Open Journal of Polymer Chemistry
Vol.2 No.2(2012), Article ID:19536,7 pages DOI:10.4236/ojpchem.2012.22009
Surface Modification of Waste Tire by Grafting with Styrene and Maleic Anhydride
1Chemistry Department, College of Science, King Khalid University, Abha, KSA
2Chemistry Department, College of Science, Damietta Branch, Mansoura University, New-Damietta, Egypt
Email: *Yassina9@yahoo.com
Received March 21, 2012; revised May 7, 2012; accepted May 15, 2012
Keywords: Waste Rubber; Grafted Copolymerization; Styrene; Maleic Anhydride
ABSTRACT
Waste tire powder, as waste rubber WR was subjected to grafting with styrene (St) and maleic anhydride (MA). Hydrogen peroxide H2O2 was used to initiate the free radical copolymerization of St onto WR. A thermal initiation was used in case of grafting of MA onto WR. Effect of initiator and monomer concentrations together with the influence of reaction temperature and reaction time were investigated. The grafting was estimated by weight, and the grafted copolymers were characterized by FT/IR, DSC and SEM to prove the grafting. It has found that the grafting increases with increase monomer and initiator concentrations. The increase in the reaction temperature and time also causes increasing levels of the grafted St and MA.
1. Introduction
Vulcanized waste rubber especially the scrap tires, cause several environmental problems. Recycling of waste rubber by grafting or blending with polymeric material has become an important in last decades. Surface modification of ground waste tire powder by chlorination and amination reactions, and by photo grafting using UV energy, have been studied [1-3]. Recently authors are modified the surface of rubber crumb with ozone [4], the enhancement of mechanical properties by blending of polypropylene with ground waste rubber powder were done [5]. Also the scrap tires are used as adsorbents for adsorption of organic and inorganic solutes [6]. Grafting copolymerization methods are increasing employed, because of permanent modification effects [7,8]. Styrene is widely used monomer for grafting reaction, due to high reactivity of benzene ring. Therefore, many efforts have been devoted to investigate the grafting of styrene onto various substrates [9,10]. Surface graft polymerization is important because it can provide materials with tailored properties for practical application. The ground tire rubber particles can be modified by grafting with styrene and acrylate [11]. Zhang et al., show that the grafted styrene onto waste rubber powder form core-shell structure [12]. The main advantages of polystyrene are its transparency, high stiffness, excellent process ability and good dielectric properties. Pukkata et al. [13], found that graft polymerization depend not only on the number of active site generated on rubber particles but also on feed of styrene.
Modification of different kinds of rubber using maleic anhydride (MA) is useful to enhance compatibility of immiscible blends as well as improving interfacial adhesion in polymeric composites [14]. Maleic anhydride was successfully photo grafted onto low density polyethylene film, and the surface hydrophilic properties of the grafted films were improved [15,16]. The grafting of maleic anhydride onto natural rubber, was generally carried out in the molten state [17-19]. The initiation system used in the grafting was peroxide initiator [20], or the shearing action [16-18] of the materials in an internal mixer at high temperature. Very limited data on preparation of MA grafted natural rubber in the solution state [21]. Different techniques have been reported to know the MA content grafted onto rubber, such as titration of acid group, gravimetry, infrared spectroscopy and so on [22].
In this work, powdered waste tires (as waste rubber), were used as a polymer backbone for the grafting reaction with styrene and maleic anhydride.
The effect of monomers (St or MA), initiator concentration (H2O2) (in case of St) as well as the reaction time and reaction temperature was studied. The surface chemistry of untreated and treated waste rubber (WR) was characterized by FT/IR spectroscopy, DSC thermal analysis and by scanning electron microscope (SEM).
2. Experimental
2.1. Materials
The ground scrap tires with an average particle size 0.2 - 0.4 mm was prepared from waste tires SBR (styrenebutadiene rubber), USA, which contain about 60% SBR and 40% various additives. Styrene and maleic anhydride (Aldrich) are used without further purification. H2O2, fuming H2SO4 (Fluke). The other chemical solvents and reagents used are of analytical pure grade.
2.2. Surface Grafting Process
The appropriate quantities of WR and St were added in a special designed steel reactor. The requisite amount of H2O2 (initiator) were added and the reactor closed tightly at the required temperature for certain demand time. After the reaction completed, reactor is cooled and opened carefully, then the polymerization mixture was poured into acetone and leaved for 24 h. Washing of grafted polymer carried using chlorobenzene, followed by drying at 40˚C for two days to constant weight.
The above procedure was used for grafting of MA onto WR without using chemical initiator, only by thermal initiation. Sulfonation of WR and WR-g-St was carried using fuming conc. H2SO4 at 80˚C for 8 h. All treated waste rubber material was dried in an oven at 40˚C for 24 h prior to further use.
2.3. Determination of Grafting Yields
Grafting yields were characterized by the following parameters:
Grafting percentage: Gp% = (A – B/B) × 100 Weight conversion: Wc% = (A ÷ B) × 100 where A and B are the weights of the grafted product and WR respectively.
