistan Teaching Center of Gastroenterology & Hepatology in Sulaimani City were assessed, during the period of December 2012 to March 2014. They underwent gastroscopy, and biopsies were taken from the corpus and antral portions of antral portion for histopathological exam. Patients’ serum samples were tested for H. pylori infection using ELISA method to detect (IgG & IgA) anti-bodies and stool samples were examined using rapid immunoassay method to detect H. pylori antigens. hs-CRP was assessed using ELISA. Results: One hundred dyspeptic patients were included in the study. The mean age was 34.2 years and male comprised 54% of the study samples. The common findings in oesophagogastroduodenoscopy (OGD) examination were antral gastropathy (59%) and duodenal ulcer (21%). A statistically significant (P < 0.01) correlation was found between hs-CRP and H. pylori IgG and IgA levels (titer). There was a highly significant (P < 0.01) correlation between the level of H. pylori IgG and the endoscopic findings. The highest serum level of H. pylori IgG was found in duodenal ulcer and antral gastritis, (88.86 ± 42.0) and (70.05 ± 35.2) Au/ml, respectively. There was a highly significant correlation (P < 0.01) between endoscopic findings and H. pylori positive antral biopsy, in duodenal ulcer, antral gastritis and duodenitis was 100%, 94.9% and 75% respectively. Also duodenal ulcer and antral gastritis showed high mean and percentage but no significant differences in both H. pylori IgA and stool Antigen.
Keywords:
H. pylori, Serology, Antral Biopsy, Stool Antigen, hs-CRP, Sulaimani

1. Introduction
Dyspepsia is a prevalent complaint in general practice and gastrointestinal clinics [1] . Helicobacter pylori infection is the most common chronic bacterial infection in the world. This bacterium colonizes human gastric mucosa and can elicit lifelong inflammatory and immune responses, with release of various bacterial and host- dependent cytotoxic substances [2] [3] . It causes chronic and active gastritis, peptic ulcer disease and associated with increased risk of developing gastric cancer [4] .
Testing for H. pylori is recommended in many clinical settings such as peptic ulcer disease, low grade gastric mucosa associated lymphoid tissue lymphoma, after endoscopic resection of early gastric cancer or if there are first degree relatives with gastric cancer, and in certain cases of dyspepsia but it is not a routine test [5] [6] . Several ways of testing methods exist.
1.1. Serology
Serological tests involve detection of antibodies against H. pylori and they are very accurate. These antibodies may remain positive for years after successful eradication of H. pylori and therefore they are not used for checking the success of treatment because the antibody levels in the blood decreases slowly [7] [8] .
1.2. Stool Antigen Test
It is a recently developed alternative to the urea breath test that needs further evaluation in the clinical setting. It appears to be a useful alternative for assessing active infection, with the same caveats about its use. However, it appears somewhat less accurate for assessing treatment success [8] - [10] .
1.3. Histology
H. pylori infection can be diagnosed accurately by histology if special stains are used. The distribution of gastritis may give information on disease risk if biopsies are taken from antrum and corpus. Histology can also give information on whether gastric atrophy or intestinal metaplasia-markers of increased risk of gastric adenocarcinoma is present [8] [11] .
An important downstream marker of inflammation, C-reactive protein (CRP), is one of the acute phase proteins that increase during systemic inflammation [12] [13] . It has also been reported that measurement of serum levels of CRP using a high sensitivity assay (hs-CRP) can reveal subclinical inflammatory states that may reflect vascular inflammation [14] . Elevated levels of markers such as CRP, are associated with pathological changes, and hence their values give useful information for exact diagnosis and therapy [15] .
The aims of the study were to assess:
1) the relationship between endoscopic findings and different H. pylori tests.
2) the relationship between H. pylori infection and hs-CRP.
2. Materials and Methods
This was a prospective study, 100 patients with dyspepsia, attending Kurdistan Teaching Center of Gastroenterology & Hepatology in Sulaimani City were assessed, during the period of December 2012 to March 2014.Those who were pregnant, had fever, previously treated for H. pylori infection or who had received antibiotics, proton pump inhibitors or bismuth compounds in the preceding 4 weeks had been excluded.
