Journal of Materials Science and Chemical Engineering
Vol.05 No.11(2017), Article ID:80389,9 pages
10.4236/msce.2017.511005
Synthesis and Characterization of GLBCO-123 Phase: Gd1-xLxBa2Cu3O7-δ (x = 0.0 - 0.5)
Made Sumadiyasa, I. Gusti Agung Putra Adnyana, Nyoman Wendri, Putu Suardana
Department of Physics, Faculty of Mathematics and Natural Sciences, Udayana University, Badung, Indonesia
Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 14, 2017; Accepted: November 14, 2017; Published: November 17, 2017
ABSTRACT
Investigation of the substitution process can provide a better understanding of the superconducting mechanisms in cuprous oxide materials. In this work the effects of substitution Lanthanum (La) for Gadolinium (Gd) on the structure and oxygen content for x = 0.0 - 0.5 in the compound Gd1-xLaxBa2Cu3O7-δ (GLBCO-123 phase) have been investigated. Samples were synthesized by using a wet-mixing method from powders of Gd2O2, La2O2, BaO, CuO, and solution of HNO3. Based on the analysis of XRD data and SEM-EDXA, it confirms that the sample has formed the GLBCO-123 phase, as expected. It has been obtained that the lattice parameters a and c are increased while the parameter b is slightly decreased with increasing content of Lanthanum. The oxygen content slightly decreased and structure of the Gd1-xLaxBa2Cu3O7-δ phase changed from orthorhombic to tetragonal with increasing the content of Lanthanum.
Keywords:
Gd1-xLaxBa2Cu3O7-δ Phase, La Substitution, Lattice Parameters, Orthorhombic, Oxygen Content

1. Introduction
Since the discovery of ceramic superconductor with critical temperature Tc ~30 K on the system Ba-La-Cu-O by Bednorz and Muller [1] , much attention has been focused on the ceramic superconductors. Because the simplicity of its structure and in an effort to study the nature and its superconducting mechanism than the system Ba-La-Cu-O is still widely studied [2] . Next, shortly after the discovery of superconducting systems La-Ba-Cu-O was isolated high-temperature superconductor Tc ~90 K of YBa2Cu3O7-δ. Then some papers appeared in which Y is fully replaced by the most of the rare earth elements, (RE)Ba2Cu3O7-δ phases [3] [4] [5] .
One of the (RE)Ba2Cu3O7-δ phases is the Gd1Ba2Cu3O7-δ phase which is usually abbreviated by GBCO-123. This phase can be superconducting with critical temperatures Tc higher than 90 K, and still can be superconducting in the magnetic field of 17.6 T at a temperature of 26 K [6] [7] . The origin of superconductivity in cuprates based on perovskite has attracted a lot of interest among researchers in recent years [5] [8] [9] . It is important to do some research to find an effective method to synthesize the Gd1Ba2Cu3O7-δ phase if we want to study the properties of superconductivity and optimize it for the purpose of practical applications. Simultaneously also it has been studied the overall understanding of the phenomenon of phase transition from orthorhombic to tetragonal phase or vice versa which generally occurs in (RE)Ba2Cu3O7-δ phases.
Ion substitution has been widely used to study the mechanism of superconductivity in cuprate superconductors which provides important information about the mechanism of the stability of the lattice structure of the superconducting phase. Therefore, in this research the effects of substitution of La to Gd on the Gd1Ba2Cu3O7 phase in the form of Gd1-xLaxBa2Cu3O7-δ, will be investigated as a continuation of our previous research [10] . This is done in order to investigate the effects of La on the formation of the Gd1Ba2Cu3O7 phase in the form of Gd1-xLaxBa2Cu3O7-δ, and the crystal lattice structure which involves changes in unit cell parameters, or thorhombicity, and oxygen content.
