Open Journal of Organic Polymer Materials
Vol.3 No.2(2013), Article ID:30536,6 pages DOI:10.4236/ojopm.2013.32009
A Novel Biodegradable Poly (Hydroxybutanedioic Acid-co-2-hydroxypropane-1,2,3-tricarboxylic Acid) Copolymer for Water Treatment Applications
Composites Research Group, Department of Mechanical Engineering, Durban University of Technology, Kwa-Zulu Natal, South Africa Email: *kannyk@dut.ac.za
Copyright © 2013 N. Mithil Kumar, K. Kanny. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 19, 2012; revised November 20, 2012; accepted November 29, 2012
Keywords: Biodegradable Polymers; Bulk Melt Condensation; Copolymers and Calcium Carbonate Scale
ABSTRACT
Minimizing the formation of inorganic scale deposits in industrial water continues to be a challenge for water treatment systems. In order to meet this challenge, a novel biodegradable poly (DL-malic acid-co-citric acid) copolymer, effective in providing calcium carbonate scale inhibition was developed. Synthesis and characterization of the biodegradable, water-soluble and polyester copolymer was performed. Synthesis was done by direct bulk melt condensation in the absence of a catalyst above 150˚C. Characterization of the copolymer was carried out using infrared absorption spectra (FTIR), differential scanning calorimetry (DSC) and thermo gravimetric analysis (TGA) equipment. In the present work the precipitation of calcium carbonate from relative supersaturated solutions at different weight ratios of comonomer inhibition rates have been studied. The results indicate that the copolymer is an effective calcium carbonate descaling inhibitor that suppresses the growth process against calcium mineral scale deposits.
1. Introduction
Mineral scale formation in water is a global problem which persists to this day. Mineral scale deposit occurs primarily because of the presence of mineral and salts in the water. Wastewater is continuously discharged from industries such as mining, water treatment sectors, oil refineries, pulp and paper industries, glass and ceramic production industries, nuclear plants, and uranium refineries. This wastewater contains minerals and salts which may contaminate the groundwater. It is well documented that impurities present in feed water and re-circulating water markedly influence the rate of scale deposit [1-2].
Scale formation in cooling water systems shortens the life of equipment. The major causes of industrial water system failures is the deposition of scale on equipment such as heat exchangers and reverse osmosis (RO) on membrane surfaces and this results in increased operational costs [3]. The mineral scale deposits commonly encountered include carbonates, sulfates and phosphates salts of alkaline earth metals.
During the last few decades control of mineral scales formation in industrial waters have been extensively researched. Several reviews that compare the performance of various chemicals and polymers commonly used to control the scale formation are available [4-6]. Various anti-scaling additives have been proposed. Such additives include certain polyphosphates, polyacrylic acids, polymethacrylic acids, polyacrylamides, lignin sulphonic acid, hydrolysed polymaleic anhydride, hydrolysed copolymers as well as their salts. These polymers satisfactorily inhibit scale formation, however, other problems are encountered due to the continued presence of the above mentioned scale inhibitors once their primary function has been achieved. Generally they are difficult to degrade by the action of microorganisms. Toxicity and non-degradation cause major harm to the water systems and the environment if the above mentioned polymers are discharged into the environment. Hence, there is a need to develop a polymer that has both the good scale inhibition and biodegradation properties. Eco-friendly non-toxic biodegradable polymers are excellent candidates for water treatment applications and have been given a lot more attention since the 1970s [7].
Quality and purity are major concerns in water treatment industries. The green polymers are good descaling inhibitors without affecting taste, quality, and purity of water [8,9]. Increasingly stringent environmental restricttions and the need for water conservation, has led to the development of modern, all-organic, non-phosphorus biodegradable polymers for cooling water treatment programs [10]. Over the years a number of investigations were conducted to study the influence of biodegradable polymer on cooling water treatment system [11,12] to minimize the scale deposition [13,14].
