Open Journal of Endocrine and Metabolic Diseases
Vol.3 No.2(2013), Article ID:31651,4 pages DOI:10.4236/ojemd.2013.32019
Overt Hypothyroidism in Hospitalized Patients: Clinical Characteristics
1Department of Medicine E, Meir Medical Center, affiliated with the Sackler Faculty of Medicine, Tel Aviv University, Kfar Saba, Israel
2Department of Geriatrics, Meir Medical Center, affiliated with the Sackler Faculty of Medicine,
Tel Aviv University, Kfar Saba, Israel
3Department of Diagnostic Imaging, Meir Medical Center, affiliated with the Sackler Faculty of Medicine,
Tel Aviv University, Kfar Saba, Israel
Email: pnina.rotman@clalit.org.il
Copyright © 2013 Pnina Rotman-Pikielny et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received December 27, 2012; revised January 28, 2013; accepted February 28, 2013
Keywords: hypothyroidism; hospitalized patients; inpatients
ABSTRACT
Objectives: Hypothyroidism is usually detected in an outpatient setting, but might be diagnosed during hospitalization. The prevalent symptoms are not fully defined. This study aimed to determine the clinical characteristics of in-patients with overt hypothyroidism. Methods: The medical records of 23 inpatients (16F/7M, age 61.5 ± 21.8 years), who had 29 admissions with a primary diagnosis of hypothyroidism from January 1997 to December 2007 were retrospectively reviewed. They comprised 0.01% of all adult medical admissions during the study. Results: Fifty-five percent had a pre-admission diagnosis of hypothyroidism, 10% were nursing home residents and 38% had cognitive decline. Sixtynine percent presented with multiple complaints, mostly weakness and constipation, reported by 89% and 68%, respectively. Thyrotropin level was 74.3 ± 53.5 mIU/L (normal 0.23 - 4) and free thyroxine was 0.43 ± 0.29 ng/dL (normal 0.8 - 2). Elevated creatinine phosphokinase, anemia (hemoglobin < 12 g/dL) and hyponatremia (sodium < 135 mEq/L) were present in 89%, 62%, and 13%, respectively. Conclusions: Overt hypothyroidism during hospitalization occurs infrequently and mostly in patients with previously diagnosed hypothyroidism. Clinical manifestations include multiple non-specific symptoms, mainly weakness and constipation, while typical “hypothyroid” symptoms such as cold intolerance and weight gain are often overlooked. A high index of suspicion is needed to detect hypothyroidism in the hospital setting.
1. Introduction
Hypothyroidism, one of the most common chronic disorders in western societies, is often diagnosed and treated in an outpatient setting. Typical symptoms include weakness, cold intolerance, dyspnea, weight gain, constipation, and cognitive dysfunction [1-3]. Infrequently, however, hypothyroidism is diagnosed in the hospital setting. This patient population, often elderly with comorbidities and sometimes cognitively impaired, may present with nonspecific symptoms, as was reported previously in older studies from the 1980s [4-6]. To the best of our knowledge, there are no recent studies addressing the clinical manifestations of patients with overt hypothyroidism during hospitalization. The aim of our study was to determine the clinical characteristics of hospitalized patients who were discharged with a primary diagnosis of overt hypothyroidism.
2. Materials and Methods
This retrospective study was performed at a secondary referral hospital, an 800-bed university-affiliated medical center. A computerized search of all hospitalizations between January 1, 1997 and December 31, 2007 identified patients with a primary discharge diagnosis of hypothyroidism.
Inclusion criteria were non-pregnant adults, 18 years or older, with laboratory findings consistent with overt hypothyroidism (elevated thyrotropin [TSH] level and reduced free thyroxine [fT4]). Patients with subclinical hypothyroidism (elevated TSH with fT4 within normal limits) were excluded. Demographic and clinical data were abstracted from the medical records. The prevalence of hypothyroidism during hospitalization was calculated by dividing the number of patients with a discharge diagnosis of overt hypothyroidism by the number of adult patients admitted to all medical departments (Internal Medicine, Intensive Care, Neurology, Cardiology, and Geriatrics) during the study period.
During this period, 69 patients with a primary diagnosis of hypothyroidism were discharged from the hospital. Of these, 46 did not meet the inclusion criteria, mainly due to laboratory findings compatible with subclinical hypothyroidism. The remaining 23 patients (16F/7M, age 61.5 ± 21.8 years) who were hospitalized with overt hypothyroidism comprised the study group. Twenty-one patients were hospitalized once, whereas two patients were hospitalized two and six times each, for a total of 29 admissions.