2. Experimental Procedure
The GLBCO-123 system ceramic sample in the Gd1-xLaxBa2Cu3O7-δ phase is synthesized by using a wet-mixing method. Powders of Gd2O2, La2O2, BaO and CuO with a purity of 99.9% respectively and in accordance with the composition of the starting material x = 0.00, 0.05, 0.15, 0.25 and 0.50 weighed respectively. All of these materials dissolved in HNO3 and stirred on a magnetic stirrer, and it has been formed a blue-white solution. While stirring the solution was heated at 250˚C until it forms a gel. Gel dried at 300˚C for 6 hours, then it followed by crushed in a mortar. The resulting powder was heated at 400˚C for 2 hours, then it followed by heating at 500˚C for 2 hours and at 600˚C for 6 hours. The products were crushed and heated at 900˚C in a furnace for 15 minutes. The result is then crushed and made into pellets for sintering at 900˚C for 15 minutes.
The sample has been characterized by X-ray diffraction (diffractometer XPERT-PRO) with CuK α radiation (λ = 1.5425 Å). The diffraction data has been collected on a range 2θ = 5˚ to 60˚, and the Match 3 software is used to identify the phases and estimate the volume fraction, of the main peaks of the GBCO-123 phase and its impurities phase. The lattice parameter has been calculated by using software of Rietica. The surface morphology and estimation of the atoms distribution in the sample (grains) were characterized by using SEM-EDX (AMATEX).
3. Result and Discussion
3.1. X-ray Diffraction (XRD)
Figure 1 shows the x-ray diffraction pattern of 2θ in the range of 5˚ - 60˚ of the Gd1-xLaxBa2Cu3O7 phase for x = 0.00, 0.05, 0.15, 0.25 and 0.50. It appears that the results of XRD characterization of all samples had shown sharp peaks. This is indicates that in the sample has been formed a good crystal. In accordance with the standard data file COD No.96-153-9606, main phase 1:2:3 of GLBCO-123 have been acquired. The main reflection peak are (013) and (103) in 2θ = 32˚ to 32.8˚, (020) and (200) in 2θ = 46˚ to 47.3˚, (123) and (213) in 2θ = 57.5˚ to 58.5˚. The splitting of these reflections indicates changes in the orthorhombicity of the cell unit [5] [11] . Impurity peaks appears around 28˚ - 30˚ on samples with x > 0.15. Phase matching has been performed by using software Match 3, and found that the reflection peaks of the impurity are coming from BaCuO2 [12] .
Figure 1 reveals that the peak intensity of the impurity increases with increasing content of La, and vice versa the intensity of the reflection peaks of the main phase is decreased. This shows a decrease in the volume fraction of GLBCO-123 phase as shown in Figure 2. This indicates that the synthesis process for the formation of the GLBCO-123 was successful according to the stoichiometric expectations, but for the content of La x > 0.15 the samples are in the multiphase material.
Figure 1 show that the increasing content of La causes the diffraction peaks of the main phase of GLBCO-123 shifted slightly towards a smaller angle, i.e. the peaks reflection (103), (020) and (123) shifted from 32.56˚ to 32.45˚, 46.53˚ to 46.51˚, and 58.53˚ to 58.11˚, respectively. This shows that the lattice structure of
Figure 1. Powder x-ray diffraction data for five samples in the Gd1-xLaxBa2Cu3O7-δ for x = 0.00, 0.05, 0.15, 0.25 and 0.50. The presence of BaCuO2 in the x ≥ 0.15 sample is indicated by characteristic peaks near 2q ≈ 29˚.
Figure 2. Volume fraction as function x = La content for Gd1-xLaxBa2Cu3O7-δ with x = 0.00, 0.05, 0.15, 0.25 and 0.50.
the GLBCO-123 phase undergoes a phase transformation from orthorhombic to tetragonal [5] [11] .
Cations substitution in a layer on the cell structure and the modification of the lattice parameter is likely to have an effect on the structure of Cu-O layer and the subsequent impact on the properties of superconductivity [13] . It has been conducted an investigation of the effects of substitution of Gd with La on the lattice structure GBCO-123. The software of Rietica was used for Rietveld analysis of X-ray data in order to obtain the lattice parameters a, b and c. This summarized in Figure 3 to Figure 5, in which the lattice parameters a, b, c/3, orthorhombicity, the unit cell volume and the oxygen content plotted against the content of La.