In this regard DL-malic acid (DMA) was chosen as a descaling agent because it can be handled safely during the synthesis [15,16]. To further improve their efficacy of scale inhibition citric acid is used as a comonomer. This combinational approach enhances their scale inhibition efficacy.
2. Experimental
2.1. Materials
DL-malic acid (DMA) (Product No. 38798, Minimum assay 99%), citric acid anhydrous (CA) (Product No. 87984, Minimum assay 99%), Calcium chloride (Product No. 20070), Ethylenediaminetetraacetic acid (EDTA), Magnesium chloride (MgCl2) and Sodium bicarbonate (NaHCO3) were purchased from s.d. Fine chemicals, Mumbai, India.
2.2. Synthesis of Poly (DMA-CA) Copolymer
Copolymers were synthesized using the following procedure. Two different weight ratios of both DMA and CA were mixed together to form the copolymer as shown in Table 1. The reactants (DMA and CA) were placed in a ceramic bowl and the monomer mixture was first heated up to 100˚C for 2 h in an oven, then heated to 120˚C for about 10 minutes until the reaction mass partially melted. The temperature was thereafter increased to 130˚C and maintained for 9 h until the mass attained a yellowish colour. Finally, the temperature was increased to 200˚C for 7 h to get a complete conversion to a polymer. The synthesized copolymer was washed with distilled water several times (20 times) at ambient temperature until polymer was free of impurities. The yield of the product was 90% - 95%. The same procedure was followed for the synthesis of poly (DMA) homopolymer. Photographic images of prepared homopolymer and copolymer are shown in Figure 1.
2.3. Experimental Test Method for Calcium Carbonate Scale Inhibition
Scale Test
The polymer (10 ppm) was added to a flask in which calcium chloride, sodium bicarbonate stock solution and 25 ml water was present. Solutions of synthetic cooling wa
Table 1. Feed composition of biodegradable water soluble polymers and their % of CaCO3 inhibition.
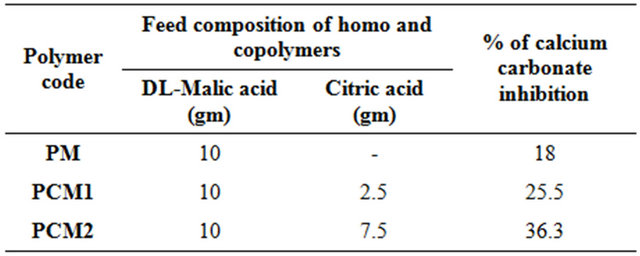

Figure 1. Photograph images of (a) homopolymer (PM) and (b) copolymer (PMC2).
Table 2. Composition of anion and cation brine.
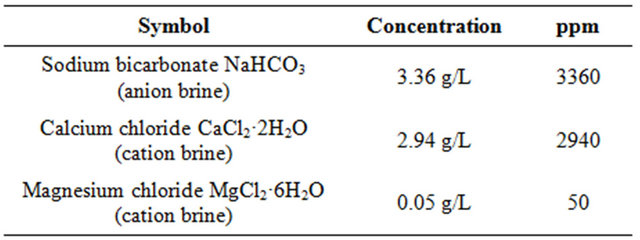
All stock solutions were prepared from analytical reagents and demineralised water (DM) without further purification.
