Journal of Applied Mathematics and Physics
Vol.06 No.12(2018), Article ID:89071,20 pages
10.4236/jamp.2018.612210
Threshold Dynamics of the Stochastic SIRC Epidemic Model
Guangyang Zhang
College of Science, University of Shanghai for Science and Technology, Shanghai, China

Copyright © 2018 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 3, 2018; Accepted: December 8, 2018; Published: December 11, 2018
ABSTRACT
In this paper, we discuss the dynamics of a stochastic SIRC epidemic model with infection rate affected by white noise. We prove that this stochastic model has a unique nonnegative solution globally. A threshold is identified. When the noise is small, the solution of the stochastic model converges to the disease-free equilibrium point of the deterministic model if , which means the basic reproductive number of the stochastic model. And if , the solution of the stochastic model fluctuates around the epidemic equilibrium of the deterministic model. When the noise is large, the disease tends to extinction. The results are illustrated by computer simulations.
Keywords:
Stochastic SIRC Model, Stability, Threshold, Persistence in Mean, The Endemic Equilibrium

1. Introduction
Mathematical models have become important instruments in the analysis and control of infectious diseases. Several models corresponding to epidemic with different characteristics have been proposed and discussed in literature [1] [2] [3] [4] [5] . The fundamental assumption in epidemic models is that the population can be divided into distinct groups; the most common are: the susceptible (S) that are the subjects that may catch the disease; the infected (I) that are the subjects that are already infected and can spread the disease to susceptible individuals; the removed (R) that are the subjects that are immune for life. Therefore, these models are referred to as SIR models.
SI, SIS, SIR models are the basic epidemic models. The differences of these models are that the population is divided into different distinct groups and the relationships between groups in these models are different. The SI model is suitable for the disease without recurrent seizures. The SIS model can describe the patient getting sick repeatedly. The SIR model is used when the patient are immunized after infection. Other descriptions may include the presence of subjects in the quarantine state (Q) and in this situation SIRQ models are analyzed [6] . The models are more and more complex with the development of epidemiology.
Recently the class of cross-immune individuals (C) in the population has been introduced [7] : it is an intermediate state between the fully susceptible state (S) and the fully protected one (R). The obtained SIRC model takes temporary partial immunity into account. The author gave a brief description of the deterministic SIRC model and calculated the basic regeneration number [8] . In 2013, Iacoviello and Stasio studied the SIRC model more closely [9] , and discussed the optimal control method in the case of the outbreak of the SIRC model based on the mathematical results.
In the study of stochastic epidemiological model, the authors give the proof of asymptotic stability of stochastic epidemic models and the theoretical results are verified by simulation experiments. The global uniqueness and asymptotic stability of the solution of the stochastic SIR model [10] were deduced in 2011. Zhao studied the stochastic SIS model in 2013 [11] , and the global uniqueness and asymptotic stability of the solution of the stochastic SIS model were deduced based on the basic regeneration number. Liu published a study of the stochastic SIRS model in 2017 [12] ; the global uniqueness and asymptotic stability of the solution of a stochastic SIRS model are deduced under the condition that the basic regeneration number is greater than 1 or less than 1.
In the existing research, there is no literature to give the basic regeneration number of stochastic SIRC model and other related characteristics. Therefore, this paper mainly studies the stochastic SIRC model, and gives the basic regeneration number of the model. It is proved that when the basic regeneration number is less than 1, the disease-free equilibrium point is randomly asymptotically stable, and when the basic regeneration number is more than 1, the solution of the stochastic model revolves around the endemic equilibrium point of the corresponding deterministic model, and computer simulation is used to verify the correctness of the conclusions.