2.4. Characterization Methods
FT/IR spectra were recorded using JASCO FT/IR 460 plus spectrophotometer. Scanning electron microscope (SEM) 6360 (LA) were used to investigate the microstructure of the polymers. Thermal date was obtained by using Shimadzu DSC-50 instrument.
3. Results and Discussion
The grafting of St or MA onto WR was performed at various conditions to get the most suitable conditions for grafting. The variables studied were temperature, time, and the amounts of monomers and initiator.
3.1. Effect of the Reaction Temperature
The graft copolymerization of St onto WR was carried out at four different temperatures ranging from 75˚C to 150˚C. The initiator and WR concentration were 0.5 and 0.25 g/ml respectively, for three days. In Table 1, we see that at higher temperature up to 125˚C, higher level of grafted St and Gp% and Wc% increases. These are attributed to high temperature lead to dissociation of H2O2, and the free radicals increased on the WR chains leading to improve the grafting. Also at such temperature the mobility of WR chains will improved, which enhance the reaction process. Higher temperature than 125˚C cause the grafting decreased, which could be due to degradation of the grafted polymer [23,24].
Table 2 shows the effect of temperature on the grafting of MA onto WR, at temperature ranged from 130 to 250˚C without initiator, at time 5 h and concentrations of MA and WR were 0.3 and 0.25 g. Again as temperature increase the mobility of WR chains increase, leading to reach of MA onto active sites of WR and grafting increase. However, at elevated temperature cause decompose of grafted polymer, resulting Gp% and Wc% declined.
3.2. Effect of the Reaction Time
The effect of the reaction time on the grafting of St onto WR was carried out in range of one to ten days, at 125˚C with the monomers and initiator concentrations of 0.3 and 0.6 g/ml respectively. Figure 1 shows the relationship between the reaction time and, Gp% and Wc%. It can be seen that as the reaction time increases there is an
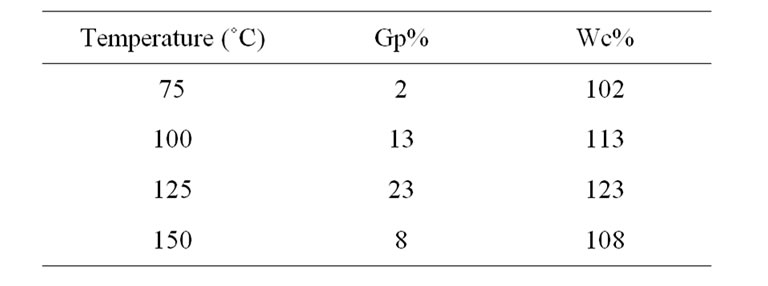
Table 1. Effect of temperature on the grafting of styrene onto waste rubber, at time 3 days; WR 3 g/12 ml; [I] 6 ml/12 ml; [St] 3 g/12 ml.
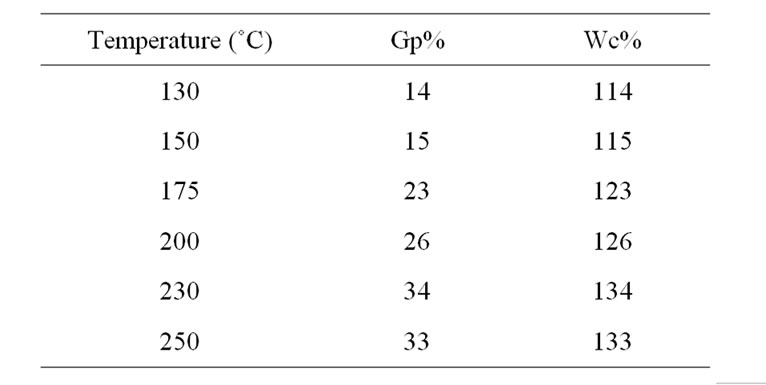
Table 2. Effect of temperature on the grafting of MA onto waste rubber at time 5 h.
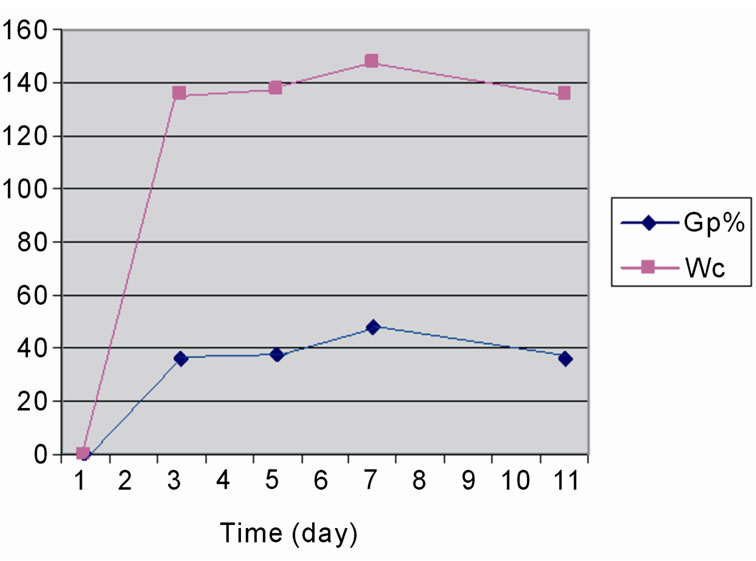
Figure 1. Influence of time on the grafting of styrene onto WR, at 125˚C; [St] 3 g/10 ml; [H2O2] 6 ml/10 ml and WR 3 g/10 ml.
increase in Gp% and Wc% reach to their maximum after seven days, while at longer time 10 days the grafting was decreased as result of destroyed of the polymer chains.