ter (CaCO3 scale) were made by mixing 50 ml of anionbrine to 25 ml of cation brine solution and 25 ml of DM (demineralized) water. Thereafter 25 ml of demineralized water was added to 25 ml of CaCl2 stock solution along with 10 ppm of the polymer solutions (scale inhibitor) in a 250 ml reagent bottle. Thereafter 50 ml of NaHCO3 of stock solution was added to the solution. Another 250 ml reagent bottle was prepared with the same contents expect the polymer solution (scale inhibitor) and was used as the control solution. The pH of both solutions was adjusted to 8.0 by the addition of standard potassium hydroxide solution. Test conditions were set as the follows: without polymer (Blank, hot), with polymer (homopolymer (PM), copolymer (PMC1) and copolymer (PMC2) dose (10 ppm), respectively. The reagent bottles were incubated for 16 hours at 70˚C. After the incubation period, the reagent bottles were removed from the heat source and filtered through a 0.42 micro meter (μm) membrane filters. The filtrate was titrated for calcium using 0.01 molarity (M) EDTA and Eriochrome Black T (EBT) indicator, the percent calcium carbonate inhibition was calculated using the following equation (1) [17]:
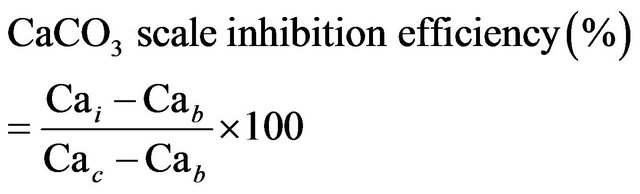 (1)
(1)
Cai = Calcium ion concentration with the inhibitor.
Cab = Calcium ion concentration in the blank.
Cac = Calcium ion concentration before the test.
3. Characterization
FTIR plots in the range of 500 to 4000 cm−1 was done on a FTIR spectrometer MB3000 Model (Quebec, Canada) using the KBr disk method. The software used was Horizon software from ABB Company. Differential scanning calorimetry (DSC) of poly (DMA) and poly (DMACA) were studied by using a SDT Q 600 DSC instrument (T.A. Instruments-Water LLC, Newcastle, DE 19720, USA) at a heating ramp of 20˚C/min under a constant nitrogen flow (100 ml/min). The samples were heated from 30˚C to 600˚C. The thermogravimetric (TGA) analysis of poly (DMA) and poly (DMA-CA) was evaluated on a SDT Q 600 TGA instrument (T.A. Instruments-Water LLC, Newcastle, DE 19720, USA) at a heating rate of 10˚C/min under a constant nitrogen flow (100 mL/min). The samples were run from 30˚C to 600˚C.
4. Results and Discussion
It was found that the addition of copolymer with higher monomer ratio of citric acid has an effect on the scale inhibition performance. The study demonstrated that the addition of small amounts of aliphatic tricarboxylic acid monomer to malic acid, transforms the polymerization from a solid state to a melt state reaction between 130˚C and 200˚C. In order to understand these copolymers were characterized by the following analysis.
4.1. FTIR Analysis
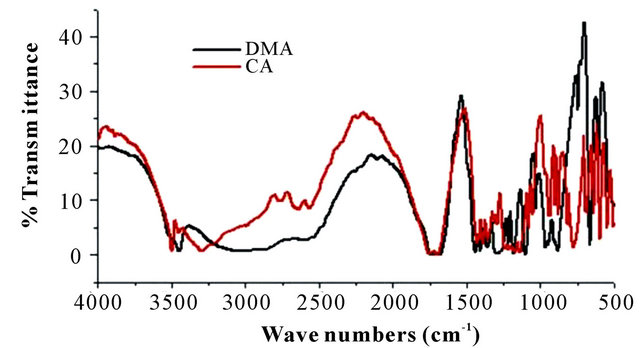
The FTIR spectra of DMA-CA monomers and poly (malic acid-citric acid) copolymer are shown in Figure 2. The peaks in the range from 3100 cm−1 to 3400 cm−1 are assigned to the fundamental stretching vibration of different -OH groups. The double and single -CO stretch peaks of DMA are located at 1719 and 1272 cm−1, respectively [18,19]. The asymmetric and symmetric -CO stretch bands are situated at 1453 cm−1 and 1376 cm−1 [20]. The spectra of CA shows a broad peak around 3505 cm−1 which indicates -OH stretching. The very distinct peaks at 2773 cm−1 and 2656 cm−1 indicate hydrogen bond formation between -OH groups and carboxylic acid groups. In the spectra two prominent peaks at 1714 cm−1 and 1428 cm−1 is observed from the asymmetrical and
 (a)
(a)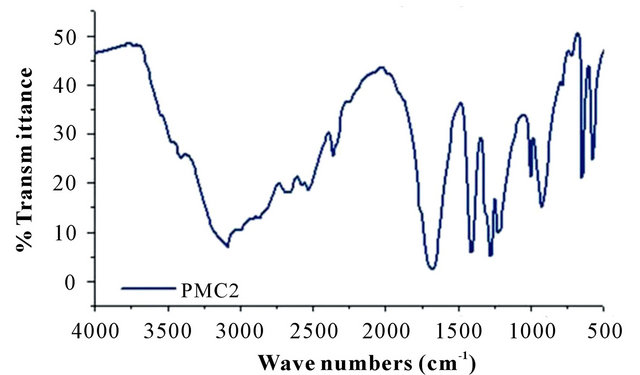 (b)
(b)
Figure 2. FTIR spectra of (a) pure DMA and CA; (b) copolymer (PMC2).