The study was approved by the Ethics Committee for Analysis of Research Projects-Clinical Direction of the Hospital and the Faculty of Medicine, University of Sulaimani. Written informed consent was obtained from
each patient prior to participation in the study.
A form designed to collect demographic data; name, age, gender, chief complain of patients and duration of illness. Under aseptic condition, five ml venous blood aspirated then centrifuged at 5000 r/min for 5 minutes and serum was stored at −20˚C for later analysis for H. pylori IgG, IgA and hs-CRP.
2.1. Serum ELISA Test
Sera were tested for H. pylori IgG & IgA antibodies at Sulaimani Central Laboratories, using ELISA tests (NovaLisa, NovaTec, Germany), and according to the standard operating procedures. The test has a sensitivity of 97% and a specificity of 98.8%. The normal (negative) value of H. pylori for IgG < 8.0 AU/ml and IgA < 1.2 Ndx [16] . Briefly, diluted serum samples were added to the coated wells with biotinylated conjugate solution and incubated before addition of a peroxidase-bound secondary immunoglobulin, incubation, and finally addition of a substrate showing H. pylori status.
hs-C-reactive protein (CRP) was determined based on immunoenzymometric assay (Monobind Inc., Accu- Bind ELISA kit, hs-CRP: 3125-300, USA). Normal range as follows: low risk: <1.0 mg/L, average risk: 1.0 - 3.0 mg/L, high risk: >3.0 mg/L.
2.2. Stool Antigen Immunoassay Test
Stool specimens were tested using the stool antigen test kits (Rapid Immunoassay method, Coris Bioconcept C-1019, Belgium). A diluted feces sample and a peroxidase conjugated to antibody were added to the wells and incubated for1 hour at room temperature. A wash was performed to remove unbound material. The substrate was added and incubated for 10 min at room temperature. Color developed in the presence of bound enzyme Stop solution was added and the results were interpreted by spectrophotometer.
2.3. Endoscopy and Histology
After fasting overnight, esophago-gastro-duodenoscopy was performed with an Olympus Videoscope OEV203/ Japan, 4 gastric biopsies (from antrum and body) were taken from each patient. Biopsies were sent to the laboratory in 10% formalin solution for histological examination (Hematoxylin and eosin stain and modified Giemsa stain). The histological findings from the section were scored according to the updated Sydney system of classification and grading of gastritis [17] .
2.4. Statistical Analysis
Statistical analysis were performed using SPSS (statistical package of social science), version 21. Results were presented as mean ± standard deviation for quantitative variables and number (percentages) for qualitative variables. The differences in such level was considered as significant when P < 0.05.
3. Results
The study sample included 100 patients, 54% were males and 46% were females, giving a male to female ratio of 1.2:1. The age ranged from 20 - 49 years with a mean of 34.2 ± 8.5 years (Figure 1).
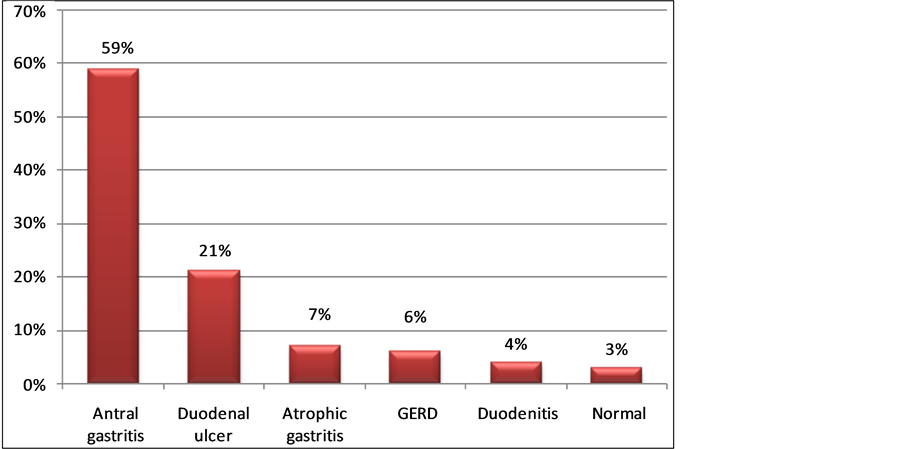
The commonest OGD findings were antral gastropathy (59%) and duodenal ulcer (21%) (Figure 2).
