Advances in Materials Physics and Chemistry
Vol. 2 No. 1 (2012) , Article ID: 17896 , 6 pages DOI:10.4236/ampc.2012.21005
Effect of Strontium on Structure and Superficial Area of La2O3
1Laboratoire de Matériaux et Catalyse, Département de Chimie, Faculté des Sciences, Université Djillali Liabes, Sidi Bel Abbès, Algérie
2Laboratoire de Matériaux, Applications et Environnement, Département des Sciences de la Matière, Faculté des Sciences et de la Technologie, Université de Mascara, Mascara, Algérie
3Centre Universitaire d’Aïn-Témouchent (CUAT), Aïn-Témouchent, Algérie
Email: *rfertout@yahoo.fr
Received June 27, 2011; revised October 23, 2011; accepted December 19, 2011
Keywords: La2O3; Strontium; Calcination; Surface; Structure; TG-DTA
ABSTRACT
Recently, lanthanum oxide doped by group IIA elements may strongly influence solid state reaction. A series of samples, noted LaSrX (where X = % atomic of strontium) have been prepared by hydrolysis, in neutral medium from La2O3 and SrCO3. These samples were calcined under air at 450˚C and 1150˚C then characterized by specific surface area (BET), X-ray diffraction, scanning electron microscopy (SEM), thermogravimetry (TG) and differential thermal analysis (DTA). Obtained results show that after calcinations at: 1) 450˚C, the addition of strontium is without effect on surface of La2O3 and the XRD analysis revealed no interaction between lanthanum oxide and strontium carbonate; 2) 1150˚C, the sintering of the samples is very important, reduction of 70% of surfaces compared to the samples calcined at 450˚C; XRD results show that LaSrX are formed principally by two oxides: SrLa2O4 and La2O3, which is confirmed by SEM method. The reaction between La2O3 and SrCO3 showed three endothermic weight losses; elimination of water, a partial de-hydroxylation of La(OH)3 and formation of La2O2CO3 and La2(CO3)3.
1. Introduction
The literatures [1-5] show that the rare earth oxides (REO) are very interesting in the conversion of greenhouse gases; particularly the valorisation of methane by direct oxidation or dry reforming and the reduction of nitrogen oxides. The ceria and lanthanum oxides are the most used and studied in catalysis. The literatures show that these oxides suffer significant damage under the reactions conditions: lower activities, reduction in surfaces… Recently we showed that the barium stabilizes La2O3 by its interaction and insertion [6].
We are interested in the effect of the elements of the group IIA on the REO; in this paper we reported the results of a study on the effect of strontium on La2O3.
2. Experimental
2.1. Preparation of the Samples
The samples are obtained according to the method used previously [6,7] in our laboratory. These samples are noted LaSrX, X (= 0, 5, 10, 15 and 20) indicates the atomic percentage of strontium (Table 1). These samples are then calcined under air (3 L/h) at 450˚C and 1150˚C during 3 hours.
2.2. Characterizations of the Samples
2.2.1. Surfaces Specific Measurements (BET)
Measurements of surfaces samples are carried out, by adsorption of N2 at 77 K, on 1 gram of powder using Micrometrics ASAP 2000 apparatus.
2.2.2. Analysis by X-Rays Diffraction (XRD)
The apparatus used is a standard diffractometer Philips PW 1800 with cylindrical chamber of 36 mm. The tension of this apparatus is of 40 kV, the current is around 30 mA. The radiations are the Kα of copper: Kα1 (1.554056 Å) and Kα2 (1.54439 Å); these can be easy separated because the ratio of their intensities is about 0.500. A nickel filter is used to eliminate the radiation Kβ from copper. The spectra obtained are indexed according to JCPDS file (Joint Committee one Powder Diffraction Standards).

Table 1. Composition of LaSrX samples.
2.2.3. Scanning Electron Microscopy (SEM)
A JEOL JSM-35 scanning electron microscope (SEM) was employed to study the particle morphology. Prior to SEM observations, samples were deposited from ethalonic solution on to holey-carbon copper grids.
