Journal of Materials Science and Chemical Engineering
Vol.2 No.5(2014), Article ID:46122,8 pages DOI:10.4236/msce.2014.25005
Influence of pH and Ultrasonic Treatment on Preparation of Titanium Phosphates and Their Powder Properties
Hiroaki Onoda, Syohei Fujikado
Department of Informatics and Environmental Sciences, Kyoto Prefectural University, Kyoto, Japan
Email: onoda@kpu.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 7 April 2014; revised 8 May 2014; accepted 17 May 2014
ABSTRACT
Titanium oxide that has the photocatalytic activity is used as the white pigment for cosmetics. A certain degree of sebum on the skin is decomposed by the ultraviolet radiation in sunlight. In this work, as a novel white pigment, titanium phosphates were prepared from titanium sulfate and phosphoric acid at pH 5, 7, and 9, with/without ultrasonic treatment for cosmetics. Their chemical composition, powder properties, photocatalytic activity, color phase, moisture retention, and smoothness were studied. These titanium phosphates had less photocatalytic activity to protect the sebum on the skin. Sample prepared at pH 7 without ultrasonic treatment had higher moisture retention than other samples. All samples obtained in this work had the suitable smoothness for cosmetics.
Keywords:White Pigment, Titanium Phosphates, Photocatalytic Activity, Smoothness

1. Introduction
As a white pigment, titanium dioxide is used for cosmetic applications [1] . This oxide is well known to have a photocatalytic activity. Therefore, a certain degree of sebum on the skin is decomposed by the ultraviolet radiation in sunlight. To repress this effect, technical processes of several kinds have been investigated and used. For example, as one such technique, composite particles with silicon oxide have been used [2] . However, these particle materials are too hard for use on a human face. Mild materials are required for use as a white pigment on a human face. In addition, one report has described that microfine titanium dioxide is adsorbed through the skin [3] . A novel white pigment that is not adsorbed must be used.
Phosphates have been used for ceramic materials, catalysts, adsorbent, fluorescent materials, dielectric substances, biomaterials, for metal surface treatment, as fertilizer, detergents, food additives, in fuel cells, pigments, and in other applications [4] [5] . Phosphate materials are well known to have high affinity for living organisms. Therefore, as a novel white pigment, phosphates are expected to be useful as cosmetics.
In earlier studies [6] [7] , we prepared a titanium phosphate pigment that had no catalytic activity with titanium chloride. The titanium chloride is difficult to treat because of the formation of smoke and undesirable precipitate. Generally, raw materials have influence on the formation and properties of materials. In the group of titanium compounds, titanium sulfate is also important compound to obtain titanium oxide as well as titanium chloride. Furthermore, the pH value in preparation is an important factor on the particle shape and size of phosphate materials [8] . The ultrasonic treatment was also found to be a useful method to control the particle shape [9] [10] .
In this work, titanium phosphates were prepared from titanium sulfate and phosphoric acid at pH 5, 7, and 9 with/without ultrasonic treatment. Their respective chemical compositions, powder properties, photocatalytic activity, colour phases, moisture retention, and smoothness of the obtained precipitates and their thermal products were studied for application to cosmetics.
2. Experimental
The 0.1 mol/L of titanium sulfate solution was mixed with 0.1 mol/L of phosphoric acid solution in molar ratio of Ti/P = 3/4, and then adjusted to pH 5, 7, 9 with ammonia solution, respectively. This Ti/P ratio was decided from the chemical composition of Ti3(PO4)4. The mixed solutions were treated with ultrasound for 10 minutes (26W, CITIZEN SW5800). For comparison, samples without ultrasound were also prepared. The precipitates were filtered off, washed with water, and dried. All chemicals were of commercial purity from Wako Chemical Industries Ltd. (Osaka Japan) and used without further purification.
A part of the precipitates was dissolved in a sulfuric acid solution. The ratios of phosphorus and titanium in the precipitates were also calculated based on the ICP results of these solutions using an SPS1500VR from Seiko Instruments, Inc. The chemical compositions of these materials were analyzed using X-ray diffraction (XRD). The XRD patterns were recorded on an X-ray diffractometer (MiniFlex; Rigaku Corp.) using monochromated CuKα radiation. Samples were heated at 100˚C in air conditions. These thermal products were also analyzed according to their XRD patterns.
