International Journal of Clinical Medicine
Vol.3 No.7(2012), Article ID:25603,9 pages DOI:10.4236/ijcm.2012.37106
Potential Medical Benefits of Eating Curry: A Self-Reported Case and Review
![]()
Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, USA.
Email: BSeshi@labiomed.org
Received July 18th, 2012; revised November 10th, 2012; accepted November 20th, 2012
Keywords: Curry; Turmeric; Curcumin; Neuropathic Pain; Memory Disturbances; Prostate-Specific Antigen
ABSTRACT
Background: This report describes the case of a 61-year-old man who ate curry for 50 years. Three months after he stopped eating curry, he developed neuropathic pain. After 6 years of not eating curry, he experienced visuospatial disturbances, and his serum prostate-specific antigen (PSA) increased from 1.91 to 5.38 ng/mL. He tested whether normal aging or the dietary change accounted for his PSA data and symptoms. This self-reported case is the first of its kind in the medical literature. Method and Results: The subject developed a vegetable curry recipe that included turmeric/ curcumin. After 7 to 10 days of eating meals containing this curry twice daily, his pain decreased noticeably, and his visuospatial memory returned. After 8 to 9 weeks of eating curry, his PSA level dropped to 3.85 ng/mL. Using a sensitive high-performance liquid chromatography method with a detection limit of 1 ng/mL curcumin, his plasma unmodified curcumin level was 2.89 ng/mL after eating a curry meal and 4.56 ng/mL after fasting for 13 h. Detection of curcumin in the blood is important because curcumin has very low oral bioavailability, and plasma curcumin has not been detected in several previous clinical trials even after administration of gram quantities of unformulated 95% curcuminoid extract. This report also presents several converging lines of evidence that may account for the apparent salutary effect of restoring curry to one’s diet. Conclusions: The results advance our understanding of curcumin effects at the level of the individual. This original case report should be of interest to a wide clinical audience that spans several clinical specialties, including neurology, urology and diabetology.
1. Introduction