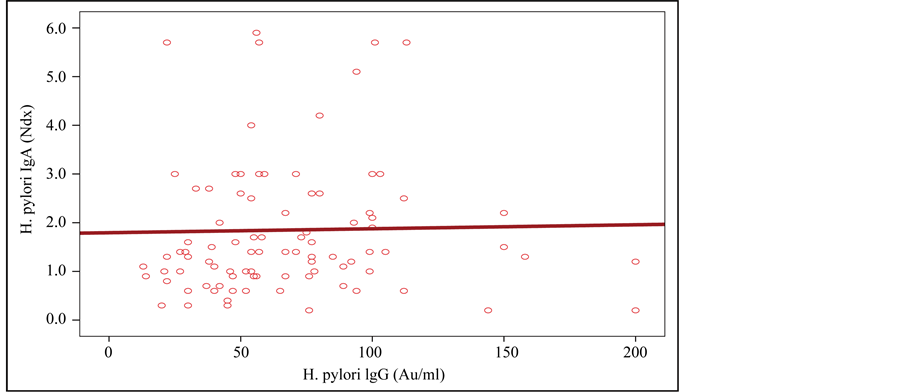
No correlation between H. pylori IgG level and H. pylori IgA level was found (P > 0.05) (Figure 3).
A statistically significant correlation (P < 0.01) between hs-CRP and H. pylori antibodies (IgG, IgA) levels was found (Table 1).
There were statistically significant associations between the level of H. pylori IgG and the results of endoscopic findings, (P < 0.01). The highest titer level of H. pylori IgG found in duodenal ulcer and antral gastritis, (88.86 ± 42.0) and (70.05 ± 35.2) Au/ml, respectively, while the highest level of H. pylori IgA found in antral gastritis and duodenal ulcer, (2.09 ± 1.58) and (1.76 ± 1.29) Ndx, respectively but statistically was not significant P > 0.05 (Table 2).
There were highly significant associations between endoscopic findings and Antral biopsy (P < 0.01), the highest percentage found in duodenal ulcer, antral gastritis and duodenitis 100%, 94.9% and 75% respectively (Table 3).
Figure 1. Distribution of patients by gender.
Figure 2. Oesophagogastroduodenoscopy findings in the study sample.
Figure 3. Relationship between H. pylori IgG level and H. pylori IgA level.
The relationship between endoscopic findings and stool antigen for H. pylori was not statistically significant, P > 0.05 (Table 4).
Comparing antigen for H. pylori serologic testes and stool antigen with antral histological examination as gold standard for diagnosis of H. pylori infection is shown in Table 5.
Table 1. Relationship between H. pylori and hs-CRP in patients.
Table 2. Relationship between endoscopic diagnosis and H. pylori IgG, H. pylori IgA.
**Highly significant difference P < 0.01.
Table 3. Relation of endoscopic findings and antral biopsy.
Chi = 77.5, df = 5, P < 0.01.
Table 4. Relationship between endoscopic findings and antigen for H. pylori.
Chi = 4.5, DF = 5, P > 0.05.
4. Discussion
Helicobacter pylori is worldwide in distribution, it is one of the commonest and probably most chronic infection throughout the world [18] . In this study we found that H. pylori infection is more prevalent in male than female patients and this agrees with some studies [19] [20] whereas other studies did not find a significant gender difference for aquision of H. pylori [21] .
The age pattern is closely similar to those of other studies, with very few presenting before the age of 20 years, peaking in the fifth decade and a mean age of 42 ± 1.1 years probably because upper gastrointestinal tract
Table 5. Test performance with its corresponding 95% confidence intervals for each diagnostic test.
diseases are prevalent in this population groups [22] [23] . The reason for the differences in the sex and age distribution is probably due to varying sample size; age groups, geographical locations and time periods the studies were carried out [24] .
In this study, UGI endoscopy study revealed abnormal findings in 97% of the patients, similar finding found by Nkrumah in Saudi Arabia that noted 94% [25] while Agbakwuru in Nigeria recorded 66% [22] .