The particle shapes and sizes of the precipitates, as well as their thermal products at 100°C, were estimated based on scanning electron microscopy (SEM) images and particle size distributions. The SEM images of the titanium phosphates were observed (JGM-5510LV; JEOL). The particle size distributions of these materials were measured using a centrifugal precipitation particle-size distribution (SA-CP3L, Shimadzu Corp.).
The cosmetic properties were estimated according to the photocatalytic activity, the color phase, the moisture retention, and the smoothness. The photocatalytic activity of samples was estimated with the decomposition of methylene blue by 365 nm radiation [11] [12] . The 0.01 g of sample was placed in 4 mL of methylene blue solution (1.0 × 10−5 mol/L), and then this solution was radiated. The decrease of the absorption at about 660 nm was estimated for 120 min. The color phase of phosphate pigments was estimated using ultraviolet-visible (UV-Vis) reflectance spectra with a UV2100 (Shimadzu Corp., reference compound; BaSO4). The whiteness was also estimated with TES135 plus color analyser (TES Electrical Electronic Corp). For the moisture retention of the samples, 0.3 g per sample was mixed with 0.1 g of water, and the weight loss was then evaluated at 50°C (MS-70 Moisture Analyzer, A and D Instruments Co. Ltd.). The same weight loss over longer time meant high water retention of samples. The particle smoothness was measured on artificial leather with KES-SE objective evaluation of surface friction property (Kato Tech Co. Ltd.). The values of MIU and MMD respectively represent the slipping resistance and roughness of powders. Sample powders were spread on the leather. Then a sensor was run over these powders. The values of MIU and MMD were calculated respectively from the power to move a sensor and the pitching of a sensor. The values of MIU and MMD have no unit because these values are related with coefficient of friction and scattering, respectively.
3. Results and Discussion
3.1. Chemical Composition and Powder Properties of Precipitates
Table 1 shows the Ti/P ratios of samples prepared under various conditions. This Ti/P ratio in experimental procedure is 3/4 settled from Ti3(PO4)4. All samples indicated higher Ti/P ratio than 0.75. This was caused from the formation of titanium oxide and hydroxide. Samples prepared at pH 7 had relative lower Ti/P ratio, on the other hand samples prepared at pH 9 had higher Ti/P ratio. At pH 9, titanium oxide and hydroxide were easy to form. Figure 1 presents XRD patterns of samples prepared under various conditions. All samples were amorphous state in XRD analyses. Samples heated at 100˚C were also amorphous (not shown). These results were the same with those prepared from titanium chloride [7] . Titanium phosphates were easy to form amorphous state.
From the viewpoint of particle shape, spherical particles are suitable for cosmetic applications. Figure 2 portrays SEM images of samples prepared under various conditions. Specified shape was not observed in all samples. The pH and ultrasonic treatment had less influence on particle shapes of titanium phosphates. Figure 3 presents the particle size distribution of samples prepared at pH 5, 7, and 9 without ultrasonic treatment. All samples indicated high ratio at 15 µm in particle size distribution. The pH in preparation had less influence on particle size distribution of titanium phosphate. The particle size distribution also had little change by ultrasonic treatment and heat treatment at 100˚C (not shown). For cosmetic applications, small and homogeneous particles are suitable. However, overly small particles showed the difficult shortcoming of entering pores in the skin [3] . The standard size of the white pigment for cosmetics is difficult to determine because the skin pore size is affected by factors such as age, gender, and climate. Furthermore, overly large particles are inappropriate because of the cracking of the coating on the skin. It is important to control the pigment particle size.
3.2. Cosmetic Properties of Titanium Phosphate
Figure 4 shows the respective photocatalytic activities of samples prepared under various conditions. Because titanium dioxide is used as a white pigment in cosmetics, this compound was evaluated for comparison with titanium phosphate [1] . Methylene blue was decomposed with titanium dioxide using UV radiation (Figure 4(b)).