2.2.4. Thermogravimetric Analysis (TG and DTA)
The thermobalance used is a SETARAM TG-DTA92-16 including an electric balance with continuous signal, a furnace equipped with a regulator-programmer of temperature and a recorder. Measurement is made under oxidizing atmosphere (air: 3 L·h–1) with a speed of heating of 10˚C∙min–1 from ambient to 1200˚C.
3. Results and Discussion
3.1. Effects of Strontium on Surfaces of La2O3
The calcinations at 450˚C showed that the strontium addition is without effect on surfaces of lanthanum oxide (Figure 1). Surfaces of the LaSrX samples are practically the algebraic sums “Equation 1” of surfaces of La2O3 and SrCO3. This result is in favour of the segregation of lanthanum (III) and strontium carbonate.
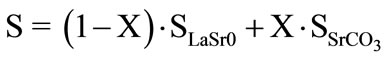 (1)
(1)
Nevertheless, one notes a light surface enrichment of strontium for the samples LaSr5 and LaSr10, starting from X = 20 this tendency is reversed.
At 1150˚C, the addition of 5 and 10% of strontium make slightly increase the surface of lanthanum oxide. This increase is 3.37% and 9.16% respectively for LaSr5 and LaSr10. Beyond 15% no effect on surface of La2O3 is detected. Reported on Figure 1, the measurements of surfaces show a surface enrichment with lanthanum for the samples LaSr5 and LaSr15 and particularly for LaSr10.
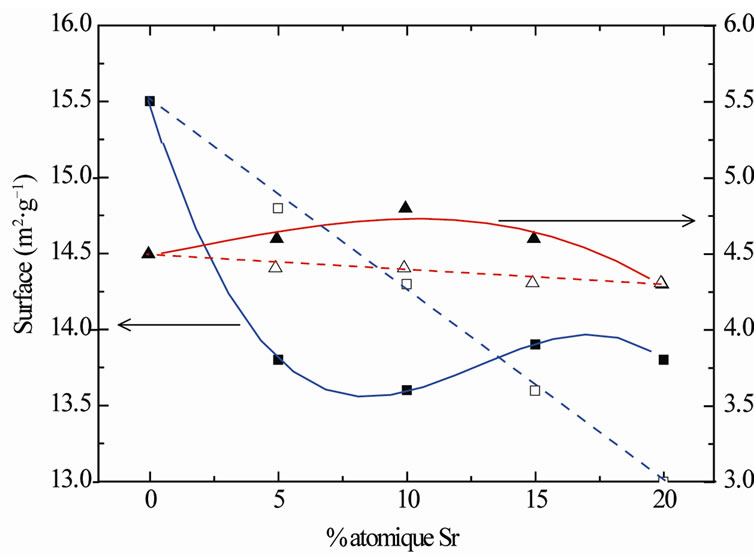
Figure 1. Surfaces of the LaSrX samples calcined under air at 450˚C; (■: measured, □: calculated) and at 1150˚C (▲: measured, △: calculated).
3.2. Characterization of the Samples by XRD and Microscopy SEM
3.2.1. Samples Calcined at 450˚C
The XRD patterns of LaSrX calcined at 450˚C are reported on Figure 2. The analysis of these spectra shows that the samples are mixtures of composed containing lanthanum (carbonates, oxides, oxy-hydroxides, hydroxides and oxy-carbonates) and of strontium carbonate. La(OH)3 is in a majority [6] and crystallizes according to a hexagonal system pertaining to the space group P63/mmc with parameters a = 4.06 Å and c = 6.43 Å, in accordance with