From Figure 3(a), it can be observed that the lattice parameter of a increase significantly, c is slight increases with increasing of La content while the lattice parameter b slight decreases with increasing of La content. It indicates a change of orthorhombicity as shown in Figure 3(b), where orthorhombicity has been calculated by the equation [11] ,
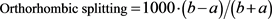 (1)
(1)
Equation (1) widely used to describe the change of orthorhombicity unit cell of Y-123 with oxygen content. In this research, it used to describe the changes of orthorhombicity cell unit of the GLBCO-123 because of the change of La content. Figure 3(b) shows that orthorhombicity decrease with increasing content of La. It shows the transformation in the structure Gd1-xLaxBa2Cu3O7-δ from orthorhombic to tetragonal phase with increasing of La content.
As the nomenclature that has been given by Chen [14] : T for tetragonal phase if a = b < c/3, Oii to orthorhombic phase when a < b < c/3, and Oi to the orthorhombic phase when a < b = c/3. The c-axis is taken along the direction of stacking cation of Gd-Ba-Ba. The lattice parameters for Gd1-xLaxBa2Cu3O7-δ with x = 0.05 to 0.50 is shown in Figure 3(a). The conditions of lattice parameter is a < b < c/3, therefore it has obtained orthorhombic phase of Oii. This is consistent


Figure 3. Orthorhombic lattice parameters (a) a (・), b (¨) and c (□), and (b) the orthorhombicity of the Gd1-xLaxBa2Cu3O7-δ phase as a function of x (content of La).
with the main peaks in the XRD spectrum in Figure 1 in which the reflection peaks (013) and (110) are overlap, but the two peaks are separated by the (103).
In the high-Tc superconductor the oxygen content plays an important role in change of the orthorhombic to tetragonal structure [3] [5] . The deficiency of oxygen δ is calculated by using the relationship between the oxygen content of 7-δ and the length of the lattice parameter c by using equation 7-δ = 74.843 − 5.814c [13] . The oxygen content as a function x for sample of Gd1-xLaxBa2Cu3O7-δ phase is shown in Figure 4. From these figures can be observed that the oxygen content decreased slightly from 6.80 to 6.77, or deficiency of oxygen δ increased slightly with increasing of La content.
According to the scheme of the unit cell structure [14] [15] , the site of oxygen O(5) and O(1) each are in the a and b axes lie in the basal plane. Therefore, from Figure 4 and in accordance with the change in lattice parameter b which decreases as a function of x as demonstrated in Figure 3(a), then for x (La) < 0.5 the amount of oxygen which occupies the site of O(1) along the b axis decreases with increasing content La. Reduced oxygen content in the unit cells means more oxygen vacancies in the unit cells, especially in O(1), consequently the lattice parameter b contraction. As shown in Figure 4, the oxygen content in the unit cell slowly decreases with increasing of La content. On the other hand, the lattice parameter a have increased significantly and the lattice parameter c relatively constant with increasing of La content. Because of the ionic radii La3+ = 1.17 Å is greater than ionic radii Gd3+ = 1.08 Å [16] than as a consequence the unit cell volume increases with the content of La as shown in Figure 5. It suggested that there was no oxygen in the site placement oxygen O(5) along the a-axis. It can be concluded that the addition of La content will result in a decrease in the oxygen content in a unit cell of the GLBCO-123 phase.
3.2. Characterization of SEM-EDX
Figure 6 shows SEM and TEM image of the compound of Gd0.95La0.05Ba2Cu3O7-δ. It seen that an aggregate consist of stacks of a lot of small grains, and the grain is
Figure 4. The oxygen content as a function x for sample of Gd1-xLaxBa2Cu3O7-δ system.
Figure 5. Unit cell volume dependence of La content.


Figure 6. (a) SEM image and (b) TEM image of compound of Gd0.95La0.05Ba2Cu3O7-δ sintering in 0.25 hour.
in the rod shape, it describes the characteristic of the orthorhombic shape. The particle size distribution as shown in Figure 7 was determined by using the software ImageJ 1.34 s. The particle size is in nanometer range. The percentage of particle size in the range of 25 - 150 nm was 98%, and the average particle size is 69.02 nm. These results are consistent with previous reports [17] .