On the other hand study the influence of time on the grafting of MA onto WR at 200˚C for 3, 5, 7, 12 h and at concentration of MA was 0.3 g WR. As show in Figure 2" target="_self"> Figure 2, when the reaction time increases the grafting are increased these can be attributed to, at longer time the reaction between the reactive sites on WR chains and MA may be more pronounced [21].
3.3. Effect of Initiator Concentrations
The effect of H2O2 concentrations on the level of grafted St onto WR, at 125˚C, for 7 days and concentration of St of 0.2 g/ml was showed in Figure 3. As clear from the figure, the grafted St increase with an increase of the initiator concentration. This is because the increase in the imitator concentration produces more free radicals to initiate the graft copolymerization. However at the higher H2O2 concentrations leads to formation of more reactive sites on WR molecules. Reactions of reactive sites on WR cause termination of backbone prior St addition. The results are in agreement with other previous studies [23,25].
Grafting of MA onto WR using H2O2 as initiator was not giving a good results. So the grafting was studied by thermal initiation method, which gives relatively good grafting yields as mentioned before (Section 3.1).
3.4. Effect of Monomer Concentrations
The backbone of WR was grafted with different quantities of St. Experiments were performed at 125˚C, for 7 days and at initiator concentration of 0.4 g/ml, and using 0.25 g/ml of dry WR. Figure 4 shows the increase of Gp% and Wc% as St concentration increase. However at

Figure 2. Influence of time on the grafting of MA onto WR, at 200˚C.
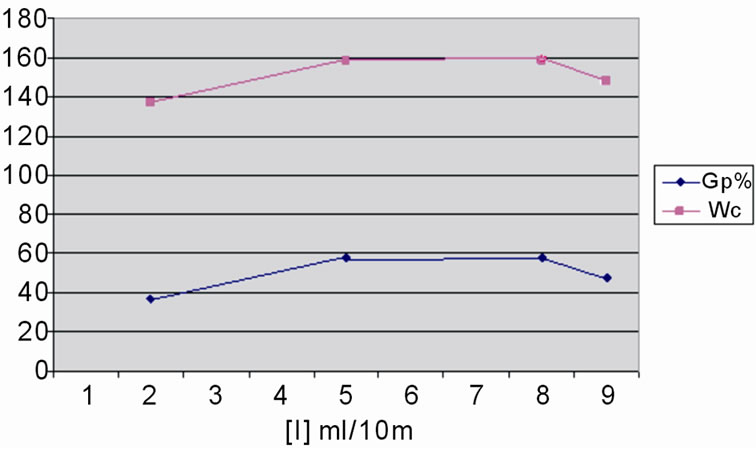
Figure 3. Influence of [H2O2] on the grafting of styrene onto WR, at 125˚C; [St] 3 g/16 ml; and WR 3 g/16 ml.
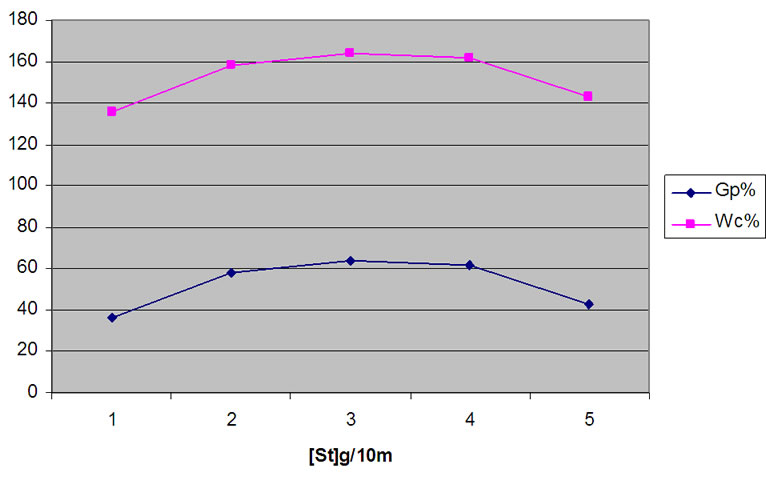
Figure 4. Influence of [St] on the grafting of styrene onto WR, at 7 days; 125˚C; [H2O2] 6 ml/15 ml; and WR 3 g/15 ml.
the higher monomer concentration grafting was decreased. This may be attributed to the increasing trend of side reactions, such as chain transfer to monomer [22].