JCPDS files. These results showed no interaction between lanthanum oxide and strontium carbonate. Sure enough the comparison between the spectra of the LaSrX samples and that of LaSr0 indicates a whole of lines at 2q = 25˚, 26˚, 37˚ and 44˚ (Figure 2) specific to SrCO3. The intensity of these lines increases with X, the addition of strontium. These results are in agreement with those of the literature [8]. After calcinations at 450˚C, one observes partial or total de-hydroxylation of the hydroxide of lanthanum La(OH)3 and probably according to following reactions:
La(OH)3 → LaO(OH) + H2O (2)
2 LaO(OH) → La2O3 + H2O(3)
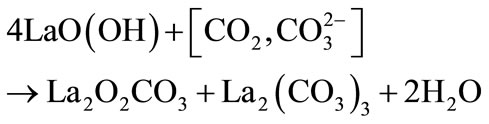 (4)
(4)
The formation of lanthanum oxy-carbonates is very frequent when La2O3 is used in catalysis [9-11]. Indeed
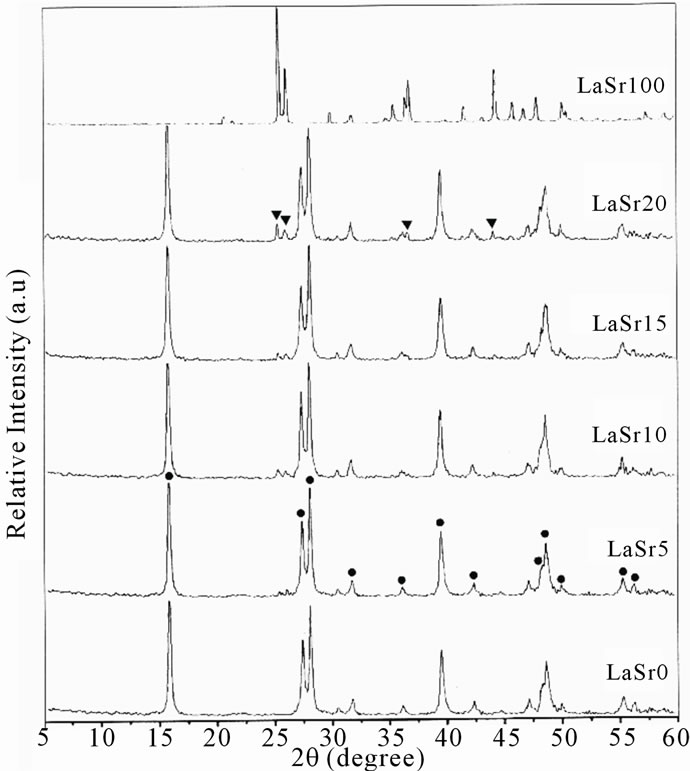
Figure 2. XRD patterns of LaSrX samples calcined under air at 450˚C; Mixture of La(OH)3 and/or La2O2CO3 (●), and SrCO3 (▼).
La2O3 activates CO2 in the form of La2O2CO3, during the reactions using CO2 [12,13]. XRD patterns of the samples show clearly the crystalline phase of La2O2CO3 (Figure 2) this one is promoted by the presence of strontium.
3.2.2. Samples Calcined at 1150˚C
The analysis of XRD patterns reported in Figure 3, shows that samples are formed principally by two oxides: a mixed compounds with structure of SrLa2O4 and La2O3 [6]; the La4Sr3O9, La4SrO7 and La2Sr2O5 appears after treatment at 1500˚C [14].
The interaction and the insertion of strontium in La2O3 are confirmed by the absence of the position of SrO on the spectra of the LaSrX samples. The literature [2] shows that the strontium oxide is not detected by XRD after calcinations between 800˚C and 950˚C. This result are confirmed by scanning electron microscopy, the SEM of sample LaSr15 calcined at 1150˚C (Figure 4), shows porous phase with two homogenous regions probably for SrLa2O4 and La2O3.
3.3. Thermogravimetric Analysis
Thermogravimetric measurements (TG and DTA), of samples from ambient to 1000˚C, are reported on Figures 5(a)-(f).