Curry is an Indian dish known for centuries. The word curry by itself is rarely used; instead, the terms “bean curry”, “potato curry”, and “spinach curry”, etc., signify the dish. Eating curry is thought to be beneficial to human health. General epidemiologic investigations have reported an increased incidence of cancer and other diseases in Indian immigrants in western countries, presumably a consequence of a cessation of eating curry, the adoption of a poor diet, or both [1,2]. One specific epidemiologic study reported an association between better cognitive performance and increased curry consumption in elderly people [3]. However, no published work has documented a direct effect of curry consumption on human health.
The individual documented in this case report ate food containing curry daily for over 50 years and then stopped eating it because of frequent gastrointestinal upset occurring after eating curry. He developed several medical problems that were reversed when he resumed eating curry, thus documenting for the first time the presumed direct effects of curry consumption on human health.
2. Case Study
This self-report describes a 61-year-old man who was born and raised in southern India and who has lived in the US for 31 years. He is a physician (hematopathologist) and a biomedical research investigator.
2.1. Neuropathic Pain
In the ensuing 3 months after the subject stopped eating curry, he developed persistent pain and burning sensation of unknown etiology in his legs, especially in the feet, with the intensity generally increasing over the course of the day. All available laboratory and radiologic investigations including magnetic resonance imaging were negative or showed minimal age-related changes (Table 1). Specifically, electromyography and nerve conduction velocity tests were normal, although these tests do not detect C-fiber or small-fiber neuropathy. No skin biopsy was performed to assess nerve fiber endings. The pain
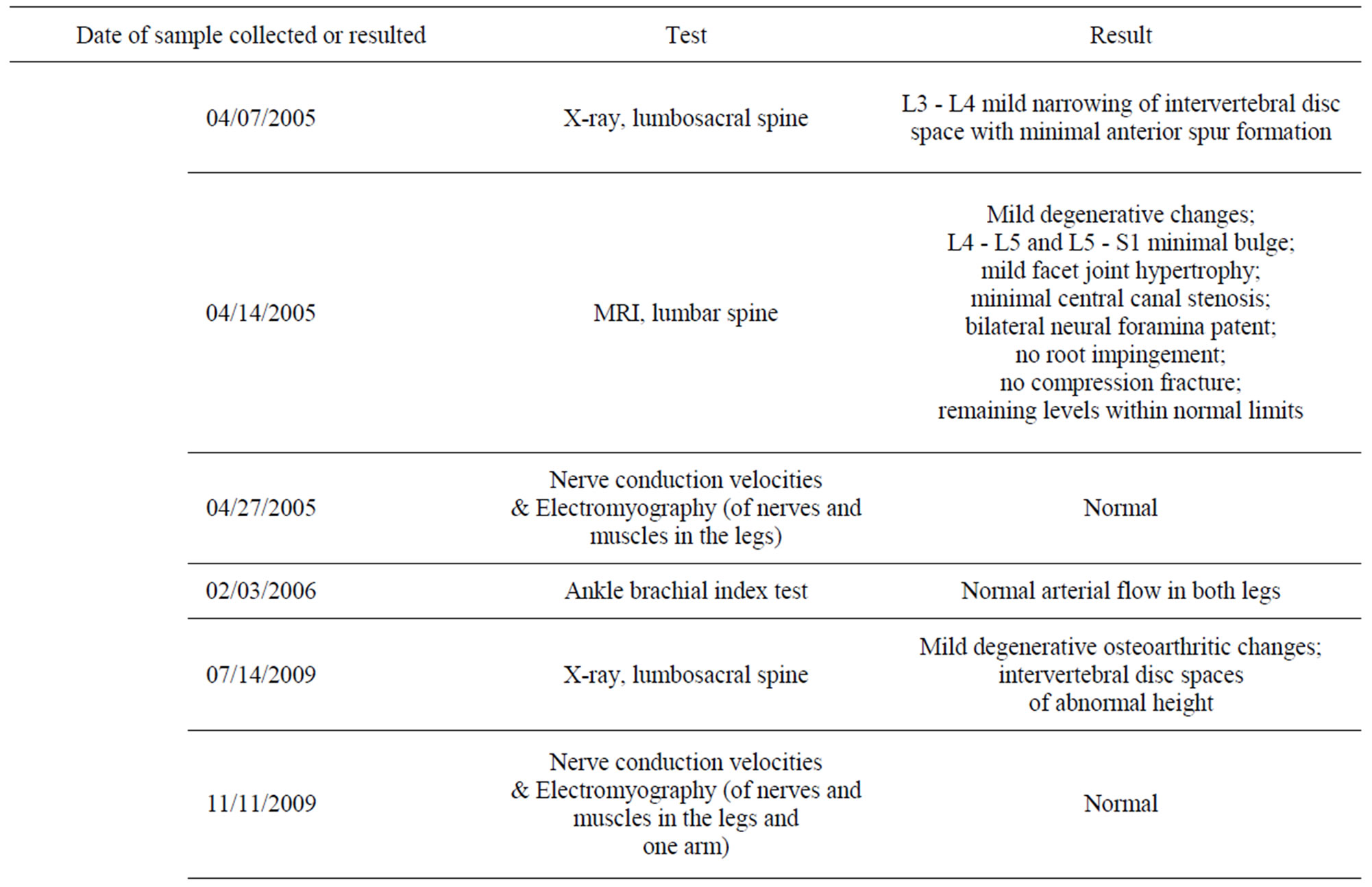
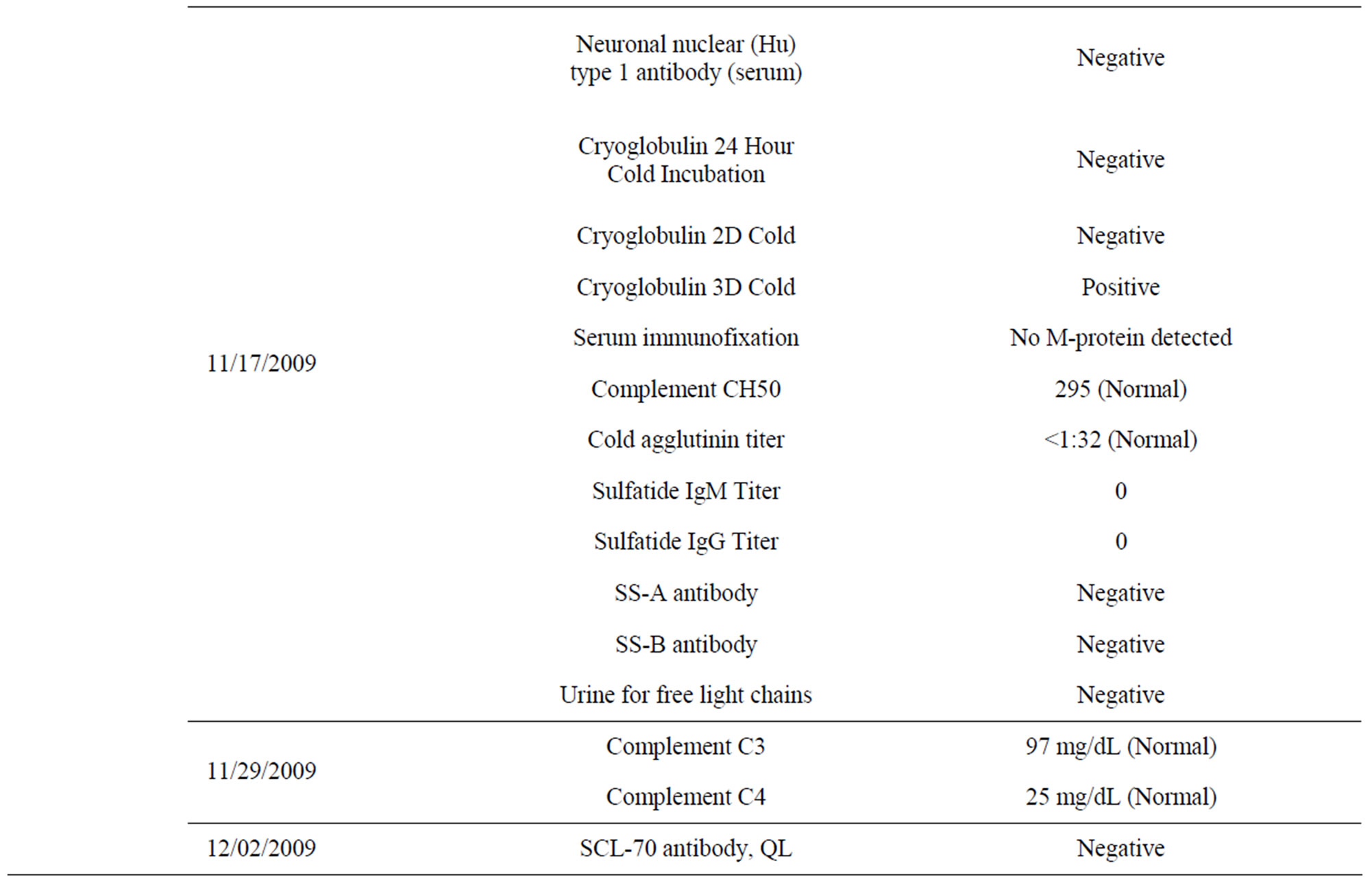
Table 1. Summary of neuropathy workup for the subject in this case study.
persisted for 6 years. During that period, whenever the subject had the opportunity to eat curry, he felt better; and his pains were greatly relieved, although temporarily. He began to suspect that resuming curry consumption might reduce his pain. However, he rarely ate curry during this time because he is a busy professional, cooking a curry meal is time consuming, and the curry dishes caused gastrointestinal disturbances because they generally included a variety of spices.
2.2. Problems with Visuospatial Memory
After approximately 6 years of not eating curry, the subject experienced problems with visuospatial memory and became confused and spatially disoriented on the street. This was frightening because he took pride in his excellent memory.