symmetrical stretching of -COO groups respectively. Poly (DMA-CA) spectra shows hydroxyl absorption bands at 3101 cm−1, which correspond to stretching frequency of the OH groups present in poly (malic acidcitric acid). The FTIR spectrum of this high amount of citric acid sample (Figure 2) (data not shown) shows large absorbance at 1618 cm−1 corresponding to carbonyl stretching in the copolymer. Additional peaks were observed at 2681 cm−1, 2530 cm−1 and 1189 cm−1 due to incorporation of CA and DMA (respectively) in copolymer structure. All the above peaks confirm the incorporation of DMA and CA units in the copolymer networks.
4.2. Thermal Analysis
4.2.1. Differential Scanning Calorimetry (DSC)
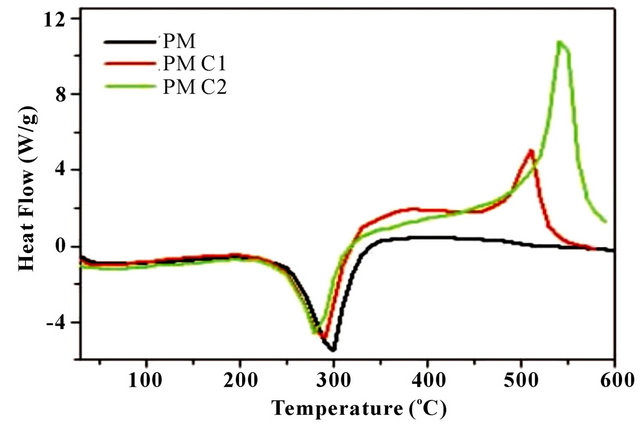
Figure 3(a) shows the DSC thermograms of the homopolymer (PM) and copolymer (PMC1 and PMC2). DSC of homopolymer and copolymers gives Tg as 300˚C, 288˚C and 280˚C respectively. The glass transition temperature decreases with the increase of citric acid content in copolymer. This is due to the incorporation of citric acid units (aliphatic) which provide more flexibility and chain length to the biodegradable copolymers. Similar observation was reported by Singh et al. [21]. In case of the physical mixture of DMA and CA [PMC2 (Figure 3(c))] shows separate endotherms corresponding to malic acid (137˚C) and citric acid (244˚C) peaks, indicating no
 (a)
(a)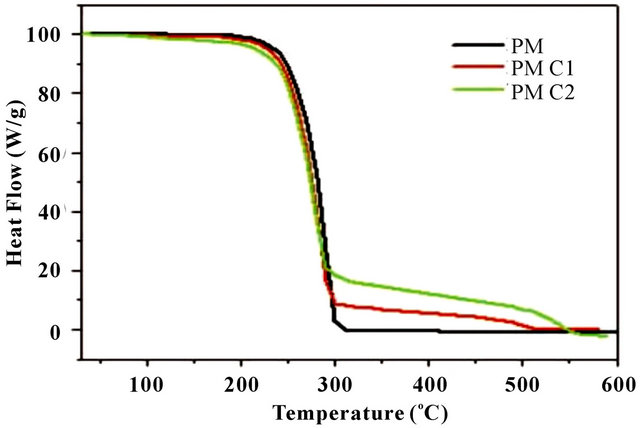 (b)
(b)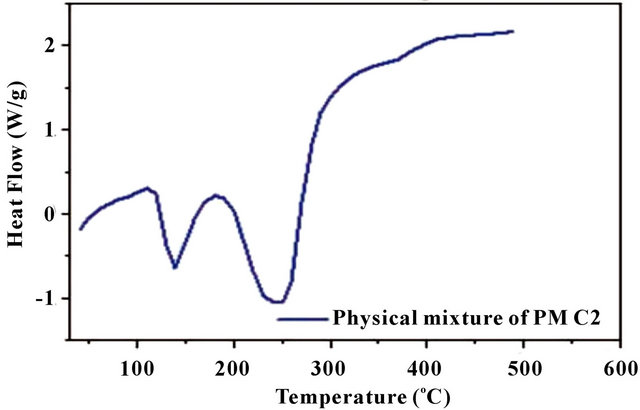 (c)
(c)
Figure 3. (a) DSC spectra of homopolymer and copolymers; (b) Physical mixture of PMC2 (malic acid (74.57 mM)-citric acid (39.03 mM)); and (c) TGA spectra of homopolymer (PM) and copolymers (PMC1-PMC2).
copolymer formation when compared to PMC2 copolymer.
4.2.2. Thermogravimetric Analysis (TGA)
TGA analysis of the samples exhibited initial degradation at 220˚C, 218˚C and 214˚C (respectively) as shown in Figure 3(b). Final degradation is shown at 301˚C, 295˚C and 289˚C. These studies indicate that copolymers with citric acid have a reduced degradation temperature threshold, due to the lower thermal stability of citric acid with respect to malic acid homopolymer. With homopolymer (malic acid as monomer), copolymer with less monomer ratio of citric acid and copolymer with higher monomer ratio of citric acid have exhibited similar trends in both DSC and TGA studies. In addition greater weight loss occurs due to the presence of citric acid in copolymers.