In the current study, the most common endoscopic findings were gastritis and duodenal ulcer occurring in 59% and 21% respectively. Walker et al. also observed duodenal ulcer as the commonest findings in their studies i.e. 20.1% [26] . The prevalence of duodenal ulceration encountered was also comparable to those seen in Kenya (21%) and pooled African data (26%) [27] [28] . We found gastritis in 59% of patients while it was 35% obtained by Agbakwuru et al. in Nigeria [23] and 31% by Nkrumah in Saudi Arabia [23] . The majority of patients infected with H. pylori develop acute gastritis [29] .
Some studies have focused on the effect of H. pylori infection on hs-CRP levels and showed higher serum levels of hs-CRP in H. pylori-infected patients than in non-infected healthy control groups [30] [31] .
We found a significant correlation between hs-CRP levels and H. pylori-infected patients, and these results agreed with Kebapcilar et al. reports [32] .
CRP is a marker of inflammation and infection of the gastric mucosa with H. pylori, which causes an inflammatory reaction, the higher concentration of hs-CRP in the H. pylori-infected group may be due to induction of subclinical micro-inflammatory reactions by H. pylori while low concentration of hs-CRP in the healthy H. pylori-negative group might represent physiological status [30] [33] .
It was previously demonstrated that serum levels of hs-CRP were significantly reduced in most H. pylori- infected patients after successful eradication of H. pylori [34] .
Although several diagnostic tests are available for the detection of H. pylori infection, all of them have both advantages and disadvantages. To define the value or usefulness of a diagnostic test, each test has to be compared to a gold standard test [35] .
Bhat et al. showed that the mucosal CagA-specific IgA antibodies are produced during the acute phase of gastric inflammation and are of poor sensitivity [36] . Nearly all infected individuals (>90%) exhibit H. pylori-specific IgG antibodies and these can be used for diagnosis of infection, for these reasons there is no relation between IgG and IgA [37] .
Antral gastritis, duodenal ulcer and duodenitis were higher among infected patients with positive H. pylori in Antral biopsy, stool antigen and high mean and SD in serology (IgG and IgA), those explained by the mainly etiology factor of Antral gastritis and duodenal Ulcer are due to H. pylori infection [4] [38] [39] While GERD and Atrophic gastritis reported low mean and percentage through these tests because the main etiologic factor was not related to H. pylori infection [38] [40] . Normal endoscopy may represent functional dyspepsia in which the relationship with H. pylori is somewhat controversial [30] .
We observed that the immunological response of anti-H. pylori IgG antibodies were greater than that of H. pylori IgA antibodies. The lower IgA seropositivity in samples supports the transient nature of infections [41] .
It has been reported that detection of H. pylori antigens in stool is a suitable noninvasive method for clinical and epidemiologic studies [42] [43] .
5. Conclusion
In our study, the performance of stool antigen test in adult patients is good because it is simple, cheap with a sensitivity, specificity, and accuracy of 65%, 47% and 61%, respectively. False positives (low specificity) of stool antigen test can be explained by several mechanisms including the occurrence of transient H. pylori infection (spontaneous clearance of the infection) which has been reported as a common phenomenon in children [44] . The exposure to antibiotic therapy results in the conversion of the bacillary form to a coccoid form of H. pylori that is the morphological manifestation of bacterial cell death without an infective capacity. Thus, after eradication treatment, the H. pylori stool antigen test might detect antigen protein profiles resulting from the degradation of the two different morphobiological forms of H. pylori [45] - [47] .
Acknowledgements
We like to thank Dr. Hadeel Abdulqadir for her help and cooperation.
Competing Interests
None.
References
- Sobieraj, D.M., Coleman, S.M. and Coleman, C.I. (2011) US Prevalence of Upper Gastrointestinal Symptoms: A Systematic Literature Review. American Journal of Managed Care, 17, 449-458.
- Roussos, A., Philippou, N. and Gourgoulianis, K.I. (2003) Helicobacter pylori Infection and Respiratory Diseases: A Review. World Journal of Gastroenterology, 9, 5-8.