This paper is organized as follows. We give the model description in Section 2. In Section 3, we show that there is a unique positive solution of system (2.2). In Section 4, we investigate system (2.2) is exponential stability when the noise is large. In this case, the infective decays exponentially to zero. When the noise is small, we deduce the condition which will enable the disease to die out exponentially in Section 4 and the condition for the disease being persistent is given in Section 5. In Section 6, we show that , the result of stochastic model is asymptotically stable and is oscillating around the endemic equilibrium of the deterministic model when . Simulations in Section 7 are reported to support the analytical results.
2. The SIRC Model
The SIRC model and its correlations between the variables are shown in Figure 1. The population can be divided into four parts.
The natural death rates of different compartments can be different for a long period. But in a short time, we can consider that the rates are the same. In this paper, we assumed that the natural death rate of S, I, R, C compartments are the same.
Here, denotes the number of members who are susceptible to an infection at time t. denotes the number of members who are infective to an infection at time t. denotes the number of members who are immune to an infection at time t. denotes the number of members who are cross-immune to an infection at time t. The parameters in the model are summarized in the following list:
A: a consistent input of new members into the population per unit time;
μ: natural death rate of S, I, R, C compartments;
β: transmission coefficient between compartments S and I;
α: transmission coefficient between compartments I and R;
ε: transmission coefficient between compartments R and C;
γ: transmission coefficient between compartments C and S.
All parameter values are assumed to be nonnegative and .
Consider the following SIRC model:
(2.1)
The threshold of system (2.1) is
System (2.1) always has the disease-free equilibrium:
If , then is unstable and there is an epidemic equilibrium. If , then is the unique equilibrium and is globally stable in intΓ, where
Figure 1. SIRC model.
.
In fact, epidemic models are inevitably affected by environmental white noise which is an important component in realism, because it can provide an additional degree of realism in comparison to their deterministic counterparts. Many stochastic models for epidemic population have been developed in [13] - [21] . For example, stochastic models are able to take care of randomness of infectious contacts occurring in the latent and infectious periods [22] . Dalal, Greenhalgh and Mao [20] have previously used the technique of parameter perturbation to examine the effect of environmental stochasticity in a model of AIDS and condom use. They found that the introduction of stochastic noise changes the basic reproduction number of the disease and can stabilize an otherwise unstable system. Nasell [23] formulated stochastic models to show that some stochastic models are a better approach to describe epidemics for a large range of realistic parameter values in comparison with their deterministic counterparts.
In this paper, we mainly discuss while β is affected by noise,
where is standard Brownian motions with , and with the intensity of white noise . The stochastic version corresponding to the deterministic model (2.1) takes the following form:
(2.2)
3. Existence and Uniqueness of Positive Solution
In the biological dynamical system, the researcher is concerned about whether the positive solution of the system exists globally. Therefore, in this section, we show that the solution of system (2.2) is global and positive.
Theorem 3.1. There is a unique solution of system (2.2) on for any initial value , and the solution will remain in with probability 1, namely for all almost surely.
Proof. Since the coefficients of the equation are locally Lipschitz continuous for all given initial value , there is a unique local solution on , where is the explosion time (see [24] ). To show that this solution is global, we need to show that , a.s. Let be sufficiently large so that lie within the interval . For each integer , define the stopping time
where throughout this paper, we set (as usual donates the empty set). According to the definition, is increasing as . Set , whence , a.s. If we can show that , a.s., then and , a.s. for all . In other words, to complete the proof all we need to show is that , a.s. If statement is false, then there exist a pair of constants and such that
Hence there is an integer 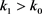 such that
such that
(3.1)
For , we can see for each k
and so
If , then , a.s. So the region
is a positively invariant set of system (2.2) on , which is similar to of system (2.1). From now on, we always assume that .
Define a C2-function W: by
The nonnegativity of this function can be seen form . Let and be arbitrary. Applying the Itô formula, we obtain
where
Therefore, we get Formula (3.2)
(3.2)
Set , and by (3.1), . Note that for every , there is at least one of , , , and that equals either k or , and hence
It then follows from (3.1) and (3.2) that
Let , we have
The former formula is contradictory and we must therefore have , a.s.