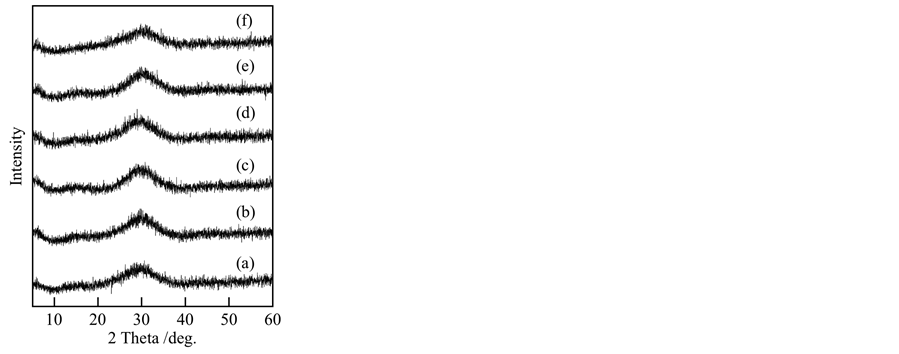
Figure 1. XRD patterns of samples prepared under various conditions: (a) pH 5, ultrasound: 0 min; (b) pH 5, 10 min; (c) pH 7, 0 min; (d) pH 7, 10 min; (e) pH 9, 0 min; (f) pH 9, 10 min.
Table 1. Ti/P ratio of samples prepared under various conditions.






Figure 2. SEM images of samples prepared under various conditions: (a) pH 5, ultrasound: 0 min; (b) pH 5, 10 min; (c) pH 7, 0 min; (d) pH 7, 10 min; (e) pH 9, 0 min; (f) pH 9, 10 min.
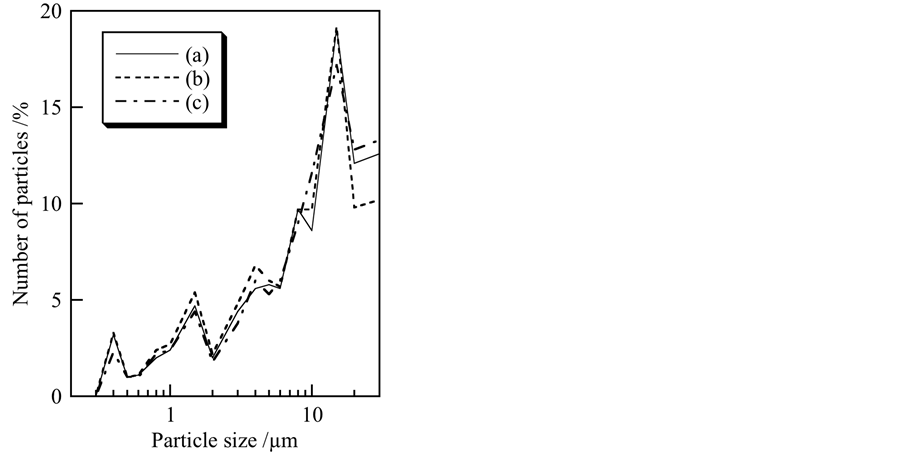
Figure 3. Particle size distribution of samples prepared under various conditions: (a) pH 5, ultrasound: 0 min; (b) pH 7, 0 min; (c) pH 9, 0 min.
Titanium phosphate had little photocatalytic activity in spite of the pH and ultrasonic treatment (Figures 4(c)-4(h)). Titanium phosphate is a mild material that can protect the sebum on the skin.
The color phase is the most important as a novel pigment. All samples without heating and heated at 100˚C were white powder. Figure 5 shows UV-Vis reflectance spectra of samples prepared at pH 7 with and without ultrasonic treatment. Samples indicated high reflectance at the range of visible light. Table 2 shows the white

Figure 4. Photocatalytic activity of samples prepared under various conditions: (a) blank; (b) TiO2; (c) pH 5, ultrasound: 0 min; (d) pH 5, 10 min; (e) pH 7, 0 min; (f) pH 7, 10 min; (g) pH 9, 0 min; (h) pH 9, 10 min.

