Open Journal of Anesthesiology
Vol.4 No.2(2014), Article ID:42773,3 pages DOI:10.4236/ojanes.2014.42008
Acute Decompensated Heart Failure Secondary to Structural Valve Degeneration of a Bioprosthetic Valve
Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, USA.
Email: CMehaffey@partners.org
Copyright © 2014 Carolyn J. Mehaffey, David Asher Cantor. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Carolyn J. Mehaffey, David Asher Cantor. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received December 17th, 2013; revised January 17th, 2014; accepted January 27th, 2014
ABSTRACT
Heart valve replacement is a common cardiac surgical procedure used to treat native valvular diseases such as aortic and mitral stenosis and regurgitation. These procedures reduce the morbidity and mortality associated with diseased native valves and yet come at the expense of prosthetic valve complications. Structural valve degeneration is one such complication. We present a case of a critically ill elderly man who had undergone mitral valve replacement 14 years prior to his current presentation. Only after admission through the emergency department was a transesophageal echocardiogram obtained and the diagnosis of prosthetic valve degeneration made. He subsequently underwent successful replacement of his diseased prosthetic valve.
KEYWORDS
Structural Valve Degeneration; Heart Failure; Mitral Valve Replacement; Bioprosthetic Mitral Valve; Endocarditis
1. Introduction
A 68-year-old male was presented to the emergency department with acute shortness of breath and hypoxia after a 2-day prodrome of general malaise. The patient’s medical history was significant for Type II diabetes mellitus, hypertension, hyperlipidemia, chronic renal insufficiency, non-sustained ventricular tachycardia, and psoriatic arthritis. His surgical history included bioprosthetic mitral valve replacement (Hancock porcine) with simultaneous implantation of an implantable cardioverter-defibrillator (ICD) 14 years prior to presentation. He had recently underwent an upgrade to a dual-chamber ICD four months before it was complicated by right ventricular lead malfunction requiring replacement one month later. His medical regimen consisted of atenolol, amoxicillin (prior to procedures) simvastatin, omeprazole, aspirin, glyburide, metformin, celecoxib, and leucovorin.
He was in extremis with jugular venous distention and crackles bilaterally to the pulmonic apicesupon presentation. No murmur was heard on the initial cardiac examination. He was treated with furosemide and nitropaste. He failed non-invasive continuous positive airway pressure (CPAP) and required intubation. Chest radiography showed pulmonary edema and bilateral lower lobe consolidation. He was admitted to the medical intensive care unit (MICU) with pa provisional diagnosis of pneumonia. His MICU course was notable for an increasing vasopressor requirement and acidosis.
Cardiology consultation was obtained. A transesophageal echocardiogram (TEE) demonstrated a flail bioprosthetic mitral valve leaflet with a severe eccentricallydirected jet of regurgitation as well as moderate mitral stenosis (Figures 1-3). An intraaortic balloon pump (IABP) was placed prior to transport to the operating room (OR) for surgical replacement of his prosthetic mitral valve.
After institution of cardiopulmonary bypass the patient’s malfunctioning mitral valve was replaced with a bovine bioprosthesis and he was successfully weaned from bypass with significant inotropic and vasopressor support. Gross pathological inspection of the explanted
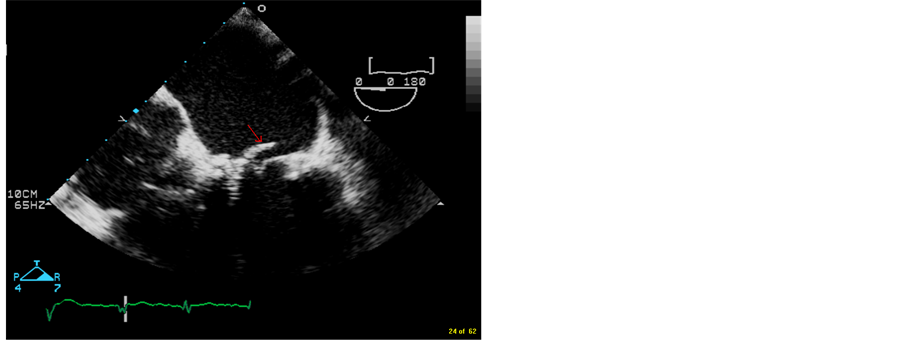
Figure 1. 4-chamber TEE image showing torn leaflet of bioprosthetic mitral valve prolapsing into the left atrium during systole.