2.3. Elevated PSA Level
Upon visiting his physician, the subject’s prostate-specific antigen (PSA) level was measured using the Roche E170 modular analytic system, an electrochemiluminescence immunoassay method. During the 6- to 7-year period of not eating curry, his PSA level rose from 1.91 to 3.19 to 5.38 ng/mL (Table 2). Urine cultures were negative for microorganisms each time. He took a course of ciprofloxacin after the last PSA measurement even though his urologist insisted that it was not indicated. During this time, the subject began to suspect that a single explanation—namely a lack of curry consumption— could account for the neuropathic pain, visuospatial memory problem, and elevated PSA level.
3. Method and Results
3.1. The Patient’s Perspective
The parsimonious explanation for the subject’s symptoms was age-related changes. However, the subject also realized that he had made a substantial change in his diet. The key question, therefore, was whether the symptoms were due to normal aging or the dietary change. He suspected that the dietary change was responsible because he was otherwise quite healthy. It was difficult for the subject and his physician to make sense of his neuropathic pain in isolation. However, two other findings provided more clues, as noted in Sections 2.2 and 2.3.
3.2. The Method
To determine the validity of his suspicion that the cessa-
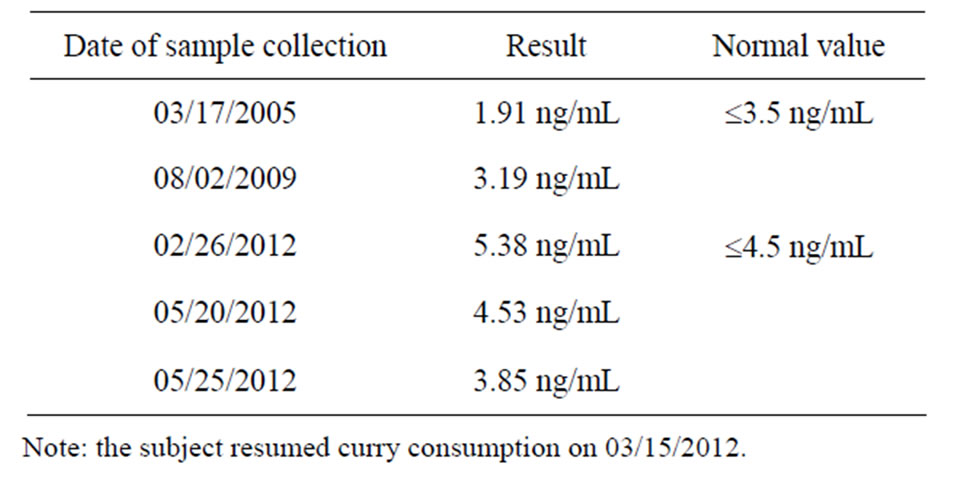
Table 2. Prostate-specific antigen (PSA) level over 7 years for the subject in this case study.
tion of eating curry was a causative factor of his ailments, the subject devised a vegetable curry recipe (Supplementary Appendix) that includes turmeric powder (Curcuma longa, also known as “the holy powder” and Indian saffron), which contains a bioactive small molecule, curcumin, that is a known regulator of hundreds of genes (curcumin is not to be confused with the similar-sounding cumin seeds). The curry recipe also included chili powder, which contains capsaicin that blocks substance P, a pain mediator. The recipe excludes many standard curry ingredients, such as, cumin seeds, fenugreek seeds, mustard seeds, chili seeds, dry chilies, cloves, coriander seeds, ginger, and garlic, all of which can adversely affect the gastrointestinal tract in some individuals. He prepared and consumed this curry daily.
3.3. Relief from Symptoms, and Laboratory Results
After 7 to 10 days of eating the new curry twice daily, his pain became manageable, and his visuospatial memory was mostly restored. After 8 to 9 weeks of eating the curry, his visuospatial memory was completely restored, and his PSA level had decreased from 5.38 ng/mL (02/26/2012) to 4.53 ng/mL (05/20/2012) (Table 2). He also realized that he had performed strenuous physical exercise and drank one cup of tea and one cup of coffee within 3 h of having his blood drawn for testing on 05/20/2012, but not at the other times. Therefore, the PSA test was repeated after abstaining from strenuous physical exercise and from drinking coffee for 48 h, and the result was 3.85 ng/mL (05/25/2012) (Table 2). Because the National Center for Complementary and Alternative Medicine, NIH, reports high doses of curcumin supplements can cause liver damage in animals (http://nccam.nih.gov/health/turmeric/ataglance.htm), liver function tests were also performed at the same time; these parameters were found to be within normal limits (Table 3). These data supported the efficacy and safety of the curry-eating regimen. It may also be noted that his fasting blood glucose was lowered from 115 mg/dL (12/09/2004) to 93 mg/dL (07/07/2012) (Table 4) (see section 4.6 under Discussion). No objective tests are available for neuropathic pain and memory disruption. A curcumin test was performed using a validated high-performance liquid

Table 3. Liver function panel for the subject in this case study.
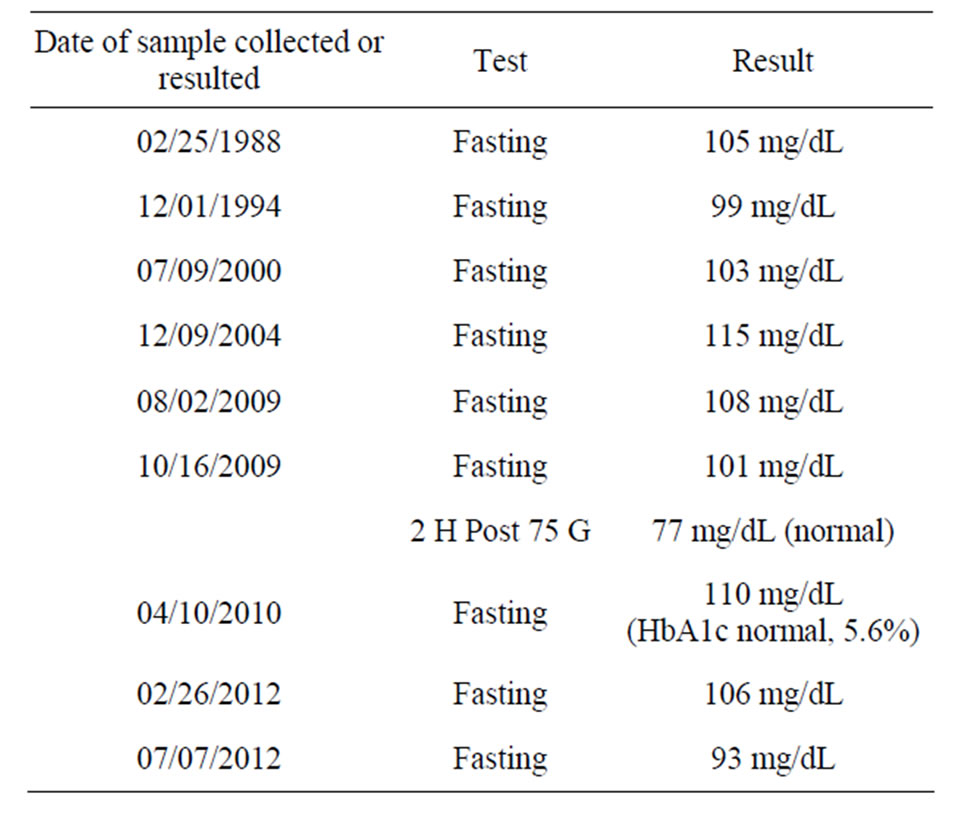
Table 4. Blood glucose level over 24 years for the subject in this case study.