- Jia, E.Z., Zhao, F.J., Hao, B., Zhu, T.B., Wang, L.S., Chen, B., et al. (2009) Helicobacter pylori Infection Is Associated with Decreased Serum Levels of High Density Lipoprotein, but Not with the Severity of Coronary Atherosclerosis. Lipids in Health and Disease, 23, 59. http://dx.doi.org/10.1186/1476-511X-8-59
- Atherton, J. (2006) The Pathogenesis of H. pylori Induced Gastro-Duodenal Diseases. Annual Review of Pathology: Mechanisms of Disease, 1, 63-96. http://dx.doi.org/10.1146/annurev.pathol.1.110304.100125
- Stenström, B., Mendis, A. and Marshall, B. (2008) Helicobacter pylori—The Latest in Diagnosis and Treatment. Australian Family Physician, 37, 608-612.
- Kusters, J.G., van Vliet, A.H. and Kuipers, E.J. (2006) Pathogenesis of Helicobacter pylori Infection. Clinical Microbiology Reviews, 19, 449-490. http://dx.doi.org/10.1128/CMR.00054-05
- Ho, B. and Marshall, B.J. (2000) Accurate Diagnosis of Helicobacter pylori. Serologic Testing. North American Journal of Clinical, 29, 853-862.
- Brown, L.M. (2000) Helicobacter pylori: Epidemiology and Routes of Transmission. Epidemiologic Reviews, 22, 283- 297. http://dx.doi.org/10.1093/oxfordjournals.epirev.a018040
- Murray, P.R., Rosental, K.S. and Pfllar, M.A. (2005) Medical Microbiology. 5th Edition.
- Debabrata, M., James, B. and John, A. (2007) Helicobacter pylori Infection and Peptic Ulcers. Journal of Medicine, 35, 204-209. http://dx.doi.org/10.1016/j.mpmed.2007.01.006
- Cirak, M.Y., Akyön, Y. and Mégraud, F. (2007) Diagnosis of Helicobacter pylori. Helicobacter, 12, 4-9. http://dx.doi.org/10.1111/j.1523-5378.2007.00542.x
- Libby, P. and Ridker, P.M. (2004) Inflammation and Atherosclerosis: Role of C-Reactive Protein in Risk Assessment. The American Journal of Medicine, 116, 9-16. http://dx.doi.org/10.1016/j.amjmed.2004.02.006
- Blake, G.J. and Ridker, P.M. (2003) C-Reactive Protein: A Surrogate Risk Marker or Mediator of Atherothrombosis? American Journal of Physiology, 285, R1250-R1252.
- Wilson, A.M., Ryan, M.C. and Boyle, A.J. (2006) The Novel Role of C-Reactive Protein in Cardiovascular Disease: Risk Marker or Pathogen. International Journal of Cardiology, 106, 291-297. http://dx.doi.org/10.1016/j.ijcard.2005.01.068
- Grdanoska, T., Zafirovska, P., Jaglikovski, B., Pavlovska, I., Zafirova, B., Tosheska-Trajkovska, K., et al. (2012) Chlamydia pneumoniae and Helicobacter pylori Serology: Importance in Patients with Coronary Heart Disease. Materia Socio Medica, 24, 151-156. http://dx.doi.org/10.5455/msm.2012.24.151-156
- Al-Balushi, M.S., Al-Busaidi, J.Z., Al-Daihani, M.S., Shafeeq, M.O. and Hasson, S.S. (2013) Sero-Prevalence of Helicobacter pylori Infection among Asymptomatic Healthy Omani Blood Donors. Asian Pacific Journal of Tropical Di- sease, 3, 146-149. http://dx.doi.org/10.1016/S2222-1808(13)60059-6
- Price, A.B. (1999) Classification of Gastritis—Yesterday, Today and Tomorrow. Verhandlungen der Deutschen Gesellschaft für Pathologie, 83, 52-55.
- Blecker, U., Lanciers, S. and Mahta, D. (1994) Familial Cluster of Helicobacter pylori Infection. Clinical Pediatrics, 13, 307-308.
- Baban, F.A. and Mohamma, M.O. (2003) The Prevalence of Helicobacter pylori in Upper Gastro Intestinal Disorders. Journal of Zankoy Sulaimani, Part A, 6, l-8.