Serum TSH levels were measured using a continuous random access analyzer (Immulite; Diagnostic Products Corp, Los Angeles, CA, USA) with normal range of 0.23 mIU/L to 4 mIU/L. Free T4 levels were measured as described, with normal values of 0.8 ng/dL to 2.0 ng/dL [7]. Serum creatine phosphokinase (CPK) level was measured on an Olympus 5200 autoanalizer (Olympus System Reagents, Dublin, Ireland).
This study was approved by the ethical committee of the medical center and informed consent was not required.
3. Results
Tables 1 and 2 summarize the relevant demographic, clinical, and laboratory findings of the study group. Emergency room diagnoses were variable, most often weakness and general deterioration. There were 2.6 ± 2.2 (range 0 - 7) admissions per year, which comprised 0.01% of all the adult medical admissions to the hospital. Whereas 10% of the patients were nursing home residents, cognitive decline and dementia were present in 38%.
Comorbidities were noted in 78%, and included hypertension (30%), atrial fibrillation (26%), and ischemic heart disease (22%). Hypothyroidism was diagnosed prior to admission in 16 (55%) patients, 10 of whom were not regularly treated, mainly due to low compliance. Only 38% of the patients had a routine (≤12 months) pre-admission TSH measurement. The etiology of the hypothyroidism was Hashimoto’s disease (72.4%), following surgery or I-131 treatment (17.2%), and amiodarone-induced hypothyroidism (10.3%).
Multiple symptoms were detected in 69% of the patients; weakness was the major complaint (89%), followed by constipation (68%; Table 3). Of note, the classic hypothyroid symptoms, weight gain and cold intolerance, were not addressed in 48% of the charts. Findings
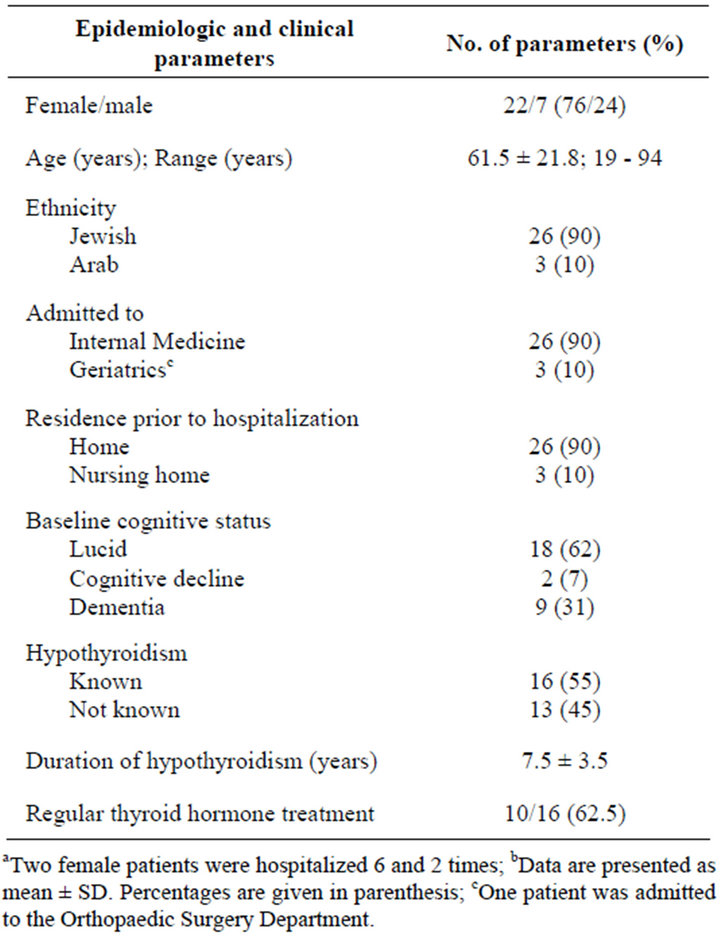
Table 1. Clinical characteristics of 23 patients with hypothyroidism diagnosed during 29 hospitalizationsa,b.