For convenience we introduce the notation:
and
4. Extinction
In this section, we investigate the conditions for the extinction of disease.
Theorem 4.1. Let be the solution of system (2) with initial value . If
(a): or
(b): and
Then , a.s.
Proof. An integration of system (2.2) yields, the Formula (4.1) is as follows
Applying the Itô formula, we obtain
Then Formula (4.2) is as follows
Let
,
which is a local continuous martingale and . Moreover
, a.s.
According to Strong Law of Large Numbers, we obtain
, a.s. (4.3)
Since , we obtain
(4.4)
Substituting (4.3) (4.4) into (4.2) yields
If the condition (a) is satisfied, the following statement is tenable.
then
If the conditions (b) are satisfied, Formula (4.5) can be rewritten in another way as follows
Lemma 4.1. Let and be two continuous adapted increasing process on with , a.s. Let be a real-valued continuous local martingale with , a.s. Let be a nonnegative -measurable random valuable such that . Define
for all . If is nonnegative, then implies that , , and , a.s.
Consider the equation
Here, is an m-dimensional Brownian motion.
Lemma 4.2. Suppose that:
1) The function h and g satisfy the local Lipschitz and line growth conditions;
2) , where is the Euclidean norm in .
Then almost every sample path of is uniformly continuous on .
Lemma 4.3. Let j be a nonnegative function defined on that is integrable on and uniformly continuous on . Then .
Theorem 4.2. Under the condition of Theorem 4.1, the disease is extinct. And
, a.s.
, a.s.
, a.s.
, a.s.
Proof. By applying Itô formula to system (2.2), Formula (4.5) is established,
Then,
where,
and
Obviously, we have
Clearly, , and
,
so and are uniformly continuous on . Next, we prove that is uniformly continuous. Noting that
,
we can easily check that the coefficients of system (2.2) satisfy the local Lipschitz and liner growth conditions. In addition,
Then, by lemma 4.2, is uniformly continuous. ,
and are uniformly continuous, so is uniformly continuous. By lemma 4.1,
By lemma 4.3, we obtain
Then,
a.s.
a.s.
a.s.
5. Persistence
Theorem 5.1. If and , then for any initial value , the solution of system (2.2) has the following property:
a.s.
Proof. From Formula (4.2), we obtain
From Formula (3.1), we have
So Formula (5.1) can be rewritten as
(5.2)
for , when , Formula (5.2) can be rewritten as
can be rewritten as (5.4)
Taking inferior limit of Formula (5.4), we have Formula (5.5)
where , if , . By substituting (5.5) into (5.3),
therefore, when , .
6. Asymptotic Property around the Epidemic Equilibrium
Consider the basic reproductive number of stochastic model , if , the disease becomes extinct and the solution of stochastic model converges to the disease-free equilibrium point of the deterministic model when . Therefore, the following part is to introduce the characteristic of stochastic model when the disease survives.
Theorem 6.1. When , the result of stochastic model is asymptotically stable and is oscillating around the endemic equilibrium of the deterministic model when . That is
where
Proof. When , . And the disease-free equilibrium of the deterministic model satisfies following equality:
that is
Define a C2-function W: by
by Itô formula,
where
And
from (6.1), we obtain Formula (6.2)
for , Formula (6.2) can be rewritten like this
similarly,
for , we have
Similar to , can be rewritten in the same way
And
Then we obtain
Let
, ,
so we have
then,
, a.s.
therefore,
7. Computer Simulations
In order to verify the above conclusions, the numerical simulation results of deterministic and stochastic models and their comparisons are given below. In these figures, , , and denote the amount of , , , that change over time in stochastic model. , , and denote the amount of , , , that change over time in deterministic model separately.
We use R to solve these three examples and get figures.