3.3.1. TG and DTA of the Precursors Alone
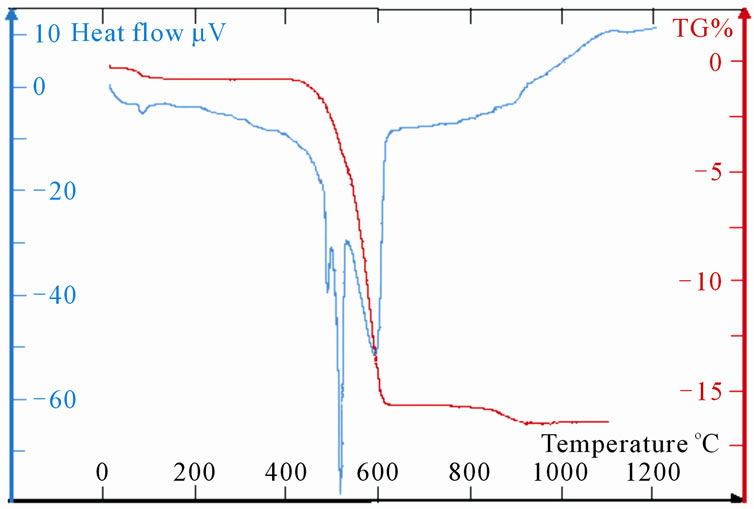
The TG curve, represented on the Figure 5(a), indicates three losses of mass for LaSr100. The first change of
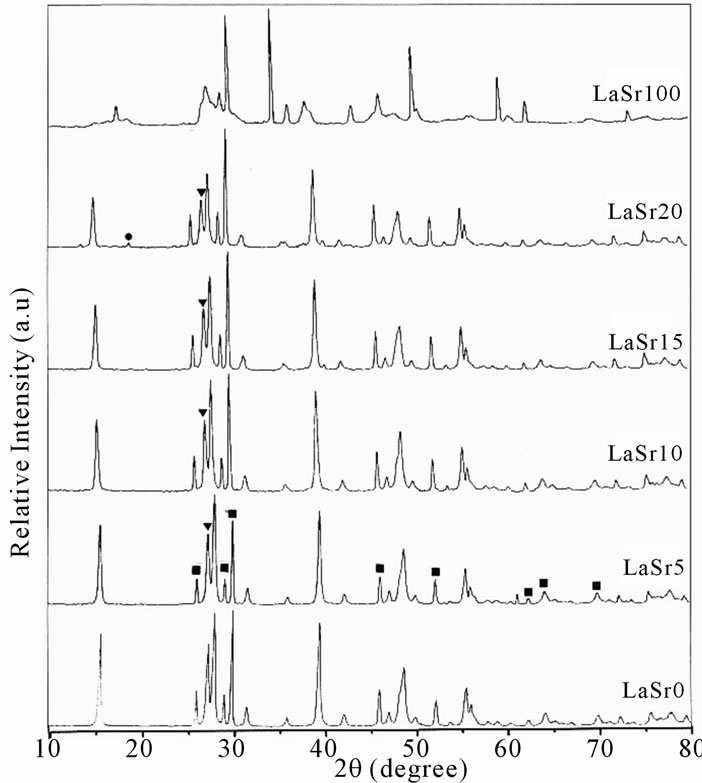
Figure 3. XRD patterns of LaSrX samples calcined under air at 1150˚C. Mixtures of SrLa2O4 (raies ■), La2O3 (▼) and metallic strontium (●).
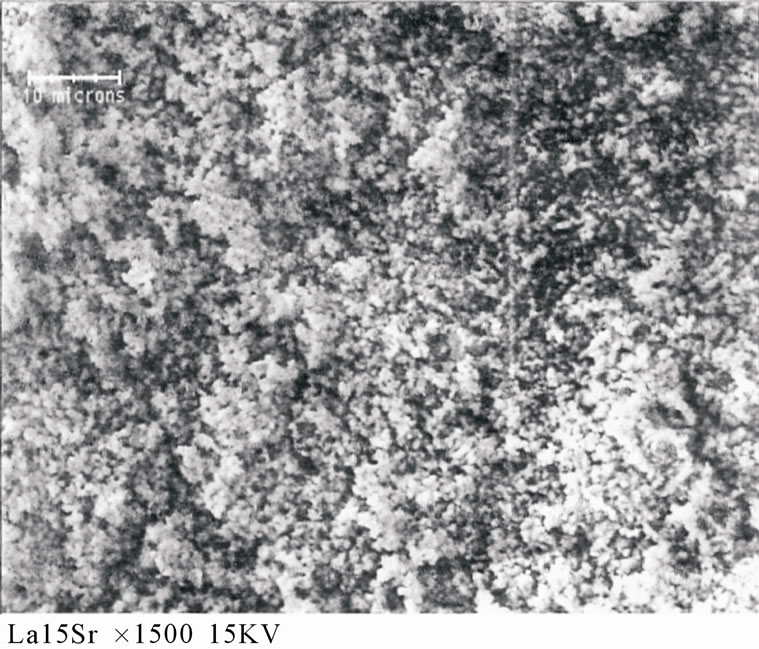

Figure 4. Micrography SEM for sample LaSr15 calcined at 1150˚C, Above: LaSr15 ×1500 KV, Below: LaSr15 ×3500 KV.