Figure 5. UV-Vis. reflectance spectra of samples prepared under various conditions: (a) pH 7, ultrasound: 0 min; (b) pH 7, 10 min.
ness of samples prepared under various conditions. These values were L* value in L*a*b* color space. All samples indicated high brightness in spite of the pH and ultrasonic treatment. Samples without heating had higher whiteness than samples heated at 100˚C. Samples heated at 100˚C indicated lower whiteness by ultrasonic treatment. These results were opposite with that without heating. This color change was generally related with the surface of particles, particle sizes, crystalline structure, and defect of crystalline structure. In this work, the particle size had less change by pH, ultrasonic treatment, and heating at 100˚C. Because samples without heating and heated at 100˚C were amorphous in XRD patterns, the crystalline structure and their defects were not clear. Therefore, the reason that samples prepared in this work became a little dark is difficult to clear.
Moisture helps to prevent the itchiness and damage to the skin. It is important that the pigments for used in cosmetics retain the moisture on the skin [12] . Figure 6 shows the moisture retention of the samples prepared under various conditions and then heated at 100˚C. Because sample without heating had the small amount water, the moisture retention was difficult to estimate on sample without heating. At the same weight loss, the later time indicate higher moisture retention. For example, at 20% of weight loss, sample prepared at pH 7 without ultrasonic treatment indicated 15.5 minutes, on the other hand, sample prepared at pH 7 with ultrasonic treatment indicated 9.2 minutes. Sample prepared at pH 7 without ultrasonic treatment had higher moisture retention than other samples. The influence of pH and ultrasonic treatment in preparation process was not clear in the moisture retention of titanium phosphates.
As described above, pigment with high smoothness spreads well on the skin. The powder smoothness is also important for cosmetics [13] . Table 3 shows the smoothness of samples prepared under various conditions. Generally, for a cosmetic application, the suitable MIU and MMD values are smaller than 0.6 and smaller than 0.04, respectively. All samples indicated the suitable MIU and MMD values.
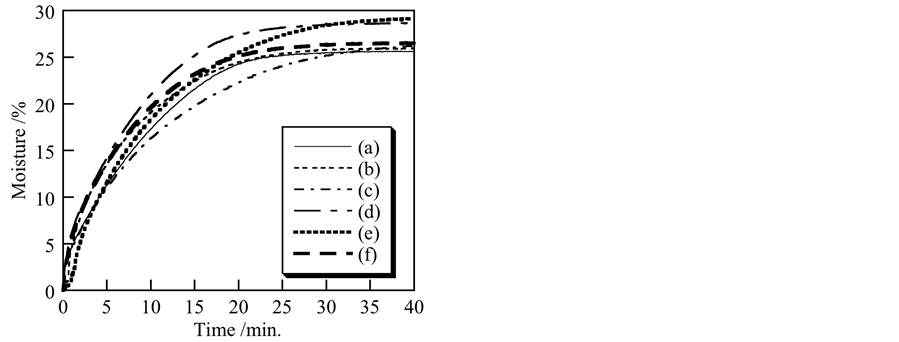
Figure 6. Moisture retention of samples prepared under various conditions and heated at 100˚C: (a) pH 5, ultrasound: 0 min; (b) pH 5, 10 min; (c) pH7, 0 min; (d) pH 7, 10 min; (e) pH 9, 0 min, (f) pH 9, 10 min.
Table 2. Whiteness of samples prepared under various conditions by color analyzer.
Table 3. Smoothness of samples prepared under various conditions.
4. Conclusion
Titanium phosphates were prepared from titanium sulfate and phosphoric acid at pH 5, 7 and 9 with/without ultrasonic treatment. All samples indicated higher Ti/P ratio than 0.75, which ratio is corresponding with Ti3(PO4)4. This was caused from the formation of titanium oxide and hydroxide. All samples indicated high ratio at 15 µm in particle size distribution. These titanium phosphates had less photocatalytic activity to protect the sebum on the skin. Samples without heating indicated higher whiteness by ultrasonic treatment. Sample prepared at pH 7 without ultrasonic treatment had higher moisture retention than other samples. All samples obtained in this work had the suitable smoothness for cosmetics.
Acknowledgements
The authors are grateful to Dr. Takeshi Toyama, Nihon University, Japan, for smoothness measurements.