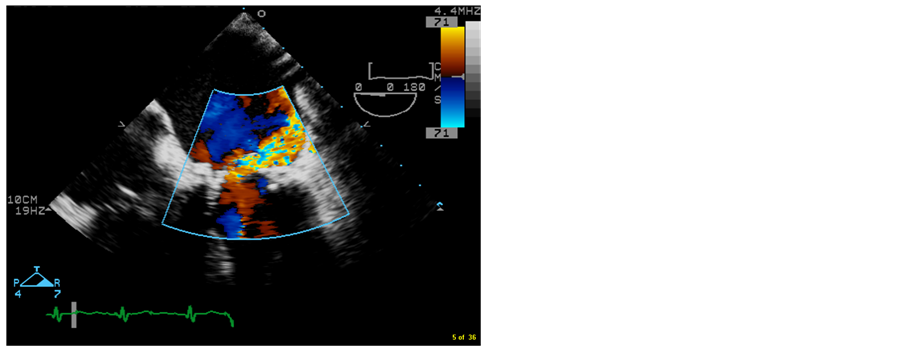
Figure 2. 4-chamber TEE color image showing severe mitral regurgitation through degenerative bioprosthetic mitral valve. Note the posteriorly directed jet due to the prolapse of the anteriorly located torn leaflet.
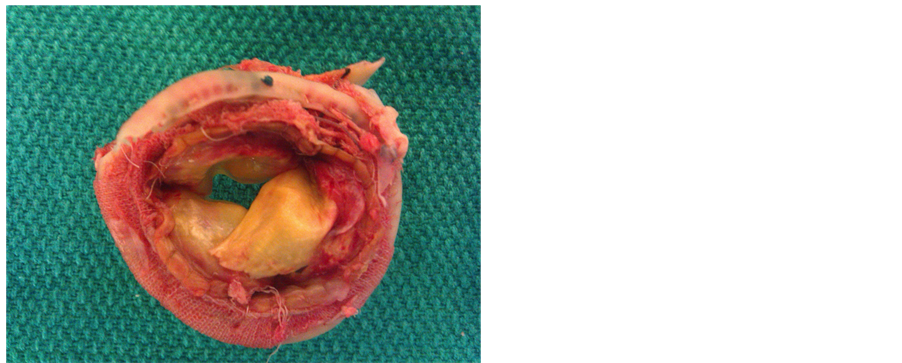
Figure 3. Photograph of degenerative explanted bioprosthetic mitral valve with torn leaflet evident. Also note the presence of pannus around the sewing ring.
valve demonstrated soft tissue overgrowth and at least one prosthetic leaflet tear thought to be consistent with bioprosthetic valve endocarditis. Subsequent microbiological and pathological analysis of the valve tissue failed to demonstrate organisms or vegetations. Blood cultures did not grow an organism, and infectious disease consultation was ordered with determination that valvular endocarditis was highly unlikely and that structural valvular degeneration (SVD) was more likely the cause for his mitral regurgitation. Given his acute presentation without clinical evidence of endocarditis and yet a 14- year-old prosthetic valve, this was mostly acute upon chronic SVD.
Perioperative issues included coagulopathy and hemorrhage requiring multiple blood transfusions, acute on chronic renal insufficiency requiring continuous venovenous hemofiltration, atrial fibrillation rate-controlled with beta blockade and pulmonary hypertension treated with sildenafil. The patient’s chest remained open until closure on postoperative day 1. The IABP was weaned on postoperative day 2. He was extubated on postoperative day 6, and discharged home on postoperative day 21 without sequellae.
2. Discussion
Valve replacement is an effective procedure to treat various valve pathologies including stenosis, regurgitation, and endocarditis. The two main options for valve replacements in the current era include mechanical and bioprosthetic valves. Bioprostheses are further subdivided by tissue origin—porcine and bovine pericardial valves or cadaveric homografts.
The decision to implant a mechanical versus a bioprosthetic valve is patient-specific, as each type has advantages and limitations. Younger patients are more likely to receive a mechanical valve as they have the advantage of increased durability. Mechanical valves are plagued by increased thrombogenicity and require lifelong anticoagulation. Bioprostheses are the implantation of choice in the older patient population. Bioprosthesessuffer from an increased rate of structural valve degeneration (SVD), defined as any process which results in stenosis or regurgitation of the prostheses [1]. These processes include calcification, leaflet tear or perforation, and stenosis due to increased leaflet stiffness or pannus formation. SVD excludes valvular degeneration secondary to endocarditis. The most recent ACC/AHA guidelines state it is reasonable to implant a mechanical valve rather thana bioprosthetic valve in patients less than 65 years old for isolated aortic valve replacement and for patients less than 65 years old requiringisolated mitral valve replacement [2]. Patient-specific considerations such as atrial fibrillation as well as patient preference will play significant roles in this decision.