The same behavior was observed in case of studying the effect of MA concentration on the Gp% and Wc%. The results were shown in Figure 5.
3.5. Infrared Characterization
Figure 6 shows the FT/IR spectra of sulfonated WR and
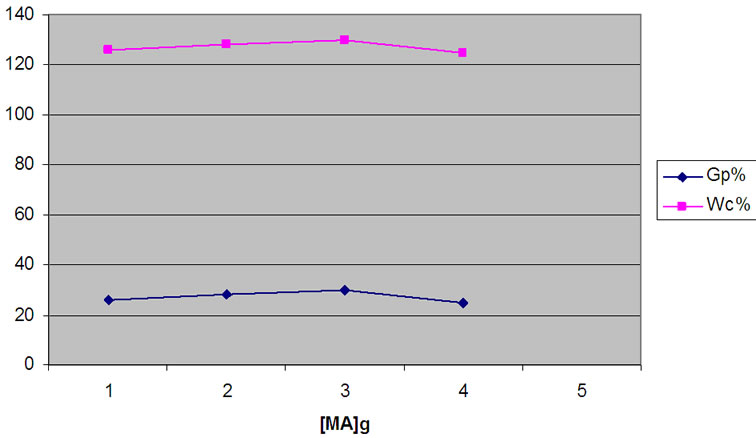
Figure 5. Influence of [MA] on the grafting of MA onto WR, at 7 h; 125˚C and WR, 1 g.
sulfonated WR-g-St focused on the range of wave number 400 - 4000 cm–1. The spectra of the grafted sample ,shows two sharp bands at 1120 and 1162 cm-1 which are assigned to sulfonic group. Also, the appearance of sharp bands at 1625 cm–1, are corresponding to aromatic ring. These will indicate the occurrence of grafting styrene onto the WR backbone. On the other hand the spectra of sulfonated WR show a relatively small band at 1157 cm–1 for sulfonic group compared with those in case of St-g-WR, which indicate the grafting of styrene ring onto rubber structure, which are available for the sulfonation. The spetra of the grafted WR with MA (WR-g-MA) are indicated in Figure 7. There is a broad characteristic band at about 1705 cm–1 and a weak absorption band at 1833 cm–1. These peaks are assigned to C=O group in anhydride group present in grafted maleic anhydride, which are due to symmetric (strong) and asymmetric (weak) C=O stretching vibrations of succinic anhydride rings, respectively [26,27].
3.6. Morphology Studies
SEM photographs of the fractured waste rubber (WR), WR-g-St and WR-g-MA are shown in Figures 8(a), 8(b), 8(c), respectively. Figure 8(a), show the micrograph fracture surface of untreated WR, which indicate the inhomogeneity in structure and larger particle size. When the WR was grafted with St, the porous surface are formed due to divide of large size particles to many segments and the particle size become smaller (Figure 8(b))
In case of WR-g-MA picture, a porous surface was observed and surface exhibit a large particle size, and indicate the penetrating of maleic anhydride onto waste rubber backbone structure (Figure 8(c)).
3.7. Thermal Analyses
The DSC thermogram obtained for waste rubber is showed in Figure 9 (A), it exhibit three exothermic peaks at 252˚C, 351˚C and 505˚C and two endothermic peaks at 282˚C and 406˚C respectively. Based on reaction transition temperature of exotherms and endotherms, the transition pyrolysis temperature ranges can be set at 206˚C - 282˚C, 283˚C - 406˚C and 407˚C - 500˚C. The first region are due to major product of pyrolysis are dipentene, the second are due to C4 hydrocarbons, and the third stage given high yields of CH4, C2 and C3 indicating the complete decomposition of rubber, according to data of Chen & Qian [28], and by Miguel et al. [29].