chromatography method at the Department of Clinical Pharmacology, ACTREC, Tata Memorial Centre, Navi Mumbai, India. The limit of detection of the assay was 1 ng/mL curcumin. The subject’s plasma curcumin level (unmodified) was 2.89 ng/mL (postprandial, 1 h 45 min after eating a curry meal consisting of green bell pepper curry plus yogurt, 06/28/2012) and 4.56 ng/mL (fasting, 13 h after eating a curry meal, consisting of valor bean curry plus yogurt, 06/29/2012) (Table 5).
4. Discussion
There are no published case reports or clinical trials documenting the direct effects of curry consumption on human health. As such, the following is a review of the epidemiologic and basic biological effects of curcumin, highlighting the possibility that the absence of the curcumin component of curry is responsible for certain ailments. Several converging lines of data may explain the neuropathic pain, problems with visuospatial memory, and rising PSA levels.
4.1. General Epidemiologic Data on Curcumin Effects
The people of India have a very low (and perhaps the lowest) incidence of Alzheimer’s disease (for review [4]) and prostate cancer (for review [5]), both of which have been attributed to eating curry. In particular, one component of curry, turmeric, contains curcumin [4,5].
4.2. Neuroprotective and Anti-Inflammatory Effects of Curcumin
The neuroprotective, anti-amyloid, and anti-inflammatory effects of curcumin are emerging as important [4,6].
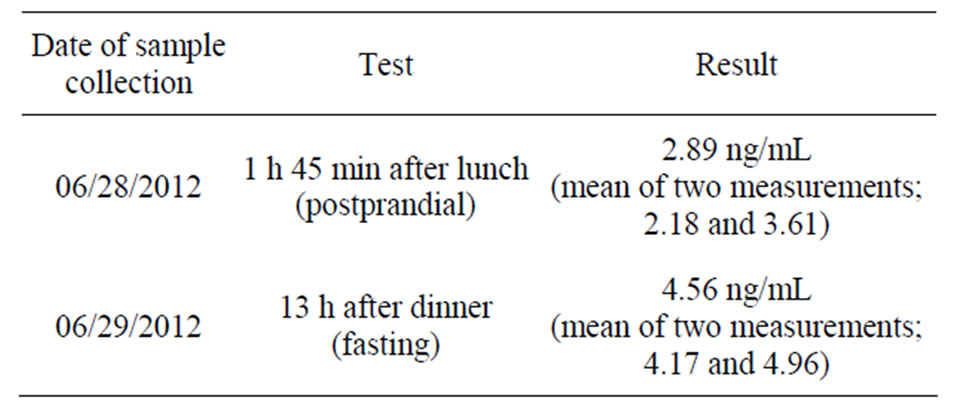
Table 5. Plasma curcumin level of the subject in this case study. Data represent the mean of readings from two assays.
Curcumin stimulates proliferation of embryonic neural progenitor cells [7], promotes neurogenesis in the hippocampus (the part of brain responsible for the formation of memories) in adult mice [7], modulates levels of neurotransmitters such as serotonin and dopamine in the mouse brain [8], and reverses impaired hippocampal regeneration in rats [9]. Finally, one epidemiologic study in humans reported an association between better cognitive performance and increased curry consumption in elderly people [3].
4.3. Anticancer Effects of Curcumin
Curcumin may induce epigenetic changes and thereby regulate gene expression without altering the DNA sequence [10]. The anticancer effects of curcumin are similarly striking as curcumin seems to control the upregulation or downregulation of hundreds of genes encompassing a variety of cancer-related genetic and signaling pathways, including those of prostate cancer [5, 11]. In addition, curcumin downregulates PSA expression in human prostate cancer cells [12]. Regardless of the recent dispute about the value of PSA testing for the diagnosis of prostate cancer (http://www.redorbit.com/ news/entertainment/1112539261/aua_disputes_panels_recommendations_on_prostate_cancer_screening/), the PSA finding in the subject may be a clinical validation of basic science data. In the current report, the subject did not develop prostate cancer, and instead the presumptive clinical diagnosis was prostatic hypertrophy.
4.4. Dietary Conditioning of Neuron Function
Curcumin induces a variety of epigenetic changes [10]. The majority of neurons, including those of the peripheral nervous system, normally do not divide during an individual’s lifetime [13]. Thus, whatever epigenetic changes may occur to the neuronal DNA as a result of exposure to curcumin during the early stages of life (prenatally, via a translactational route, or oral consumption) may be permanent. Therefore, neuronal function may be permanently modified and also dependent on eating curcumin for maintenance of optimal functionality. The following explanation is thus offered to account for published data and the results of the current self-reported case.