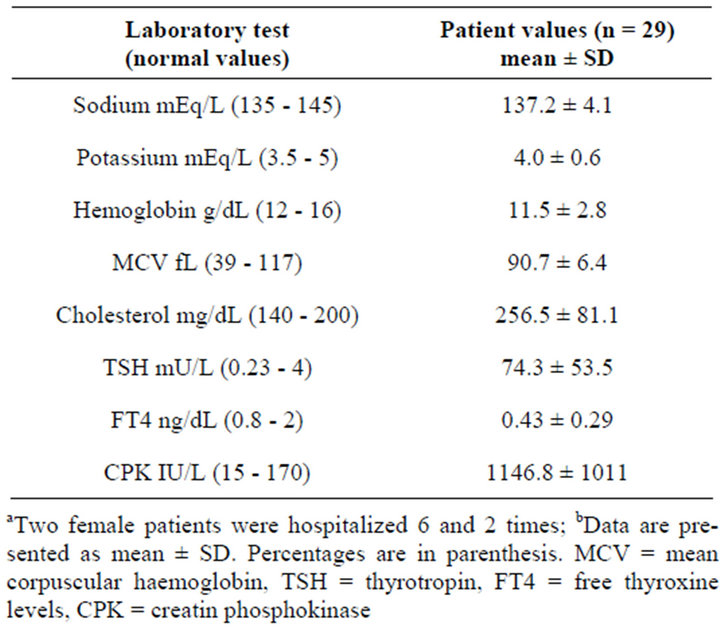
Table 2. Laboratory data of 23 patients with hypothyroidism diagnosed during 29 hospitalizationsa,b.
such as puffy eyes or muscle weakness were not mentioned in the medical reports. Moreover, despite profound hypothyroidism (TSH 74.3 ± 53.5 mIU/L, fT4 0.43 ± 0.29 ng/dL), hyponatremia (sodium < 135 mEq/L) was noted in only 13% of patients. Anemia (hemoglobin < 12 g/dL) and macrocytosis (mean corpuscular volume
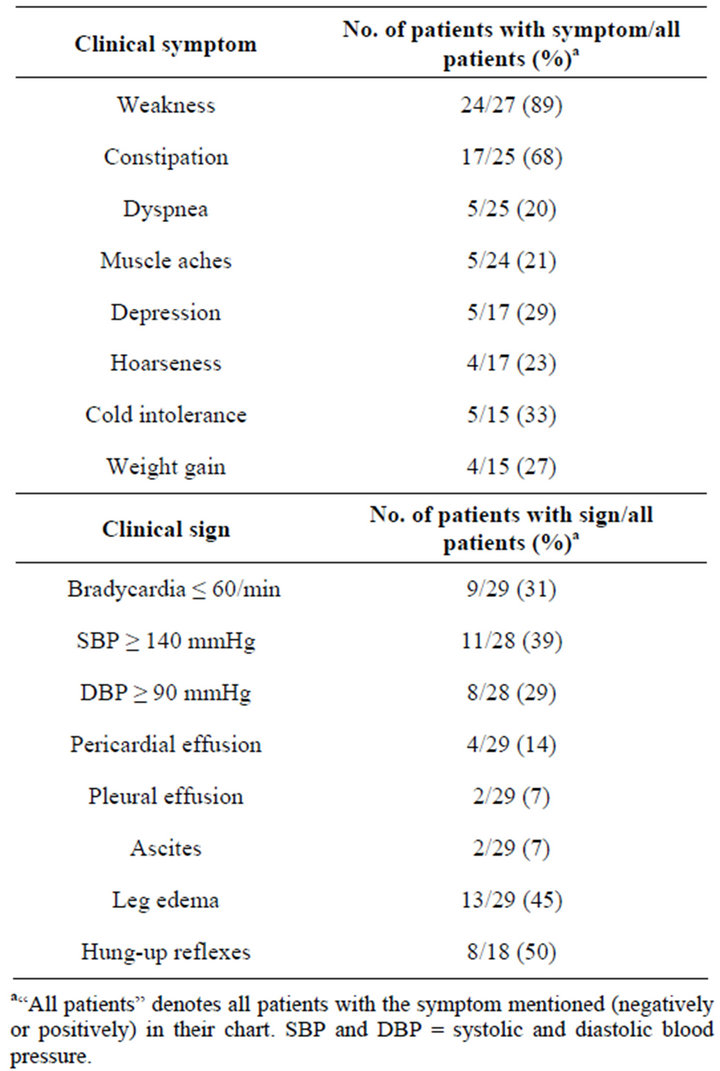
Table 3. Symptoms and signs of 23 patients with hypothyroidism diagnosed during 29 hospitalizations.