The EDX results of the compound of Gd0.95La0.05Ba2Cu3O7-δ were collected in Table 1. The second column in Table 1 shows the average of the atomics content (%) in the sample. This is accordance with the contents of atomic of the Gd0.96La0.05Ba2.01Cu3.05O6.75 phase, which the oxygen content consistent with the oxygen content in Figure 4.
4. Summary
The effects of La content on the formation of GdBa2Cu3O7-δ, have been studied by X-ray diffraction technique and SEM-EDXA. The XRD and SEM-EDXA data, confirmed that the GLBCO-123 phase with a crystal size in the order of nanometers can be synthesized by using wet mixing method with HNO3 as the solvent. La substitution gave a significant effect on the structure of the phase GLBCO-123: resulting in a change of lattice parameters, oxygen content and orthorhombicity. The GLBCO-123 phase formed in an orthorhombic structure, and with increasing substitution of La to Gd in the GLBCO-123 phase the orthorhombic structure transformed onto a tetragonal.
Figure 7. Particle size distribution.
Table 1. EDX of compound Gd0.95La0.05Ba2Cu3O7-δ.
The critical temperature (Tc) of the Gd1Ba2Cu3O7-δ phase is closely related to its oxygen content, therefore it is important to keep its oxygen content qualified to be superconducting at high temperatures (i.e. 7-δ > 6.75) with high orthorhobicity. The decrease in oxygen content due to La’s substitution will have an impact on Tc reduction, therefore, in the synthesis of the Gd1-xLaxBa2Cu3O7-δ compound should be carried out in an oxygen gas atmosphere.
Acknowledgements
This report is part of the fundamental research report with contract No. 486 127/UN14.2 /PNL.01.03.00/2016. The authors are thankful to RISTEKDIKTI and LPPM of Udayana University.
Cite this paper
Sumadiyasa, M., Adnyana, I.G.A.P., Wendri, N. and Suardana, P. (2017) Synthesis and Characterization of GLBCO-123 Phase: Gd1-xLxBa2Cu3O7-δ (x = 0.0 - 0.5). Journal of Materials Science and Chemical Engineering, 5, 49-57. https://doi.org/10.4236/msce.2017.511005
References
- 1. Bernorz, J.G. and Muller, K.A. (1986) Posible High Tc Superconductivity in the Ba-La-Cu-O System. Zeitschrift für Physik B Condensed Matter, 64, 189-193 https://doi.org/10.1007/BF01303701
- 2. Morales Rivera, A.M., G’omez Cuaspud, J.A., Parra V’argas, C.A. and Brijaldo Ramirez, M.H. (2016) Synthesis and Characterization of LaBa2Cu3O7-δ System, by Combustion Technique. Journal of Superconductivity and Novel Magnetism, 29, 1163-1171. http://link.springer.com/content/pdf/10.1007%2Fs10948-015-3311-3.pdf
- 3. Ishikawa, M., Takabatake, T. and Nakazawa, Y. (1988) Characterization of High Tc Phase and Its Metamorphic Phases Ba2RCu3O7-δ (R=Y, Gd, Dy, Ho, Er, Tm). JJAP Series I, Superconducting Materials, 28-33.
- 4. Mori, K., Sasakawa, M., Isikawa, Y., Khobayashi, K. and Sato, K. (1988) Electrical Resistivities, Transition Temperature and Latice Parameters of LnBa2Cu3O7-y (Ln=Y, Eu, Gd, Ho, Er, La) Sintered in the Flow of Oxigen Gas and Air. JJAP Series I, Superconducting Materials, 81-84.