On the other hand, the DSC curves for the WR-g-St grafted waste rubber Figure 9" target="_self"> Figure 9 (B) exhibit one endothermic peak at about 190˚C which are attributed to transition glass temperature (Tg), and other exothermic peak at about 330˚C. The DSC thermogram for WR-g-MA in
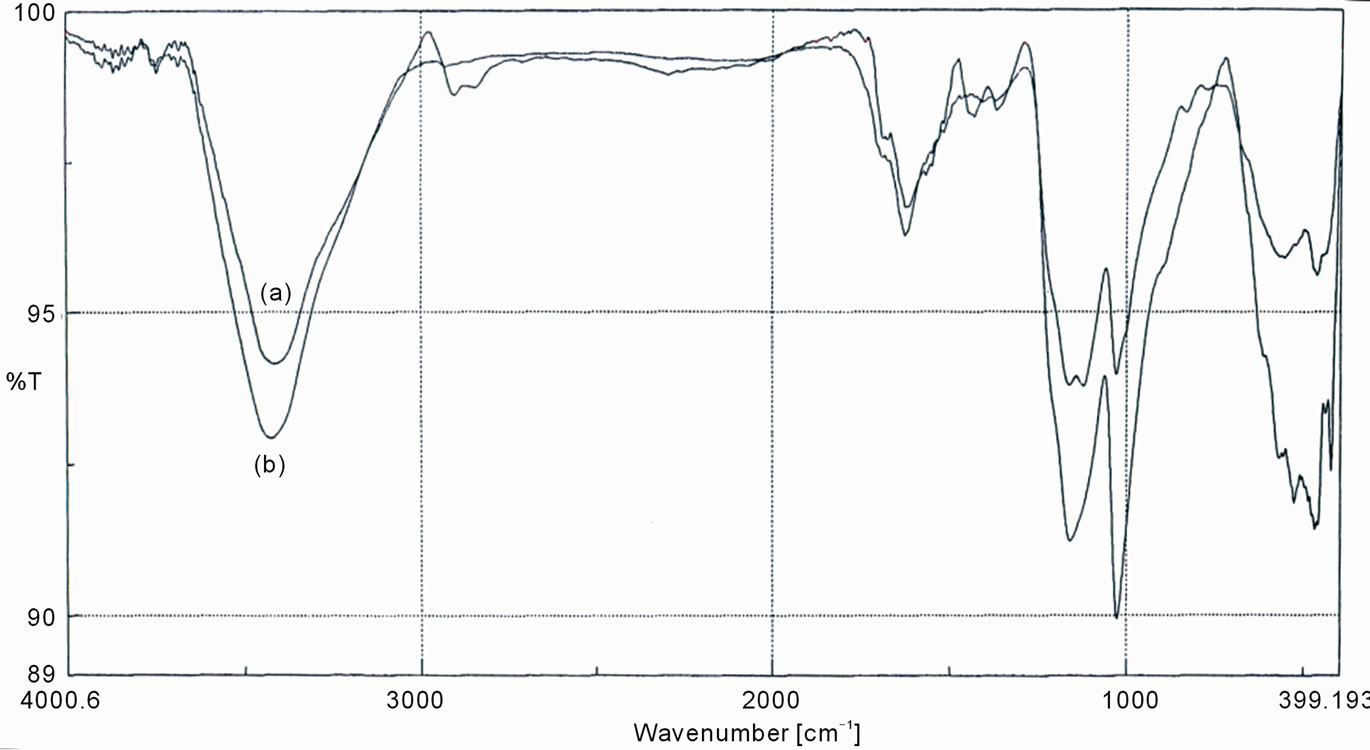
Figure 6. FT-IR spectra of (a) sulfonated WR-g-St; and (b) sulfonated waste rubber.
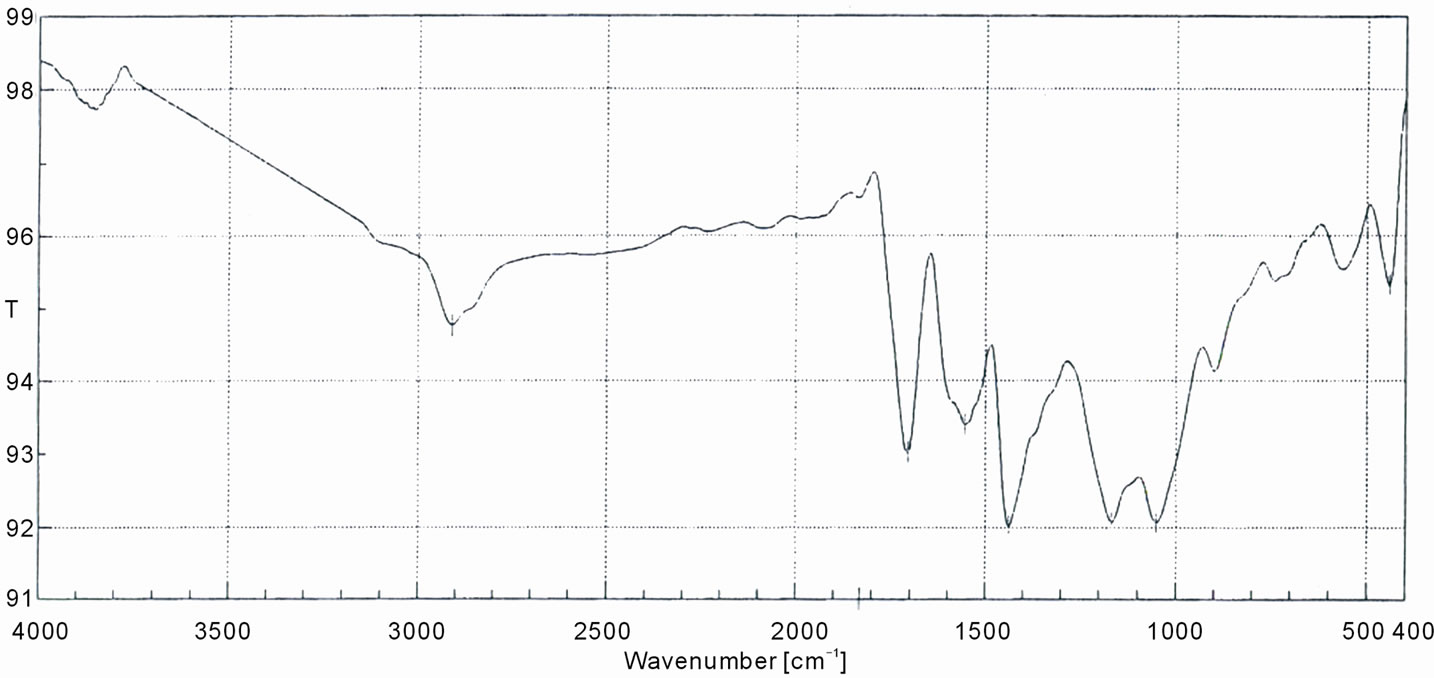
Figure 7. FT-IR spectrum of WR-g-MA copolymer.