The DNA of individuals who grew up eating curry may become permanently modified epigenetically (or in other ways) such that they are dependent on certain curry components—in particular curcumin—for optimal gene function. Consequently, prolonged cessation of eating curry may result in a variety of “withdrawal” symptoms. Resumption of eating curry could possibly reverse these ailments. Such permanent dietary conditioning of neuronal function may occur with foods consumed by people of other ethnicities, but the effects may not be as powerful as those of curry.
This idea remains to be tested. Many Indian emigrants to other countries may stop or reduce their curry intake. No direct studies exist suggesting that the absence of curry leads to illness in Indians in western countries. However, general epidemiologic investigations have reported an increased incidence of cancer and other diseases in Indian immigrants in western countries [1,2]. These reports raise the question of whether such outcomes are due to the absence of eating curry, the adoption of a poor diet, or both. The diet of the male subject described in this case study was reasonably healthy in the 6-year period during which he did not consume curry. During the first 4 years of not consuming curry, he ate no breakfast but only a cup of strong coffee, ate lunch with other physicians, and ate dinners that generally consisted of steamed white rice bought from a Chinese restaurant mixed with a hot sauce, or more frequently mixed with fresh lime or lemon juice, salt, and olive oil. One invariant feature of the subject’s daily diet from childhood was a minimum of two servings of yogurt made of 2% fat or whole milk that was always mixed with rice (or other grains) and the curry all together. Traditionally, Indians generally do not mix “curry and yogurt,” whereas they always mix “rice and curry” and “rice and yogurt”. During the next 2 years, the subject’s breakfast included smoothies containing protein powder and several different fruits and vegetables. Lunches and dinners included a variety of whole grains cooked like rice or crepes, steamed vegetables, and salmon or chicken marinated in lemon juice, salt, olive oil, and saffron, and baked. For convenience, he ate one or two meals per week at a Persian or a Lebanese restaurant. On occasion, he also ate at Indian restaurants. There was never a change in the level of his consumption of yogurt. The subject believed that he ate healthy meals and was healthy in other respects, even though his specific health problems worsened.
4.5. Curcumin Effects on First Exposure
The total beneficial effects of curcumin may be due to a variety of mechanisms acting at different levels, including direct regulation of the levels of neurotransmitters in the brain [8]. Thus, even in individuals who have never consistently eaten curry-containing dishes, beginning such a diet may result in beneficial epigenetic or other changes. This idea is supported by studies showing beneficial effects of curcumin in animals that had never been exposed to it [7-9]. Thus, it is possible that the improvement of the three symptoms described for the male subject in this report was due to three separate mechanisms mediated by curcumin.
4.6. Curcumin Effects on Fasting Blood Glucose
The man in this report has a history of mildly elevated fasting blood glucose (but no diabetes). Impaired glucose tolerance is known to cause small-fiber neuropathy that is potentially reversible [14]. As shown in Table 4, however, the history of elevated fasting glucose predated the onset of problems described for this male subject, there was no specific trend in blood glucose level except that the levels were slightly high throughout, and the results of HbA1c and a glucose tolerance tests were normal. Thus, the improvement in neuropathy was not likely due to major shifts in glucose. After almost 4 months of eating curry (twice daily, involving different vegetables) and regularly exercising (6 days a week, during the latter 2 months), the subject’s blood glucose level ultimately was lowered from 115 mg/dL (12/09/2004) to 93 mg/dL (07/07/2012) (Table 4). The subject never previously ate curry with such regularity as during the most recent 3 months and 3 weeks. Because dietary curcumin has been shown to significantly improve blood glucose levels in mouse models [15], and because of the well-known impact of exercise on blood glucose, the beneficial drop in blood glucose may be due to a combined or synergistic effect of eating curry and exercising. Prospective controlled human studies should help distinguish the contributions of curcumin and exercise to fasting blood glucose level.
5. Conclusion and Future Directions
The subject’s plasma curcumin levels are consistent with the conclusion that relief from his ailments was due to eating curry. Curcumin has many physiological and pharmacological effects. It is unclear, however, how this information about curcumin, especially in culinary amounts, translates to an individual’s plasma curcumin level and what the health status is of people who grew up eating curry but who stopped eating it for a prolonged period. This section reviews the literature on the pharmacokinetics of curcumin, again with the hypothesis that curcumin might be the key medicinally relevant component of curry. Further, this section presents possible reasons why curcumin levels are detectable in this first case and provides future directions to establish the clinical relevance of consuming curry.
5.1. Lack of Clinical Studies Comparing Curcumin Effects in Pure Form vs Curry Form
The present case may represent an unintended “experiment of nurture”, involving a relatively lengthy departure from a “normal” diet¾the one to which one is accustomed¾that resulted in pathophysiological consequences. Resuming such a diet may provide important insights, both theoretical and practical. Replicating the symptoms by repeating the curry deprivation would provide further support for the symptoms being due to the absence of curry, but the subject does not wish to re-experience the frightening visuospatial memory disturbances. Although the curry diet appears safe and effective, the limitations of this report are that it is based on one individual, and it was not blinded. The existing scientific literature focuses on the direct testing of curcumin itself, the active component of turmeric, rather than turmeric as a component of a meal. The chemical structure of curcumin (diferuloylmethane) is similar to that of the Congo Red pigment that stains amyloid (for review [4]). Based on human studies, curcumin’s bioavailability is reduced by hepatic and intestinal glucuronidation (for review [4]). Because it is poorly absorbed, co-administration of piperine (an alkaloid present in black pepper), which inhibits glucuronidation, may be needed to enhance its absorption [16]. Curcumin in doses > 2000 mg was needed to achieve measurable levels in human blood (for review [4]). In several human studies, curcumin up to 1200 mg/day was well tolerated (for review [4]). Although the literature on curcumin is extensive, basic or clinical studies comparing the bioavailability and effects of curcumin in pure form vs. curry form have not been published.