- 5. Wong-Ng, W. and Cook, L.P., Su, H.B., Vaudin, M.D., and Chiang, C.K., Welch, D.R., Fuller, E.R., Yang, Jr, Z., and Bennett, L.H. (2006) Phase Transformations in the High-Tc Superconducting Compounds, Ba2RCu3O7-δ (R = Nd, Sm, Gd, Y, Ho, and Er). Journal of Research of the National Institute of Standards and Technology, 111, 41-55. https://www.ncbi.nlm.nih.gov/pubmed/27274916 https://doi.org/10.6028/jres.111.004
- 6. Nariki, S., Sakai, N. and Murakami, M. (2005) Melt-processed Gd-Ba-Cu-O superconductor with trapped field of 3 T at 77 K. Superconductor Science and Technology, 18, S126. https://doi.org/10.1088/0953-2048/18/2/026
- 7. Durrell, J.H., Dennis, A.R., Jaroszynski, J., Ainslie, M.D., Palmer, K.G.B., Shi, Y.H., Campbell, A.M., Hull, J., Strasik, M., Hellstrom, E.E., and Cardwell, D.A. (2014) A Trapped Field of 17.6T in Melt-Processed, Bulk Gd-Ba-Cu-O Reinforced With Shrink-Fitsteel. Superconductor Science and Technology, 27, 082001. http://iopscience.iop.org/article/10.1088/0953-2048/27/8/082001 https://doi.org/10.1088/0953-2048/27/8/082001
- 8. Narlikar, A.V. (2004) High Temperature Superconductivity, Materials Aspect of High-Temperature Superconductors for Applicat. Springer, Berlin, 1.
- 9. David Hawthorn, et al. (2016) Physicists Discover New Properties of Superconductivity. http://phys.org/news/2016-02-physicists-properties-superconductivity.html
- 10. Sumadiyasa, M., Adnyana, I.G.A.P., Widagda, I.G.A. and Suharta, W.G. (2016) Study Synthesis of (La1-xGdx)Ba2Cu3O7-δ Superconductors at Low Temperature. Journal of Physics: Conference Series, 725, 012001. https://doi.org/10.1088/1742-6596/725/1/012001
- 11. Yossefov, P., Shter, G.E., Reisner, G.M., Friedman, A. and Yeshurun, G.S. (1997) Relationship of Solubility Parameter (x), Powder Properties and Phase Formation in the Ndl÷xBa2-xCu3O6.5÷x/2÷ 8 System. Physica C, 275, 299-310.
- 12. Zhangt, K., Dabrowski, B., Segret, C.U., Hinks, D.G., Schullert, I.K., Jorgensen, J.D. and Slaski, M. (1987) Solubility and Superconductivity in RE(Ba2-xREx)Cu3O7+δ Systems (RE = Nd, Sm, Eu, Gd, Dy). Journal of Physics C: Solid State Physics, 20, L935-L940.
- 13. Stoyanova-Ivanova, A., Georgieva, St., Nedeltcheva, T., Dimova, L. and Shivachev, B. (2011) Variation of the Unit Cell Parameters of the REBa2Cu3Oy (RE = Gd, Er) Ceramics in Function of the Oxygen Content. Bulgarian Chemical Communications, 43, 320-324.
- 14. Chen, I.W., Keating, S.J., Keating, C.Y., Wu, X., Xu, J., Reyes-Morel, P.E. and Tien, T.Y. (1987) Structural Behavior and Superconductivity of YBa2Cu3Ox. Solid State Communications, 63, 997-1001.
- 15. Lundqvist, P. (1998) Structure and Physical Properties of Doped 123-Superconductors, and Search for New Superconducting Hydrides. Department of Solid State Physics Royal Institute of Technology, S-10044 Stockholm.
- 16. Liang, J.M., Liu, R.S., Huang, Y.T., Wu, S.F. and Wu, P.T. (1990) Superconductivity with Tc(zero) above 105 K in Tl-Containg Septenary Oxides with Y, Ba2Cu30,-Like Stucture. Physica C, 165, 347-353.
- 17. Vinila, V.S., Jacob, R., Mony, A., Nair, H.G., Issac, S., Rajan, S., Nair, A.S., Satheesh, D.J. and Isac, J. (2014) Ceramic Nano-Crystalline Superconductor Gadolinium Barium Copper Oxide (GdBaCuO) at Different Treating Temperatures. Journal of Crystallization Process and Technology, 4, 168-178. https://doi.org/10.4236/jcpt.2014.43021