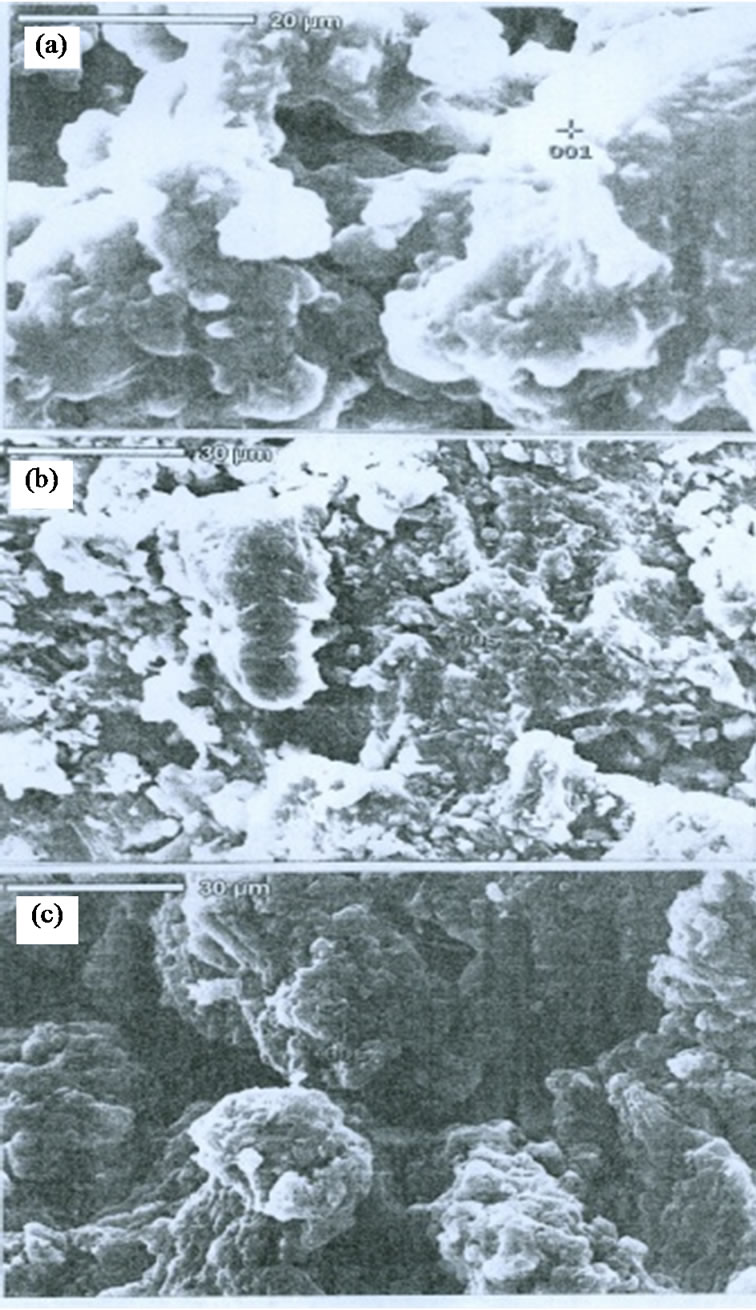
Figure 8. SEM micrographs of (a) WR; (b) WR-g-St; and (c) WR-g-MA.
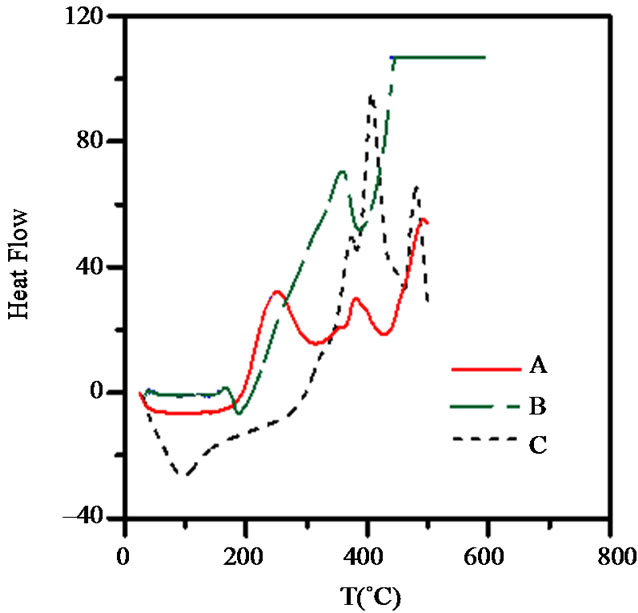
Figure 9. DSC curves for (A) WR; (B) WR-g-St; and (C) WR-g-MA.