5.2. First Report of Curcumin Levels in a Curry Eater
Considering the beneficial effects reported in the present case report and epidemiologic data from India, it is reasonable to conclude that the curry formulation described here likely provides a therapeutically adequate amount of curcumin, although no published studies have documented curcumin levels in the blood and/or other tissues of curry eaters. However, unpublished results of V. Gota et al. involving three healthy curry eaters and a large number of participants with various illnesses in their clinical trials prior to dosing with curcumin (ACTREC, Tata Memorial Centre, Navi Mumbai, India, cited here with permission) showed undetectable plasma curcumin levels. This observation is likely because the levels were below the detection limit, in contrast to the plasma curcumin levels of the individual in the current study of 2.89 ng/mL (postprandial) and 4.56 ng/mL (fasting) (Table 5). All the participants in the above-mentioned study were regular curry eaters; however, it appears that they may not necessarily have cooked their curry dishes the same way as described in this report, and/or those participants may not have eaten curry with the same regularity as the man in this report who consumed curry over 3.5 months. The observation that the subject’s fasting level of curcumin was higher than the postprandial level is interesting and may reflect biological variation or the different curry sources consumed preceding each sample collection (green bell pepper vs valor beans). Another possibility is that the peak level in the postprandial sample had not been attained, as curcumin uptake may differ from that of glucose. These data are unlikely to be the result of intra-assay variation because a repeat analysis of a second set of sample aliquots also showed the fasting level to be higher than the postprandial level (Table 5).
5.3. Reasons for the Detectable Levels of Curcumin in a Curry Eater
The levels of curcumin detected in the subject’s blood are consistent with the literature. Curcumin has poor oral bioavailability, and no detectable plasma levels of curcumin have been observed in several previous clinical trials even after administering gram quantities of unformulated 95% curcuminoid extract [17,18]. In some studies (for example, “Curcumin and Gingerol protocol for MDS-UMASS Medical School”), even mega doses of curcumin (4 - 8 g/day, and sometimes 12 g/day) are well tolerated, a claim that is unlikely to be useful because there is no evidence that curcumin is absorbed into the bloodstream. Blood plasma seems to contain the lowest amount of curcumin compared to other tissues following oral administration in rats [19]. The reasons for the detectable levels of curcumin in the blood of the individual in the current report are probably three-fold: 1) The curry formulation favors improved absorption. Curcumin is a lipid-soluble compound, and two of the ingredients in the curry dish described are lipids (olive oil and coconut powder), which likely aided its absorption; 2) The subject not only ate curry but also mixed it with yogurt (as a habit) and some grains. Such a culinary practice apparently has a favorable clinical effect, as yogurt was a good delivery system for curcumin in a rat model [20]. Chemical or other types of interactions, if any, between curcumin and yogurt components, such as organic acids, remain to be investigated; 3) As a consequence of regular curry consumption for 3.5 months, the curcumin in the subject’s system was probably equilibrated between the blood and other organs/tissues, resulting in a detectable amount in the circulation. The amount of curcumin consumed by the subject with each meal was relatively small because he added ~1/2 tsp turmeric (~1.25 g) to the curry dish (see Supplementary Appendix), divided the curry dish, and ate it with four meals, i.e., ~312 mg turmeric per meal. Considering that curcumin represents only 2% - 5% of turmeric [21], the intake of curcumin per meal was estimated at 6 - 15 mg, which is small compared to the gram quantities of curcumin administered in clinical trials. Thus, it is indeed remarkable that curcumin was detectable in the subject’s blood. The levels more likely reflect the body’s store of curcumin that accumulated over 3.5 months rather than from recent meals; the relevant pharmacokinetic mechanisms remain to be investigated.
5.4. Summary of Observations of the Present Case
The important observations from this case study are: 1) Consumption of large quantities of curcumin may not be necessary to achieve therapeutic benefits because such benefits were attained even with relatively low-level consumption by the subject in this case study; 2) The plasma curcumin levels of the subject were high compared with levels of regular curry eaters in India, but they were not as high as the levels obtained using formulated curcumins, such as Solid Lipid Curcumin Particle (22.43 ng/mL) [17] or Theracurmin (275 ± 67 ng/mL) [18]. However, potential deleterious effects of maintaining such high levels of plasma curcumin over the long-term are not known; 3) Moreover, higher levels of curcumin may not necessarily be more biologically effective because curcumin shows biphasic effects on cultured neural progenitor cells; low concentrations stimulate cell proliferation, whereas high concentrations are cytotoxic [7]. Of course, eating curry, as opposed to taking curcumin tablets, has additional health benefits because one is simultaneously eating a variety of vegetables and greens. The curry formulation presented in this report, although complex, has at least one measurable end analyte, namely curcumin. Because the curry recipe described here contains defined ingredients and the curry dish thus prepared is easily tolerated in the diet, it is amenable to a clinical trial. A randomized controlled clinical study could best determine the possible health benefits of eating curry. To facilitate large-scale clinical trials, the current lowthroughput, labor-intensive high-performance liquid chromatography methodology for measuring plasma curcumin levels should be replaced with a relatively inexpensive and practical assay. Eating curry over a long period may result in somewhat of a biological dependency, as evidenced by some of the long-term withdrawal effects described in this case report. Perhaps Indian physicians in the US and elsewhere will come forward to report any Indian patients who may have experienced a similar phenomenon.
In summary, although this is a case report, it has a high probability of being generalizable to other curry eaters. The data on the subject’s curcumin levels reinforce the objectivity of the report. This is the first report of curcumin levels in a curry eater. This report should provide a framework for future clinical and laboratory investigations and bring rapid advances to the field, targeting the individual person as the focus of investigation, and thus filling the void that exists between population-based epidemiologic data and cellular-based fundamental biological investigations of curcumin.
6. Acknowledgements
I thank Kousalya Reddy Chada and Narayanadas Vakamudi for helpful discussions. Dr. Marshall A. Lichtman (University of Rochester, NY) critically read the manuscript and made valuable comments and suggestions. Dr. Greg M. Cole (University of California, Los Angeles) carefully read the manuscript and drew my attention to published studies on the effects of curry consumption as related to cognitive function in the elderly and cancer incidence in emigrant Indians. I am grateful to Dr. Vikram Gota (ACTREC, Tata Memorial Centre, Navi Mumbai, India) for help with measuring the plasma curcumin levels in this case. I also thank Dr. Gota for sharing the unpublished results involving the curcumin levels of curry eaters. Finally, I thank Dr. C. C. Di Stasio, Dr. A. Chopra, and Dr. N. R. Doshi, physicians at Kaiser Permanente, for reviewing this report for accuracy of the clinical data and for their comments.