Example 1. let , , , , , , and . The initial values of deterministic and stochastic models are both , , , . We compute that and . Then theorem 6.1 implies that the system will persist and the solution of stochastic model will randomly oscillate around the positive equilibrium point of its corresponding deterministic model. The simulation result is shown in Figure 2.
Example 2. let , , , , , , and . The initial values of deterministic and stochastic models are both , , , . We compute that
and .
Then theorem 4.1(b) implies that the disease will be extinct, as shown by the following four pictures in Figure 3.
Example 3. let , , , , , , and . The initial values of deterministic and stochastic models are both , , , . We compute that
and .
Figure 2. The sample paths of model (2.2) with and .
Figure 3. The sample paths of model (2.2) with and .
Then theorem 4.1(a) implies that the disease will be extinct, as shown by the following four pictures in Figure 4.
Figure 4. The sample paths of model (2.2) with and .
8. Conclusions
The solution of our stochastic SIRC model is affected by basic reproduction number and noise strength. When the noise is small, the solution of the stochastic model converges to the disease-free equilibrium point of the deterministic model if . And if , the solution of the stochastic model fluctuates around the epidemic equilibrium of the deterministic model. When the noise is large, the disease tends to extinction.
In Section 6, we get that the solution of the stochastic model fluctuates around the epidemic equilibrium of the deterministic model. Our next work is to prove this conclusion is valid by probability; that is
.
Conflicts of Interest
The author declares no conflicts of interest regarding the publication of this paper.
Cite this paper
Zhang, G.Y. (2018) Threshold Dynamics of the Stochastic SIRC Epidemic Model. Journal of Applied Mathematics and Physics, 6, 2498-2517. https://doi.org/10.4236/jamp.2018.612210
References
- 1. Xiao, D.M. and Ruan, S.H. (2007) Global Analysis of an Epidemic Model with Nonmonotone Incidence Rate. Mathematical Biosciences, 208, 419-429. https://doi.org/10.1016/j.mbs.2006.09.025
- 2. Zhang, X.-B., Huo, H.-F., Xiang, H., Shi, Q.H. and Li, D.G. (2017) The Threshold of a Stochastic SIQS Epidemic Model. Physica A: Statistical Mechanics and Its Applications, 482, 362-374. https://doi.org/10.1016/j.physa.2017.04.100
- 3. Lahrouz, A. and Omari, L. (2013) Extinction and Stationary Distribution of a Stochastic SIRS Epidemic Model with Non-Linear Incidence. Statistics and Probability Letters, 83, 960-968. https://doi.org/10.1016/j.spl.2012.12.021
- 4. Witbooi, P.J. (2013) Stability of an SEIR Epidemic Model with Independent Stochastic Perturbations. Physica A: Statistical Mechanics and Its Applications, 392, 4928-4936. https://doi.org/10.1016/j.physa.2013.06.025
- 5. Lin, Y.G., Jiang, D.P. and Wang, S.H. (2014) Stationary Distribution of a Stochastic SIS Epidemic Model with Vaccination. Physica A: Statistical Mechanics and Its Applications, 394, 187-197. https://doi.org/10.1016/j.physa.2013.10.006
- 6. Hethcote, H., Ma, Z. and Liao, S. (2002) Effects of Quarantine in Six Endemic Models for Infectious Diseases. Mathematical Biosciences, 180, 141-160. https://doi.org/10.1016/S0025-5564(02)00111-6
- 7. Andreasen, V., Lin, J. and Levin, S. (1997) The Dynamics of Cocirculating Influenza Strains Conferring Partial Crossimmunity. Mathematical Biosciences, 35, 825-842.