Figure 9 (C) show three exothermic bands at higher temperature compared with that for waste rubber, these appear at 370˚C, 400˚C and 470˚C respectively, which reflect the crystalline structure of the grafted waste rubber as a result of penetration of MA within the WR chains. These results are also detected by previous SEM micrographs.
The future of this work in our laboratory is to obtain an ion exchanger for industrial water treatments, based on waste rubber.
4. Conclusion
Graft copolymers of WR and St were prepared by using hydrogen peroxide as initiator (H2O2). However the graft copolymers of WR and MA were prepared by thermal initiation. The influences of temperature, time, and concentrations of both monomer and initiator were studied. The grafted yields increase in both cases of the grafting with increasing monomer and initiator (in case of St) concentrations. The increase of reaction time and reaction temperature also causes increasing the grafting percentage. However the higher increasing initiator concentration, reaction time and reaction temperature cause the dissociation of the grafted polymer and lead to decrease of the grafting percentage. The grafting is confirmed by FT/IR, DSC analyses and SEM photographs of the polymers. The products are used for some application as will be appear in future publications.
5. Acknowledgements
The authors thankful to the kind support of King Abdulaziz City for Science and Technology (KACST), for the Grant No. AP-25-18. The authors are also grateful for King Khalid University (KKU) for encouragements to do such research.
REFERENCES
- Y. A. Aggour, A. S. Al-Shihri and M. R. Bazzt, “Recycling of Vulcanized Waste Rubber through Halogenations and Amination Chemical Reactions,” WIT Transaction on Ecology & Environment WIT Press, Vol. 120, 2009, pp. 875-883.
- K. I. Lee and S. H. Ryu, “Ultraviolet Photo Grafting Reaction of Acrylamide onto Styrene-Butadiene Rubber,” Elastomers, Vol. 33, No. 4, 1998, p. 363.
- J. J. Yu and S. H. Ryu, “Ultraviolet-Initiated Photo Grafting of Glycidyl Methacrylate onto Styrene-Butadiene Rubber,” Journal of Applied Polymer Science, Vol. 73, No. 9, 1994, pp. 1733-1739. doi:10.1002/(SICI)1097-4628(19990829)73:9<1733::AID-APP14>3.0.CO;2-J
- F. Cataldo, O. Ursini and G. Angelini, “Surface Oxidation of Rubber Crumb with Ozone,” Polymer Degradation and Stability, Vol. 95, No. 5, 2010, pp. 803-810. doi:10.1016/j.polymdegradstab.2010.02.003
- S. L. Zheng, Z. X. Zheng, K. Pal, Z. X. Xin, J. Suh and J. K. Kim, “Prediction of Mechanical Properties of Waste Polypropylene/Waste Ground Tire Powder Blends Using Artificial Neutral Networks,” Material & Design, Vol. 31 No. 8, 2010, pp. 3624-3629. doi:10.1016/j.matdes.2010.02.039
- C. T. Torrado, M. A. Franco, C. F. Gonzalez, M. A. Domingues and V. G. Serrano, “Development of Adsorbents from Used Tires Rubber, Their Uses in the Adsorption of Organic and Inorganic Solutes in Aqueous Solution,” Fuel Processing Technology, Vol. 92, No. 2, 2011, pp. 206-212.
- C. Geismann and M. Ulbricht, “Photoreactive Functionalization of Poly(Ethylene Terphthalate) Trak-Etched Pore Surfaces with Smart Polymer Systems,” Macromolecular Chemistry and Physics, Vol. 206, No. 2, 2005, pp. 268- 281. doi:10.1002/macp.200400374
- Y. A. Aggour, A. S. Al-Shihri and M. R. Bazzt, “Chemical Modification of Scraped Tires through Grafting with AMPS,” 2012, in press.
- J. Cha and J. L. White, “Styrene Grafting onto a Polyolefin in an Internal Mixer and Twin-Screw Extruder,” Polymer Engineering Science, Vol. 41, No. 7, 2001, pp. 1238-1250. doi:10.1002/pen.10825
- T. Rager, “Pre-Irradiation Grafting of Styrene/divinylbenze onto PTFE Co-Hexafluropropylene from NonSolvents” Helvetica Chemica Acta, Vol. 86, No. 6, 2003, pp. 1966-1981.