REFERENCES
- R. Sinha, D. E. Anderson, S. S. McDonald and P. Greenwald, “Cancer Risk and Diet in India,” Journal of Postgraduate Medicine, Vol. 49, No. 3, 2003, pp. 222- 228.
- T. Rastogi, S. Devesa, P. Mangtani, A. Mathew, N. Cooper, R. Kao and R. Sinha, “Cancer Incidence Rates among South Asians in Four Geographic Regions: India, Singapore, UK and US,” International Journal of Epidemiology, Vol. 37, No. 1, 2008, pp. 147-160. doi:10.1093/ije/dym219
- T. P. Ng, P. C. Chiam, T. Lee, H. C. Chua, L. Lim and E. H. Kua, “Curry Consumption and Cognitive Function in the Elderly,” American Journal of Epidemiology, Vol. 164, No. 9, 2006, pp. 898-906. doi:10.1093/aje/kwj267
- J. M. Ringman, S. A. Frautschy, G. M. Cole, D. L. Masterman and J. L. Cummings, “A Potential Role of the Curry Spice Curcumin in Alzheimer’s Disease,” Current Alzheimer Research, Vol. 2, No. 2, 2005, pp. 131-136. doi:10.2174/1567205053585882
- B. B. Aggarwal, “Prostate Cancer and Curcumin: Add Spice to Your Life,” Cancer Biology & Therapy, Vol. 7, No. 9, 2008, pp. 1436-1440. doi:10.4161/cbt.7.9.6659
- G. M. Cole, B. Teter and S. A. Frautschy, “Neuroprotective Effects of Curcumin,” Advances in Experimental Medicine and Biology, Vol. 595, 2007, pp. 197-212. doi:10.1007/978-0-387-46401-5_8
- S. J. Kim, T. G. Son, H. R. Park, M. Park, M. S. Kim, H. S. Kim, H. Y. Chung, M. P. Mattson and J. Lee, “Curcumin Stimulates Proliferation of Embryonic Neural Progenitor Cells and Neurogenesis in the Adult Hippocampus,” The Journal of Biological Chemistry, Vol. 283, No. 21, 2008, pp. 14497-14505. doi:10.1074/jbc.M708373200
- S. K. Kulkarni, M. K. Bhutani and M. Bishnoi, “Antidepressant Activity of Curcumin: Involvement of Serotonin and Dopamine System,” Psychopharmacology, Vol. 201, No. 3, 2008, pp. 435-442. doi:10.1007/s00213-008-1300-y
- Y. Xu, B. Ku, L. Cui, X. Li, P. A. Barish, T. C. Foster and W. O. Ogle, “Curcumin Reverses Impaired Hippocampal Neurogenesis and Increases Serotonin Receptor 1A mRNA and Brain-Derived Neurotrophic Factor Expression in Chronically Stressed Rats,” Brain Research, Vol. 1162, 2007, pp. 9-18. doi:10.1016/j.brainres.2007.05.071
- S. Reuter, S. C. Gupta, B. Park, A. Goel and B. B. Aggarwal, “Epigenetic Changes Induced by Curcumin and Other Natural Compounds,” Genes & Nutrition, Vol. 6, No. 2, 2011, pp. 93-108.
- A. Goel and B. B. Aggarwal, “Curcumin, the Golden spice from Indian Saffron, Is a Chemosensitizer and Radiosensitizer for Tumors and Chemoprotector and Radioprotector for Normal Organs,” Nutrition and Cancer, Vol. 62, No. 7, 2010, pp. 919-930. doi:10.1080/01635581.2010.509835
- K. H. Tsui, T. H. Feng, C. M. Lin, P. L. Chang and H. H. Juang, “Curcumin Blocks the Activation of Androgen and Interlukin-6 on Prostate-Specific Antigen Expression in Human Prostatic Carcinoma Cells,” Journal of Andrology, Vol. 29, No. 6, 2008, pp. 661-668. doi:10.2164/jandrol.108.004911
- J. Zhao, H. He, K. Zhou, Y. Ren, Z. Shi, Z. Wu, Y. Wang, Y. Lu and J. Jiao, “Neuronal Transcription Factors Induce Conversion of Human Glioma Cells to Neurons and Inhibit Tumorigenesis,” PloS One, Vol. 7, No. 7, 2012, Article ID: e41506. doi:10.1371/journal.pone.0041506
- A. G. Smith and J. R. Singleton, “Impaired Glucose Tolerance and Neuropathy,” The Neurologist, Vol. 14, No. 1, 2008, pp. 23-29. doi:10.1097/NRL.0b013e31815a3956
- S. P. Weisberg, R. Leibel and D. V. Tortoriello, “Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity,” Endocrinology, Vol. 149, No. 7, 2008, pp. 3549-3558. doi:10.1210/en.2008-0262
- M. K. Bhutani, M. Bishnoi and S. K. Kulkarni, “AntiDepressant Like Effect of Curcumin and Its Combination with Piperine in Unpredictable Chronic Stress-Induced Behavioral, Biochemical and Neurochemical Changes,” Pharmacology, Biochemistry, and Behavior, Vol. 92, No. 1, 2009, pp. 39-43. doi:10.1016/j.pbb.2008.10.007
- V. S. Gota, G. B. Maru, T. G. Soni, T. R. Gandhi, N. Kochar and M. G. Agarwal, “Safety and Pharmacokinetics of a Solid Lipid Curcumin Particle Formulation in Osteosarcoma Patients and Healthy Volunteers,” Journal of Agricultural and Food Chemistry, Vol. 58, No. 4, 2010, pp. 2095-2099. doi:10.1021/jf9024807
- M. Kanai, A. Imaizumi, Y. Otsuka, H. Sasaki, M. Hashiguchi, K. Tsujiko, S. Matsumoto, H. Ishiguro and T. Chiba, “Dose-Escalation and Pharmacokinetic Study of Nanoparticle Curcumin, a Potential Anticancer Agent with Improved Bioavailability, in Healthy Human Volunteers,” Cancer Chemotherapy and Pharmacology, Vol. 69, No. 1, 2012, pp. 65-70. doi:10.1007/s00280-011-1673-1
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, “Bioavailability of Curcumin: Problems and Promises,” Molecular Pharmaceutics, Vol. 4, No. 6, 2007, pp. 807-818. doi:10.1021/mp700113r
- V. O. Gutierres, C. M. Pinheiro, R. P. Assis, R. C. Vendramini, M. T. Pepato and I. L. Brunetti, “CurcuminSupplemented Yoghurt Improves Physiological and Biochemical Markers of Experimental Diabetes,” The British Journal of Nutrition, Vol. 108, No. 3, 2012, pp. 440-448.