The Hancock porcine valve was the first commercially available bioprosthetic valve and was first implanted in 1970. Cohn et al. published their 15-year experience with this valve implanted in the mitral, aortic, and both positions in 1989 [3]. The probability of freedom from SVD drops sharply from 75% at 10 years after implantation to 45% at 15 years post-implantation for mitral valve replacement. Furthermore the age of the patient is correlated with the probability of freedom from SVD in that older patients (e.g. >40 years) are much more likely to have a longer-functioning valve.3These findings correlate with more recent series indicating satisfactory results up to 15 years from implantation [4]. At least one other series, however, has shown much more pessimistic results with a 14-year freedom from SVD rate of 37% [5]. This dramatic difference in results could be due to differences in the definition of SVD or average age at implantation. The Hancock II valve was a second-generation porcine valve introduced in 1982. Results in terms of freedom from SVD when placed in the mitral position were comparable to that of the Hancock I [6].
Our 68-year-old diabetic patient had a Hancock porcine valve implanted 14 years prior to presentation, making him 54 years old at the age of implantation. He had two invasive procedures 3 and 4 months prior to presentation which initially raised the suspicion of possible endocarditis leading to valve failure. Pathologic and microbiologic testing ruled this out as the cause of his valvular dysfunction. Structural valve dysfunction secondary to senescence of the valve appeared much more likely as the valve showed significant stenosis secondary to pannus (soft tissue) infiltration at the time of explantation. Given the patient’s age at implantation and that it was a mitral prosthesis, perhaps SVD should have been higher on our initial differential for valvular dysfunction and clinical deterioration.
There are many reported cases of acute mitral regurgitation due to porcine bioprosthetic valve failure [7,8]. This is a type of SVD which can result in a particularly acute decompensation in the patient’s clinical picture with a resultant high morbidity and mortality. Early diagnosis and surgical treatment of this condition can result in a favorable outcome as seen in our case.
3. Conclusion
Heart valve replacement is a common procedure that has significantly increased the longevity of many patients. The two main categories of heart valve prostheses are mechanical and bioprosthetic. Identification of which type of valve a patient has and an understanding of the advantages and limitations of each type can be helpful in diagnosis of valvular pathology in this patient group. Bioprosthetic valves generally have a durability of around 15 years, and patients with older bioprostheses should undergo thorough cardiac interrogation. Transthoracic (TTE) or transesophageal (TEE) echocardiography can provide rapid and accurate evaluation of prosthetic valvular dysfunction.
REFERENCES
[1] L. H. Edmunds Jr., R. E. Clark, L. E. Cohn, et al., “Guidelines for Reporting Morbidity and Mortality after Cardiac Valvular Operations,” The Annals of Thoracic Surgery, Vol. 62, No. 3, 1996, pp. 932-935
[2] R. O. Bonow, B. Carabello, K. Chatterjee, et al., “Focused Update Incorporated into the ACC/AHA 2006 Guidelines for the Management of Patients with Valvular Heart Disease,” Circulation, Vol. 118, 2008, pp. e523- e661. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.190748
[3] L. H. Cohn, et al., “Fifteen-Year Experience with 1678 Hancock Porcine Bioprosthetic Heart Valve Replacements,” Annals of Surgery, Vol. 210, No. 4, 1989, pp. 435-443. http://dx.doi.org/10.1097/00000658-198910000-00003
[4] F. Santini, G. B. Luciani, et al., “Over Twenty-Year Follow-Up of the Standard Hancock Porcine Bioprosthesis Implanted in the Mitral Position,” The Annals of Thoracic Surgery, Vol. 71, Suppl. 5, 2001, pp. S232-235. http://dx.doi.org/10.1016/S0003-4975(01)02524-3
[5] J. M. Bernal, J. M. Rabasa, et al., “Valve-Related Complications with the Hancock I Porcine Bioprosthesis, A Twelveto Fourteen-Year Follow-Up Study,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 101, No. 5, 1991, pp. 871-880.
[6] C. Valfre, P. Lus, G. Minniti, et al., “The Fate of Hancock II Porcine Valve Recipients 25 Years after Implant,” European Journal Cardio-Thoracic Surgery, Vol. 38, No., 2010, pp. 141-146. http://dx.doi.org/10.1016/j.ejcts.2010.01.046
[7] J. Butany, B. Dias, P. Iyengar, et al., “Acute Mitral Regurgitation Due to a Torn Porcine Bioprosthetic Cusp,” Circulation, Vol. 105, 2002, pp. e58-e60.
[8] H. Niinami, Y. Naito, H. Koyanagi, et al., “Malfunction of a Hancock Bioprosthesis in the Mitral Position 24 Years after Initial Implantation,” The Journal of Heart Valve Disease, Vol. 9, No. 6, 2000, pp. 810-812.