- S. Coiai, E. Passaglia and F. Ciardell, “Gradient Density Grafted Polymers on Ground Tire Rubber Particles by Atom Transfer Radical Polymerization,” Macromolecular Chemistry and Physics, Vol. 207, No. 4, 2005, pp. 2289- 2298. doi:10.1002/macp.200600376
- J. L. Zhang, X. H. Chen, C. M. Ke, Y. Zhou, H. Z. Lu and D. L. Wang, “Graft Polymerization of Styrene onto Waste Rubber Powder and Surface Characterization of Graft Copolymer,” Polymer Bulletin, Vol. 68, No. 3, 2012, pp. 789-801. doi:10.1007/s00289-011-0586-9
- N. Pukkate, Y. Yamamoto and S. Kawahara, “Mechanism of Graft Copolymerization of Styrene onto Deproteinized Natural Rubber,” Colloid Polymer Science, Vol. 286, No. 4, 2008, pp. 441-416. doi:10.1007/s00396-007-1787-5
- S. Newman, “Rubber Modification of Plastics,” In: D. R. Paul and S. Newman, Eds., Polymer Blend, Academic Press, New York, Vol. 2, 1978, p. 63.
- J. P. Deng, W. T. Yang and B. Ranby, “Melt-Photografting Polymerization of Maleic Anhydride onto LDPE Film,” European Polymer Journal, Vol. 38, No. 7, 2002, pp. 1449-1455. doi:10.1016/S0014-3057(02)00004-6
- J. P. Deng and W. T. Yang, “Surface Photo-Grafting Polymerization of Vinyl Acetate Malic Anhydride and Their Charge-Transfer Complex IV. MA,” Journal of Applied Polymer Science, Vol. 87, No. 14, 2003, pp. 2318-2325. doi:10.1002/app.11734
- E. Carone, U. Kipcak Jr., M. C. Goncalves and S. P. Nunes, “In Situ Compatibilization of Polyamide 6/Natural Rubber Blends with Maleic Anhydride,” Polymer, Vol. 41, No. 15, 2000, pp. 5929-5935. doi:10.1016/S0032-3861(99)00800-9
- C. Nakason, A. Kaesaman, Z. Samoh, S. Homsin and S. Kitkamjonwong, “Rheological Properties of Maleated Natural Rubber and Its Blends,” Polymer Testing, Vol. 21, No. 4, 2002, pp. 449-455. doi:10.1016/S0142-9418(01)00109-X
- C. Nakason, A. Kaesaman, S. Homsin and S. Kiatkamjonwong, “Rheological and Curing Behavior of Reactive Blending, Maleated Natural Rubber Cassava Starch,” Journal of Applied Polymer Science, Vol. 81, No. 11, 2001, pp. 2803-2813. doi:10.1002/app.1728
- M. Saedan, T. Navarat and A. Sobmbat, “Grafting of Maleic Anhydride on Natural Rubber Molecules in a Molten State,” The National Research Council of Thailand, Bangkok, 1997.
- C. Nakason, A. Kaesaman and P. Supasanthitikul, “The Grafting of Maleic Anhydride onto Natural Rubber,” Polymer Testing, Vol. 23, No. 1, 2004, pp. 35-41. doi:10.1016/S0142-9418(03)00059-X
- F. M. B. Coutinho and M. I. P. Ferreira, “Characterization of EPDM Rubber Modified with Maleic Anhydride by Diffuse Reflective FTIR,” Polymer Testing, Vol. 13, No. 1, 1994, pp. 25-34. doi:10.1016/0142-9418(94)90037-X
- Y. A. Aggour, G. Bekhat and A. M. Atia, “Copolymerization and Thermal Investigation of AMPS with Acrylonitrile,” Journal of Polymer Materials, Vol. 17, No. , 2000, p. 193.
- J. Deng and W. Yong, “Grafting Copolymerization of Styrene and Maleic Anhydride Binary Monomer System Induced by UV Irradiation,” European Polymer Journal, Vol. 41, No. 11, 2005, pp. 2658-2692. doi:10.1016/j.eurpolymj.2005.05.022
- Y. A. Aggour, “Modification of Polystyrene Properties through N-vinylcarboxamido-2-methylpropane Sulfonic Acid Monomer,” Polymer International, Vol. 53, No. 12, 2004, pp. 1930-1935. doi:10.1002/pi.999
- Z. G. Shen, H.-M. Li, H.-B. Chen and S. G. Lin, “Preparation and Characterization of MA functionalized Syndiotactic Polystyrene,” Polymer, Vol. 43, No. 20, 2002, pp. 5455-5461.
- L. J. Bellamy, “The Infrared Spectra of Complex Molecules,” Wiley, New York, 1964.
- F. Chen and J. Quian, “Studies of the Thermal Degradation of Waste Rubber,” Waste Management, Vol. 23, No. 6, 2003, pp. 463-467.
- G. S. Miguel, J. Aguodo, D. P. Serrano and J. M. Escola, “Thermal and Catalytic Conversion of Used Tyre Rubber and Its Polymeric Constituents Using Py-GC/MS,” Applied Catalysis B: Environmental, Vol. 64, No. 3-4, 2006, pp. 209-219.
NOTES
*Corresponding author.