- S. Shishodia, G. Sethi and B. B. Aggarwal, “Curcumin: Getting Back to the Roots,” Annals of the New York Academy of Sciences, Vol. 1056, 2005, pp. 206-217. doi:10.1196/annals.1352.010
Supplementary Appendix
Seshi’s Basic Curry Recipe
Three variations can be prepared: 1) plain; 2) tomatobased; and 3) tamarind-based. In the interest of time efficiency, make two curry dishes of a given variation side by side.
1) Wash the vegetable of choice (~2.0 lb) as appropriate (generally by soaking for 10 min in 1.25% distilled white vinegar, followed by rinsing in water);
2) Peel the vegetable, if required;
3) Cut the vegetable into small, bite-sized cubes;
4) Transfer the pieces to a flat-bottom pot (such as a vegetable steamer or a pressure cooker base), add just enough water to cover the pieces, and 1 tbsp iodized salt. Bring water to boil, and let vegetables boil for 2 min or until wilted;
5) Transfer the vegetable pieces to a salad spinner with running cold water, rinse 2 - 3 times, spin dry, and set them aside. Steps 4 and 5 a) minimize the rawness of vegetables, b) cleanse off pesticides, and c) ensure adequate cooking of vegetables, and consequently make vegetables easily tolerated by the gastrointestinal tract;
6) Repeat steps 1 - 5 for the second vegetable;
7) For tomato-based curry: Wash and cut four medium-sized tomatoes, and set the cut pieces aside;
8) For tamarind-based curry: Take tamarind of the size of two lemons, add 2 cups of water, microwave for 2 min, let cool, squeeze and strain the juice out, and set aside (Never use a pre-made commercial tamarind paste because it will adversely affect the taste);
9) Cut one large yellow onion using an onion chopper, and set the chopped onion aside;
10) Wash curry leaves (two twigs; Murraya koenigii), wipe the leaves dry, and set them aside;
11) Heat frying pan on low-to-medium heat (150˚F if on an induction hotplate). Then, sequentially add the following ingredients:
a) 4 - 6 tbsp cooking olive oil;
b) Curry leaves (a taste booster), once aroma begins, add the next item;
c) Chopped onion;
d) 1 tbsp iodized salt;
e) Turmeric (1/2 tsp) (provides color and enhances taste).
Continue mixing the contents until the onion pieces turn translucent (glistening).
For plain or tamarind-based curry: transfer 1/2 of the contents to a second frying pan.
For tomato-based curry: add tomato pieces to the above, and continue cooking for ~10 min until the mixture thickens. Then, transfer 1/2 of the contents to a second frying pan.
12) Add the pre-boiled vegetable and 1/4 to 1/2 cup water (depending on how soft you want to cook it, as it needs to be cooked until all the water is fully absorbed) to the above mixture in each pan. Cook until the mixture thickens and water is gone. Check and add salt to taste.
For tamarind-based curry: add 1 cup tamarind juice, in addition to or instead of water, to the mixture in each frying pan, and cook until the gravy achieves desired consistency. Add salt and sugar (to taste).
13) Finally, add 1/2 tbsp chili powder and 1 tbsp dry coconut powder (another taste booster, in addition to curry leaves) to each mixture;
14) Continue cooking for an additional 2 min. Turn off heat.
Notes
1) For plain curry, the procedure may be carried through 12 - 14 steps; or after step 11, add chili powder and coconut powder to the contents, mix, and cook for 2 min, and then add the pre-boiled vegetables (no water), mix, and transfer the contents to a baking pan, cover with aluminum foil, and bake for 20 min at 400˚F. As this baking part is performed unattended, it is a time saver;
2) Approximately 2.0 lb vegetable will yield a curry dish of approximately four servings. A serving is defined as the amount of curry eaten with a meal, such as lunch or dinner. The vegetables regularly cooked using the described method are those that are typically cooked in an Indian household: okra, green beans, gavar beans or cluster beans (Cyamopsis tetragonoloba), bitter melon, tindora (Coccinia grandis, the ivy gourd), taro root, toor dhal (Pisum sativum; pressure-cooked first), bottle gourd (Lagenaria siceraria), ridge gourd (Lufa acutangula), mixed greens (a mix of fresh spinach, mustard greens, turnip greens, and collard greens, as commercially sold), green bell pepper, broccoli, and cauliflower; onion by itself as a vegetable. Of course, other vegetables may also be considered, as per taste;
3) In the initial search to find a solution to the subject’s neuropathic pain, he first learned to cook a variety of whole grains like one would cook rice. He has been eating them for several years. He has thus been eating “healthy” otherwise and is healthy in other respects, but that did not help with the particular problems described here. He continues to eat different grains, but mixed with different curries;
4) Although the vegetable curry as described has been the main source of the subject’s curcumin, he also receives it in other minor ways, as he sometimes eats an egg omelet (prepared including salt and black pepper, plus turmeric, the latter mixed in olive oil first), and salmon and chicken dishes (prepared including turmeric, some ginger and lemon juice). Sometimes, before going to bed, or if he wakes up at night, he may drink a cup of warm whole milk with a pinch of turmeric and a spoonful of brown sugar or honey or a malted chocolate drink, Bourn-Vita, added to it. Alternatively, he may drink a cup of warm whole milk with a pinch each of salt and black pepper plus turmeric added to it. These additions fully account for his dietary sources of curcumin.