> 90 fL) were present in 62% and 48% of the patients, respectively. Serum CPK level, measured during 19 of the 29 admissions, was elevated more than 2-fold in 78%. The average hospital stay was 4.7 ± 5.5 days. Two female patients died, one, age 47-years, had 6 consecutive admissions due to hypothyroidism over 4 years.
4. Discussion
This study focuses on the clinical characteristics of patients who were diagnosed with overt hypothyroidism in an inpatient setting. Twenty-nine admissions during the 11-year study period constituted 0.01% of all adult medical hospital admissions, in comparison with previously reported 1% - 9% prevalence of hypothyroidism in nonselected hospitalized patients [4,5]. This reported prevalence is based on relatively old studies from the 1980s that used a different methodology for patient identification, i.e. thyroid function tests for consecutive hospital admissions, regardless of the clinical picture.
The main finding of our study is that hypothyroidism in inpatients present with multiple, nonspecific symptoms and signs, primarily weakness, constipation, and anemia, that are relatively common in the older inpatient population. Traditional hypothyroid symptoms such as cold intolerance, weight gain, hoarseness, and dry skin, were mentioned in the medical records infrequently. This is in agreement with previously published data showing that less than a third of hypothyroid inpatients present with specific symptoms [5]. In fact, several of the classic hypothyroid symptoms, such as fatigue, dry skin, and constipation often accompany normal aging and might be overlooked in an older population [4].
However, as only a third of our patients were older than 80 years, the lack of these symptoms in our cohort was not predominantly age-related. Other hypothyroid symptoms, including depression and lack of energy, which are difficult to detect in patients with cognitive dysfunction, were also not recorded in our series, and as nearly 40% of the patients had cognitive impairment, it is possible that these symptoms were not identified.
In line with their profound hypothyroid state, 65% of our patients had elevated CPK level, as was previously reported in up to 97% of hypothyroid patients [8,9]. Sixty-two percent of the patients in our series had anemia, while macrocytosis was noted in 48%. Since the majority of our patients had comorbidities, it is difficult to assess the real frequency of hypothyroidism-related anemia.
More than half of our patients were diagnosed with hypothyroidism several years before the current admission. Despite current recommendations to obtain an annual TSH measurement in chronic thyroid hormone users [10], it appears that the majority of our patients (62%) did not have regular follow-up care. In this regard, it was previously reported that a third of patients chronically treated with thyroid hormone have elevated TSH levels, with approximately half having a moderately elevated TSH, above 10 mIU/L [11-13].
Serum triiodothyronine levels were not included in the current analysis because it is not required for the diagnosis of hypothyroidism, is not routinely checked, and was absent from most of the charts. In addition, it is decreased in 58% of hospitalized patients as part of the sick euthyroid syndrome [14].
Although our study did not reveal any new hypothyroid-related symptoms, it emphasizes the lack of unique or specific symptoms in this inpatient population with overt hypothyroidism, indicating a need for a high clinical index of suspicion.
The main limitations of this study were its retrospective nature and its predominant reliance on two incomplete sources of data: the hospital coding system for identifying the cases and patient records for reviewing the symptoms. The imperfect coding system might have led to a few undetected cases. Moreover, misdiagnoses might have also led to an underestimation of inpatient hypothyroidism. Taken together, the actual prevalence of inhospital overt hypothyroidism might be higher than that reported in this study. In addition, our analysis depended on information found in the charts, so it lacked consistency in detecting thyroid-related symptoms and identifying comorbidities. Unfortunately, our analysis did not include data on the effect of thyroxine replacement during hospitalization on the various hypothyroid symptoms. Additionally, since our study did not have a comparator group, it could not shed light on the utility of clinical signs and symptoms such as weakness and constipation in diagnosing hypothyroidism in inpatients.
In conclusion, overt hypothyroidism is rare in non-selected medical inpatients, and is detected mainly in known hypothyroid patients. As multiple, non-specific symptoms, mostly weakness and constipation dominate the clinical picture of hypothyroidism, a high index of suspicion is needed to diagnose it in the hospital setting. Hypothyroidism should be included in the differential diagnosis in such a clinical setting, especially in cognitively impaired patients and in patients with known thyroid disease.