4.2.3. Results of Scale Inhibition Test
DL-malic acid homo polymer and copolymers were prepared in order to study their scale inhibition efficiency. These copolymers were freely water soluble in the presence of aqueous alkali conditions. Both polymers were tested under identical conditions and it was found that the copolymers had higher scale inhibition efficiency. The number of carboxyl groups in hydrolyzed poly(malic acid-citric acid) molecular structure was greater than that of polyacrylic and polymethacrylic acid thus had better scale inhibition properties. Further, the presence of the tricarboxylic acid comonomers (citric acid) had a profound effect on the ability copolymer to inhibit scale growth. Tri acids of citric acid with longer polyester chains facilitated greater functional carboxylic groups and allowed the copolymers with larger DL-malic acid/tri acid ratios superior inhibition behavior. All polymers with concentration of 10 ppm were reasonably good inhibitors, however, PMC2 was more effective than PMC1 and PM. PMC2 with concentration of 10 ppm effectively blocked all the active growth sites and had the maximum inhibition of calcium carbonate growth rate over a 16 h period. Results in Table 1, indicate that scale inhibition rate increases from 25.5% to 36.3% with increasing citric acid content from 13.01 to 39.03 (mM) respectively. Copolymer with higher concentration of citric acid has higher scale inhibition efficiency than homopolymer. Further, the scale inhibition efficiency of the homopolymer was 18% which was significantly lower than that of the copolymer. This suggests that the synergistic scale inhibition effect is due to the presence of citric acid in copolymer network.
Also poly(malic acid-co-citric acid) was found to increase the degradation rate and the non-toxic in water. It has higher scale performance than hydrolyzed DMA homopolymer (PM). Inhibitor poly(malic acid) and inhibittor poly(malic acid-co-citric acid), each at a dose level of 10 ppm (on active basis) was evaluated at 65˚C - 70˚C for their efficacy against CaCO3 scales of normal tap water. The time taken for equilibration was 16 hr. This indicated that the scale inhibition ratios increase with the increase of the citric acid concentration. However, the scale inhibition performance becomes better with the addition of citric acid. Further it was observed that even at temperatures greater than 65˚C, the CaCO3 scale inhibittion exceeded 35% with only 10 mg/L copolymer.