- Valliani, A., Khan, F., Chagani, B., Khuwaja, A.K., Majid, S., Hashmi, S., et al. (2013) Factors Associated with Helicobacter pylori Infection, Results from a Developing Country-Pakistan. Asian Pacific Journal of Cancer Prevention, 14, 53-56. http://dx.doi.org/10.7314/APJCP.2013.14.1.53
- Abdallah, T.M., Mohammed, H.B., Mohammed, M.H. and Ali, A.A. (2014) Sero-Prevalence and Factors Associated with Helicobacter pylori Infection in Eastern Sudan. Asian Pacific Journal of Tropical Disease, 4, 115-119.
- Agbakwuru, E.A., Fatusi, A.O., Ndububa, D.A., Alatise, O.I., Arigbabu, O.A. and Akinola, D.O. (2006) Pattern and Validity of Clinical Diagnosis of Upper Gastrointestinal Diseases in South-West Nigeria. African Health Sciences, 6, 98-103.
- Onyekwere, C.A., Hameed, H., Anomneze, E.E. and Chibututu, C. (2008) Upper Gastrointestinal Endoscopy Findings in Nigerians: A Review of 170 Cases in Lagos. The Nigerian Postgraduate Medical Journal, 15, 126-129.
- Jeje, E., Olajide, T. and Akande, B. (2013) Upper Gastrointestinal Endoscopy: Our Findings, Our Experience in Lagoon Hospital, Lagos, Nigeria. Macedonian Journal of Medical Sciences, 6, 168-173.
- Nkrumah, K.N. (2002) Endoscopic Evaluation of Upper Abdominal Symptoms in Adult Patients, Saudi Aramco-Ai Hasa Health Center, Saudi Arabia. West African Journal of Medicine, 21, 1-4.
- Walker, T.D., Karemera, M., Ngabonziza, F. and Kyamanywa, P. (2014) Helicobacter pylori Status and Associated Gastroscopic Diagnoses in a Tertiary Hospital Endoscopy Population in Rwanda. Transactions of the Royal Society of Tropical Medicine and Hygiene, 108, 305-307. http://dx.doi.org/10.1093/trstmh/tru029
- Lule, G.N., Sang, F. and Ogutu, E.O. (1991) Helicobacter pylori in Peptic Ulcer Disease in Kenya. East African Medical Journal, 68, 324-327.
- Kidd, M., Louw, J.A. and Marks, I.N. (1999) Helicobacter pylori in Africa: Observations on an “Enigma within an Enigma”. Journal of Gastroenterology and Hepatology, 14, 851-858. http://dx.doi.org/10.1046/j.1440-1746.1999.01975.x
- Watari, J., Chen, N., Amenta, P.S., Fukui, H., Oshima, T., Tomita, T., et al. (2014) Helicobacter pylori Associated Chronic Gastritis, Clinical Syndromes, Precancerous Lesions, and Pathogenesis of Gastric Cancer Development. World Journal of Gastroenterology, 20, 5461-5473. http://dx.doi.org/10.3748/wjg.v20.i18.5461
- Jafarzadeh, A., Hassanshahi, G.H. and Nemati, M. (2009) Serum Levels of High-Sensitivity C-Reactive Protein (hs-CRP) in Helicobacter pylori-Infected Peptic Ulcer Patients and Its Association with Bacterial CagA Virulence Factor. Digestive Diseases and Sciences, 54, 2612-2616. http://dx.doi.org/10.1007/s10620-008-0686-z
- Stettin, D., Waldmann, A., Ströhl, A. and Hahn, A. (2008) Association between Helicobacter pylori-Infection, C-Re- active Protein and Status of B Vitamins. Advances in Medical Sciences, 53, 205-213.
- Kebapcilar, L., Bilgir, O., Cetinkaya, E., Akyol, M., Bilgir, F. and Bozkaya, G. (2010) The Effect of Helicobacter pylori Eradication on Macrophage Migration Inhibitory Factor, C-Reactive Protein and Fetuin-A Levels. Clinics, 65, 799- 802. http://dx.doi.org/10.1590/S1807-59322010000800010
- Yoshiko, I., Koji, S., Kentaro, T., Toshimitsu, N., Shozo, K. and Hisao, A. (2008) Significant Association between Helicobacter pylori Infection and Serum C-Reactive Protein. Journal of Medical Sciences, 5, 224-229.