5. Acknowledgements
This work was presented in part in the 38th annual meeting of the Israeli Endocrine Society, Tel Aviv, March 2009.
The authors thank Faye Schreiber, MS for reviewing the manuscript.
The authors do not have any conflict of interest with regard to the manuscript.
REFERENCES
- T. P. R. Larsen and T. F. Davies, “Hypothyroidism and Thyroiditis,” In: P. R. Larsen, H. M. Kronenberg, S. Melmed and K.S. Polonsky, Eds., Williams Textbook of Endocrinology, W.B. Saunders Company, Philadelphia, 2003, pp. 423-446.
- J. L. Jameson and A. P. Weetman, “Disorders of the Thyroid Gland,” In: S. F. Fauci, E. Braunwald, D. L. Kasper, et al., Eds., Harrison’s Principals of Internal Medicine, McGraw-Hill, New York, 2008, pp. 2229-2233.
- C. P. Roberts and P. W. Ladenson, “Hypothyroidism,” Lancet, Vol. 363, No. 9411, 2004, pp. 793-803. doi:10.1016/S0140-6736(04)15696-1
- E. H. Livingston, J. M. Hershman, C. T. Sawin and T. T. Yoshikawa, “Prevalence of Thyroid Disease and Abnormal Thyroid Tests in Older Hospitalized and Ambulatory Persons,” Journal of the American Geriatrics Society, Vol. 35, No. 2, 1987, pp. 109-114.
- M. Bahemuka and H. M. Hodkinson, “Screening for Hypothyroidism in Elderly Inpatients,” British Medical Journal, Vol. 2, No. 5971, 1975, pp. 601-603. doi:10.1136/bmj.2.5971.601
- J. Attia, P. Margetts and G. Guyatt, “Diagnosis of Thyroid Disease in Hospitalized Inpatients,” Archives of Internal Medicine, Vol. 159, No. 7, 1999, pp. 658-665. doi:10.1001/archinte.159.7.658
- G. G. Klee and I. D. Hay, “Biochemical Testing of Thyroid Function,” Endocrinology and Metabolism Clinics of North America, Vol. 26, No. 4, 1997, pp. 763-775. doi:10.1016/S0889-8529(05)70281-4
- Z. Hekimsky and I. Kavaladi Oktem, “Serum Creatine Kinase Levels in Overt and Subclinical Hypothyroidism,” Endocrine Research, Vol. 31, No. 3, 2005, pp. 171-175. doi:10.1080/07435800500371706
- I. W. Beyer, R. Karmali, N. Demeester-Mirikine, E. Cogan and M. J. Fuss, “Serum Creatine Kinase Levels in Overt and Subclinical Hypothyroidism,” Thyroid, Vol. 8, No. 11, 1998, pp. 1029-1031. doi:10.1089/thy.1998.8.1029
- P. Ladenson, P. A. Singer, K. B. Ain, N. Bagchi, S. T. Bigos, E. G. Levy, S. A. Smith, G. H. Daniels and H. D. Cohen, “American Thyroid Association Guidelines for Detection of Thyroid Dysfunction,” Archives of Internal Medicine, Vol. 160, No. 11, 2000, pp. 1573-1575. doi:10.1001/archinte.160.11.1573
- J. V. Parle, J. A. Franklyn, K. W. Cross, S. R. Jones and M. C. Sheppard, “Thyroxine Prescription in the Community: Serum Thyroid Stimulating Hormone Level Assays as an Indicator of Undertreatment or Overtreatment,” British Journal of General Practice, Vol. 43, No. 368, 1993, pp. 107-109.
- B. Vaidya and S. H. S. Pearce, “Management of Hypothyroidism in Adults,” British Medical Journal, Vol. 337, 2008, p. a801. doi:10.1136/bmj.a801
- C. T. Sawin, A. Gellar, J. M. Hershman, W. Castelli, P. Bacharach, “The Aging Thyroid. The Use of Thyroid Hormone in Older Persons,” JAMA, Vol. 261, No. 18, 1989, pp. 2653-2655. doi:10.1001/jama.1989.03420180077034
- M. C. Sheppard and D. B. Ramsden, “Abnormalities of Thyroid Function Tests in Hospital Inpatients,” Postgraduate Medical Journal, Vol. 61, No. 721, 1985, pp. 983- 987. doi:10.1136/pgmj.61.721.983