Table 1 shows the effect of higher monomer ratio of citric acid on the scale inhibition performance of the copolymer. For copolymer with higher monomer ratio of citric treatment, the scale inhibitor was effectively absorbed and precipitated to inorganic salt. The formation of CaCO3 scale is influenced this precipitation. Thus, copolymer with higher monomer ratio of citric acid finds good application in industrial cooling water treatment plants.
5. Conclusions
Biodegradable polymers synthesized and tested in this study are effective as scale growth inhibitors. The data presented suggest that these polymers can be used effecttively to control growth of calcium carbonate mineral scale. The calcium carbonate scale performance data suggest that copolymers have different effects on the inhibitory power of polymers depending on the percentage of monomer rate of citric acid. A copolymer with higher monomer ratio of citric acid had higher scale inhibition properties. In this regard the following conclusions can be made:
1) The scale reduction by using 10 ppm DL-malic acid (PM) homopolymer was found to be 18% after 16 hrs incubation at 65˚C - 70˚C.
2) The scale inhibition rate of calcium carbonate in synthetic cooling water containing the newly developed poly (DL-malic acid-co-citric acid) (PCM1 & PCM2) inhibitors reduced to 25.5% and 36.3% respectively.
3) Due to the precipitation effect, poly (DL-malic acidco-citric acid) (PCM2) exhibited an inhibition efficiency of 36.3%.
REFERENCES
- J. A. Wohlever, Z. Amjad and R. W. Zuhl, “Performance of Anionic Polymers as Precipitation Inhibitors for Calcium Phosphonates in Advances in Crystal Growth Inhibition Technologies,” Kluwer Academic Publishers, New York, 2001.
- J. Amjad, J. F. Pugh, Zibrida and R. W. Zuhl, “Performance of Polymers in Industrial Water Systems. The Influence of Process Variables,” Material Performance, Vol. 36, No. 1, 1997, pp. 32-38.
- Z. Amjad and R. W. Zuhl, “An Evaluation of Silica Scale Control Additives for Industrial Water Systems,” NACE International, New Orleans, 2008.
- S. J. Gadaleta, A. Gericke, A. L. Boskey and R. Mendelsohn, “Two-Dimensional Infrared Correlation Spectroscopy of Synthetic and Biological Apatites,” Biospectroscopy, Vol. 2, No. 6, 1996, pp. 353-364. doi:10.1002/(SICI)1520-6343(1996)2:6<353::AID-BSPY2>3.0.CO;2-4
- Z. Amjad, “Controlling Metal Ion Fouling in Industrial Water Systems,” Association of Water Technologies, 2002.
- K. D. Demadis and S. D. Katarachia, “Metal-Phosphonate Chemistry: Synthesis, Crystal Structure of Calcium-Amnotris (Methylene Phosphonate) and Inhibition of CaCO3 Crystal Growth,” Phosphorus, Sulfur, and Silicon and the Related Elements, Vol. 179, No. 3, 2004, pp. 627-648. doi:10.1080/10426500490441514
- D. R. Lu, C. M. Xiao and S. J. Xu, “Starch-Based Completely Biodegradable Polymer Materials,” Polymer Letters, Vol. 3, No. 6, 2009, pp. 366-375. doi:10.3144/expresspolymlett.2009.46
- S. Roweton, S. J. Huang and G. Swift, “Poly (Aspartic Acid): Synthesis, Biodegradation, and Current Applications,” Journal of Environmental Polymer Degradation, Vol. 5, No. 3, 1997, pp. 175-181.