References
- Diebold, U. (2003) The Surface Science of Titanium Dioxide. Surface Science Report, 48, 53-229. http://dx.doi.org/10.1016/S0167-5729(02)00100-0
- Senzuki, M., Tamura, T., Miura, K., Ikarashi, Y., Watanabe, Y. and Fujii, M. (2010) Study on Penetration of Titanium Dioxide (TiO2) Nanoparticles into Intact and Damaged Skin in Vitro. The Journal of Toxicology Science, 35, 107-113. http://dx.doi.org/10.2131/jts.35.107
- Gamer, A.O., Leibold, E. and Van Ravenzwaay, B. (2006) The in Vitro Absorption of Microfine Zinc Oxide and Titanium Dioxide Through Porcine Skin. Toxicology in Vitro, 20, 301-307. http://dx.doi.org/10.1016/j.tiv.2005.08.008
- Jones, D.J., Aptel, G., Brandhorst, M., Jacquin, M., Jimenez-Jimenez, J., Jimenez-Lopez, A., Maireles-Torres, P., Piwonski, I., Rodrigues-Castellon, E., Zajac, J. and Roziere, J. (2000) High Surface Area Mesoporous Titanium Phosphate: Synthesis and Surface Acidity Determination. Journal of Materials Chemistry, 10, 1957-1963. http://dx.doi.org/10.1039/B002474K
- Bhaumik, A. and Inagaki, S. (2001) Titanium Phosphate Molecular Sieves with Ion-Exchange Capacity. Journal of American Chemical Society, 123, 691-696. http://dx.doi.org/10.1021/ja002481s
- Onoda, H. and Yamaguchi, T. (2012) Influence of Ultrasonic Treatment on Preparation and Powder Properties of Titanium Phosphates. Journal of Materials Chemistry, 22, 19826-19830. http://dx.doi.org/10.1039/C2JM33952H
- Onoda, H., Yamaguchi, T. and Takenaka, A. (2012) Synthesis and Pigmental Properties of Titanium Phosphates with the Addition of Urea. International Journal of Cosmetic Science, 34, 86-90. http://dx.doi.org/10.1111/j.1468-2494.2011.00685.x
- Raynaud, S., Champion, E., Bernache-Assollant, D. and Thomas, P. (2002) Calcium Phosphate Apatites with Variable Ca/P Atomic Ratio I. Synthesis, Characterisation and Thermal Stability of Powders. Biomaterials, 23, 1065-1072. http://dx.doi.org/10.1016/S0142-9612(01)00218-6
- Cai, Y., Pan, H., Xu, X., Hu, Q., Li, L. and Tang, R. (2007) Ultrasonic Controlled Morphology Transformation of Hollow Calcium Phosphate Nanospheres: A Smart and Biocompatible Drug Release System. Chemistry of Materials, 19, 3081-3083. http://dx.doi.org/10.1021/cm070298t
- Jung, S.H., Oh, E., Lim, H., Shim, D.S., Cho, S., Lee, K.H. and Jeong, S.H. (2009) Shape-Selective Fabrication of Zinc Phosphate Hexagonal Bipyramids via a Disodium Phosphate-Assisted Sonochemical Route. Crystal Growth and Design, 9, 3544-3547. http://dx.doi.org/10.1021/cg900287h
- Ramaswamy, V., Jagtap, N. B., Vijayanand, S., Bhange, D.S. and Awati, P.S. (2008) Photocatalytic Decomposition of Methylene Blue on Nanocrystalline Titania Prepared by Different Methods. Materials Research Bulletin, 43, 1145- 1152. http://dx.doi.org/10.1016/j.materresbull.2007.06.003
- Du, P., Bueno-Lopez, A., Verbaas, M., Almeida, A.R., Makkee, M., Moulijn, J.A. and Mui, G. (2008) The Effect of Surface OH-Population on the Photocatalytic Activity of Rare Earth-Doped P25-TiO2 in Methylene Blue Degradation. Journal of Catalysis, 260, 75-80. http://dx.doi.org/10.1016/j.jcat.2008.09.005
- Cheng, S.Y., Yuen, C.W.M., Kan, C.W., Cheuk, K.K.L., Tang, J.C.O. and Li, S.Y. (2009) A Comprehensive Study of Silicone-Based Cosmetic Textile Agent. Fibers and Polymers, 10, 132-140. http://dx.doi.org/10.1007/s12221-009-0132-7