- 8. Casagrandi, R., Bolzoni, L., Levin, S. and Andreasen, V. (2006) The SIRC Model and Influenza A. Mathematical Bioscience, 200, 152-169. https://doi.org/10.1016/j.mbs.2005.12.029
- 9. Iacoviello, D. and Stasio, N. (2013) Optimal Control for SIRC Epidemic Outbreak. Computer Methods and Programs in Biomedicine, 110, 333-342. https://doi.org/10.1016/j.cmpb.2013.01.006
- 10. Ji, C.Y., Jiang, D.Q. and Shi, N.Z. (2011) Multigroup SIR Epidemic Model with Stochastic Perturbation. Physica A: Statistical Mechanics and Its Applications, 390, 1747-1762. https://doi.org/10.1016/j.physa.2010.12.042
- 11. Zhao, Y.N., Jiang, D.Q. and Reganle, D. (2013) The Extinction and Persistence of the Stochastic SIS Epidemic Model with Vaccination. Physica A: Statistical Mechanics and Its Applications, 392, 4916-4927. https://doi.org/10.1016/j.physa.2013.06.009
- 12. Liu, Q., Jiang, D.Q., Shi, N.Z., Hayat, T. and Alsaedi, A. (2017) Stationary Distribution and Extinction of a Stochastic SIRS Epidemic Model with Standard Incidence. Physica A: Statistical Mechanics and Its Applications, 469, 510-517. https://doi.org/10.1016/j.physa.2016.11.077
- 13. Roberts, M.G. (2017) An Epidemic Model with Noisy Parameters. Mathematical Biosciences, 287, 36-41. https://doi.org/10.1016/j.mbs.2016.08.002
- 14. Djordjevic, J., Silva, C.J. and Torres, D.F.M. (2018) A Stochastic SICA Epidemic Model for HIV Transmission. Physica A: Applied Mathematics Letters, 84, 168-175. https://doi.org/10.1016/j.aml.2018.05.005
- 15. Liu, Q. and Jiang, Q.D. (2018) The Threshold of a Stochastic SIS Epidemic Model with Imperfect Vaccination. Physica A: Mathematics and Computers in Simulation, 144, 78-90. https://doi.org/10.1016/j.matcom.2017.06.004
- 16. Beretta, E., Kolmanovskii, V. and Shaikhet, L. (1998) Stability of Epidemic Model with Time Delays Influenced by Stochastic Perturbations. Mathematics and Computers in Simulation, 45, 269-277. https://doi.org/10.1016/S0378-4754(97)00106-7
- 17. Cao, B. and Shan, M. (2017) A Stochastic SIS Epidemic Model with Vaccination. Physica A: Statistical Mechanics and Its Applications, 486, 127-143. https://doi.org/10.1016/j.physa.2017.05.083
- 18. Carletti, M. (2002) On the Stability Properties of a Stochastic Model for Phage-Bacteria Interaction in Open Marine Environment. Mathematical Biosciences, 175, 117-131. https://doi.org/10.1016/S0025-5564(01)00089-X
- 19. Carletti, M. (2007) Mean-Square Stability of a Stochastic Model for Bacteriophage Infection with Time Delays. Mathematical Biosciences, 210, 395-414. https://doi.org/10.1016/j.mbs.2007.05.009
- 20. Dalal, N., Greenhalgh, D. and Mao, X. (2007) A Stochastic Model of AIDS and Condom Use. Journal of Mathematical Analysis and Applications, 325, 36-53. https://doi.org/10.1016/j.jmaa.2006.01.055
- 21. Tornatore, E., Buccellato, S.M. and Vetro, P. (2005) Stability of a Stochastic SIR System. Physica A: Statistical Mechanics and Its Applications, 354, 111-126. https://doi.org/10.1016/j.physa.2005.02.057
- 22. Britton, T. (2010) Stochastic Epidemic Models: A Survey. Mathematical Biosciences, 225, 24-35. https://doi.org/10.1016/j.mbs.2010.01.006
- 23. Nasell, I. (2002) Stochastic Models of Some Endemic Infections. Mathematical Biosciences, 179, 1-19. https://doi.org/10.1016/S0025-5564(02)00098-6
- 24. Mao, X. (1997) Stochastic Differential Equations and Their Applications. Horwood, Chichester.