- G. Yuhua, L. Zhenfa, Z. Lihui and L. Haihua, “Study on Scale Inhibition Performance of Polyaspartic Acid Derivative,” Advanced Material Research, Vol. 535-537, Article ID: 10.4028, 2012, pp. 2287-2290. doi:10.4028/www.scientific.net/AMR.535-537.2287
- M. T. Sunita and D. J. Bhimrao Sarwade, “Synthesis and Biodegradability of Polyaspartic Acid: A Critical Review,” Journal of Macromolecular Science, Pure and Applied Chemistry, Vol. 42, No. 9, 2005, pp. 1299-1315. doi:10.1080/10601320500189604
- G. Swift, “Directions for Environmentally Biodegradable Polymer Research,” Accounts of Chemical Research, Vol. 26, No. 3, 1993, pp.105-110. doi:10.1021/ar00027a005
- L. Randal, J. L. Shogren, W. D. Westmorel, O. S. Gonzalez, M. D. Kenneth and G. Swift, “Properties of Copolymers of Aspartic acid and Aliphatic Dicarboxylic Acids Prepared by Reactive Extrusion,” Journal of Applied Polymer Science, Vol. 110, No. 6, 2008, pp. 3348- 3354. doi:10.1002/app.28944
- B. Akin, M. Öner, Y. Bayram and K. D. Demadis, “Effects of Carboxylate-Modified, ‘Green’ Inulin Biopolymers on the Crystal Growth of Calcium Oxalate,” Crystal Growth Desalination, Vol. 8, No. 6, 2008, pp. 1997-2005. doi:10.1021/cg800092q
- K. D. Demadis and M. Öner, “Inhibitory Effects of Green Additives on the Crystal Growth of Sparingly Soluble Salts,” Nova Science Publishers, New York, 2009.
- E. R. Camargo, E. Longo, R. L. Edson and M. Kakihana, “Qualitative Measurement of Residual Carbon in Wet -Chemically Synthesized Powders,” Ceramics International, Vol. 30, No. 8, 2004, pp. 2235-2239. doi:10.1016/j.ceramint.2004.02.003
- E. R. Camargo, M. Popa and M. Kakihana, “Sodium Niobate (NaNbO3) Powders Synthesized by a Wet-Chemical Method Using a Water-Soluble Malic Acid Complex,” Chemistry of Materials, Vol. 14, No. 5, 2002, pp. 2365-2368. doi:10.1021/cm011696d
- T. Y. Soror, “Scale and Corrosion Prevention in Cooling Water Systems Part I: Calcium Carbonate,” The Open Corrosion Journal, Vol. 2, No. 1, 2009, pp. 45-50. doi:10.2174/1876503300902010045
- S. H. Yao, F. F. Zheng, H. Liu, J. Y. Wang, H. J. Zhang, T. Yan, J. B. Wu, Z. R. Xia and X. Y. Qin, “Synthesis of Stoichiometric LiNbO3 Nanopowder through a Wet Chemical Method,” Crystal Research & Technology, Vol. 44, No. 11, 2009, pp. 1235-1240. doi:10.1002/crat.200900064
- J. P. Zhao, X. R. Liu and L. S. Qiang, “Preparation and Characterization of LiNbO3 Thin Films Derived from Metal Carboxylate Gels,” Thin Solid Films, Vol. 336-338, Article ID: 10.4028, 2007, pp. 213-216. doi:10.4028/www.scientific.net/KEM.336-338.213
- J.-J. Max and C. Chapados, “Infrared Spectroscopy of Aqueous Carboxylic Acids: Malic Acid,” The Journal of Physical Chemistry A, Vol. 106, No. 27, 2002, pp. 6452- 6461. doi:10.1021/jp014377i
- K. A. Singh, L. C. Pathak and S. K. Roy, “Effect of Citric Acid on the Synthesis of Nano-Crystalline Yttria Stabilized Zirconia Powders by Nitrate-Citrate Process,” Ceramics International, Vol. 33, No. 8, 2007, pp. 1463- 1468. doi:10.1016/j.ceramint.2006.05.021
NOTES
*Corresponding author.