- Kanbay, M., Gür, G., Arslan, H., Yilmaz, U. and Boyacioĝllu, S. (2005) Does Eradication of Helicobacter pylori Infec- tion Help Normalize Serum Lipid and CRP Levels? Digestive Diseases and Sciences, 50, 1228-1231. http://dx.doi.org/10.1007/s10620-005-2764-9
- Guarner, J., Kalach, N., Elitsur, Y. and Koletzko, S. (2010) Helicobacter pylori Diagnostic Tests in Children: Review of the Literature from 1999 to 2009. European Journal of Pediatrics, 169, 15-25. http://dx.doi.org/10.1007/s00431-009-1033-x
- Bhat, N., Gaensbauer, J., Peek, R.M., Bloch, K., Tham, K.T., Blaser, M.J., et al. (2005) Local and Systemic Immune and Inflammatory Responses to Helicobacter pylori Strains. Clinical and Diagnostic Laboratory Immunology, 12, 1393-1400.
- Lenzi, C., Palazzuoli, A., Giordano, N., Alegente, G., Gonnelli, C., Campagna, M.S., et al. (2006) H pylori Infection and Systemic Antibodies to CagA and Heat Shock Protein 60 in Patients with Coronary Heart Disease. World Journal of Gastroenterology, 12, 7815-7820.
- Dixon, M.F., Genta, R.M., Yardley, J.H. and Correa, P. (1996) Classification and Grading of Gastritis: The Updated Sydney System. American Journal of Surgical Pathology, 20, 1161-1181. http://dx.doi.org/10.1097/00000478-199610000-00001
- Dixon, M. (2001) Pathology of Gastritis and Peptic Ulceration. In: Mobley, H.L.T., Mendz, G.L. and Hazell, S.L., Eds., Helicobacter pylori: Physiology and Genetics, ASM Press, Washington DC, 459-469.
- Yamada, T., Alpers, D.H., Kalloo, A.N., Kaplowitz, N., Owyang, C. and Powell, D.W. (2009) Textbook of Gastroenterology. 5th Edition, Blackwell Publishing, Oxford, 925.
- Pérez-Pérez, G.I., Sack, R.B., Reid, R., Santosham, M., Croll, J. and Blaser, M.J. (2003) Transient and Persistent Helicobacter pylori Colonization in Native American Children. Journal of Clinical Microbiology, 41, 2401-2407. http://dx.doi.org/10.1128/JCM.41.6.2401-2407.2003
- Koletzko, S. (2005) Noninvasive Diagnostic Tests for Helicobacter pylori Infection in Children. Canadian Journal of Gastroenterology, 19, 433-439.
- Pourakbari, B., Mirsalehian, A., Maleknejad, P., Mamishi, S., Azhdarkosh, H., Daryani, N.E., et al. (2011) Evaluation of a New Antigen for Diagnosis of Helicobacter pylori Infection in Stool of Adult and Children. Helicobacter, 16, 42- 46. http://dx.doi.org/10.1111/j.1523-5378.2010.00813.x
- Leal, Y.A., Flores, L.L., García-Cortés, L.B., Cedillo-Rivera, R. and Torres, J. (2008) Antibody-Based Detection Tests for the Diagnosis of Helicobacter pylori Infection in Children: A Meta-Analysis. PLoS ONE, 3, e3751. http://dx.doi.org/10.1371/journal.pone.0003751
- Forné, M., Domínguez, J., Fernández-Bañares, F., Lite, J., Esteve, M., Galí, N., et al. (2000) Accuracy of an Enzyme Immunoassay for the Detection of Helicobacter pylori in Stool Specimens in the Diagnosis of Infection and Post Treatment Check-Up. The American Journal of Gastroenterology, 95, 2200-2205. http://dx.doi.org/10.1111/j.1572-0241.2000.02303.x
- Kabir, S. (2001) Detection of Helicobacter pylori in Faeces by Culture, PCR and Enzyme Immunoassay. Journal of Medical Microbiology, 50, 1021-1029.
- Babak, P., Mona, G., Shima, M., Setareh, M., Hossein, A. and Mehri, N. (2013) Diagnosis of Helicobacter pylori Infection by Invasive and Noninvasive Tests. Brazilian Journal of Microbiology, 44, 795-798. http://dx.doi.org/10.1590/S1517-83822013005000052