mass of about 0.53%, which intervenes with 100˚C, corresponds at the beginning of the water of hydratation. One second loss of mass of 15% starts as of 480˚C to finish with 620˚C approximately. In this temperature range, it has formation of SrO2 according to the “Reaction (5)”; the decomposition of SrCO3 in SrO and CO2 usually proceeds towards 1300˚C [13]
SrCO3 → SrO2 + CO (5)
DTA curves show, in this range of temperature, three peaks of endothermic reactions. The first two fine peaks, towards 480˚C - 540˚C, correspond to the phase shift of SrCO3 [15]; the third towards 600˚C is due to the reaction (5). Indeed, the TG shows that the ratio
 is close to the stoichiometry of CO.
is close to the stoichiometry of CO.
The third change which takes place towards 900˚C shows a loss of mass of 0.62% which could be allotted to a sub-
 (a)
(a)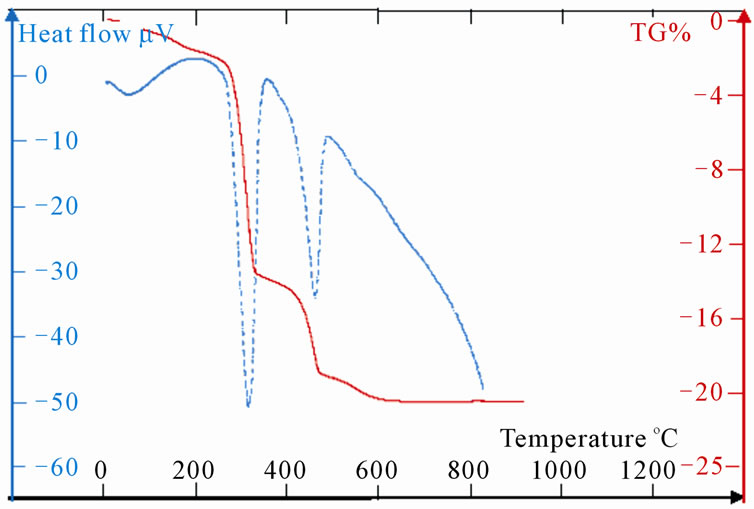 (b)
(b)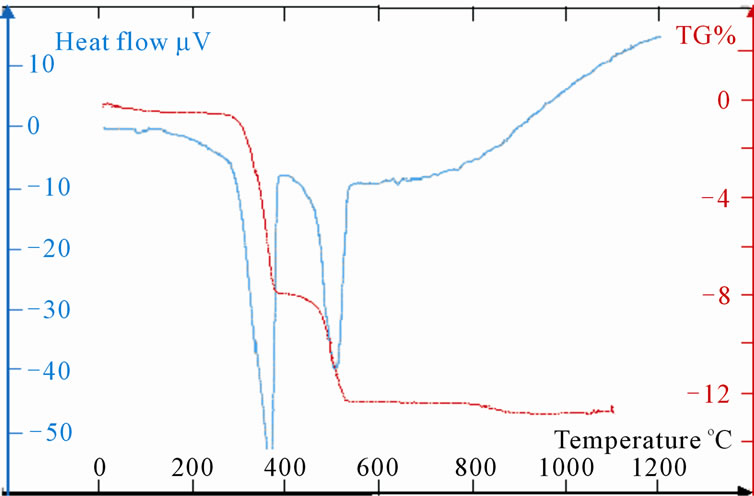 (c)
(c)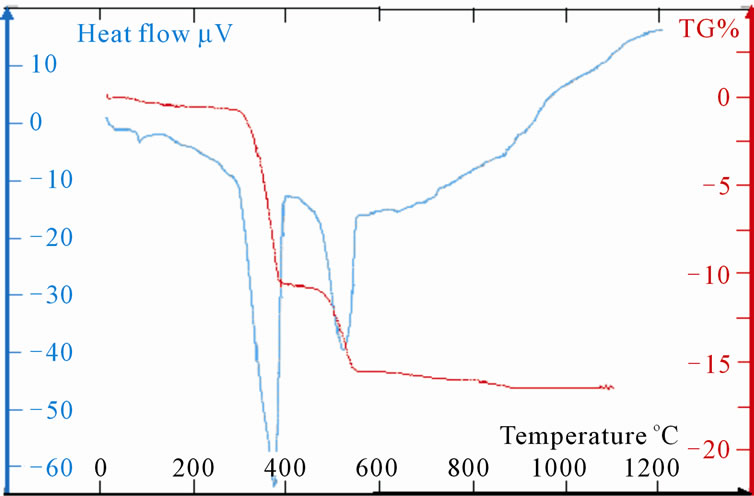 (d)
(d)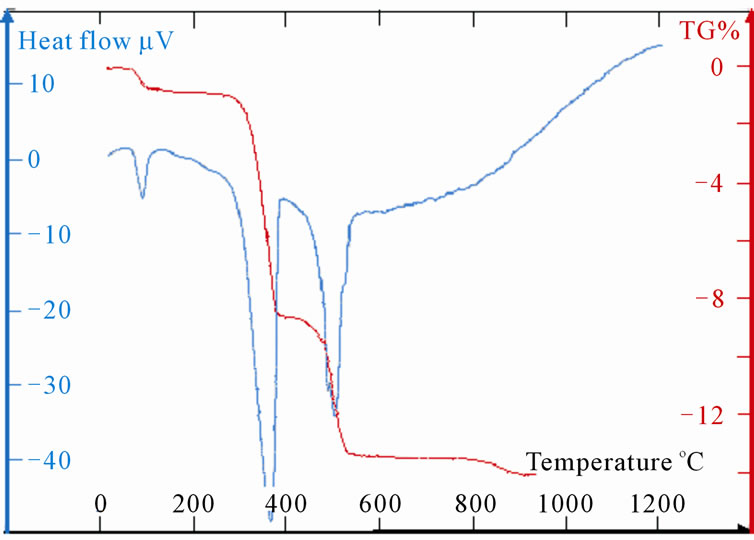 (e)
(e)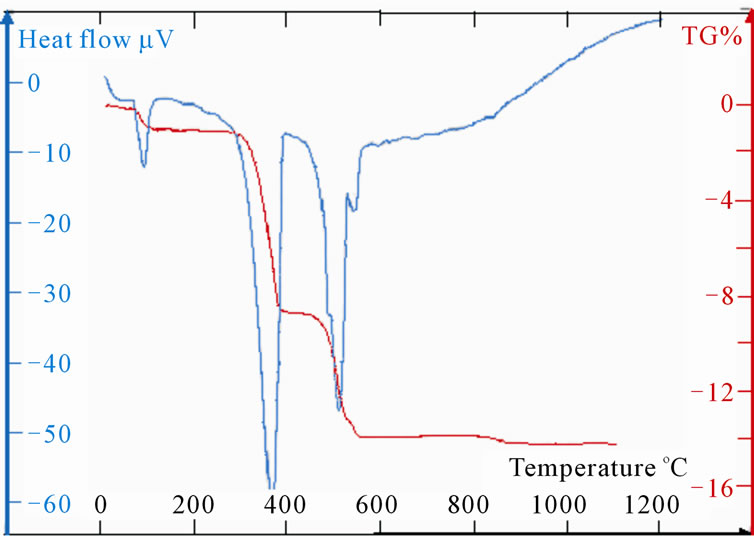 (f)
(f)
Figure 5. (a): TG and DTA of LaSr100; (b):TG and DTA of LaSr0; (c): TG and DTA of LaSr5; (d): TG and DTA of LaSr10; (e): TG and DTA of LaSr15; (f): TG and DTA of LaSr20.
limation of metal strontium coming from the decomposition of 1% of SrO2 “Reaction (6)”.
SrO 2 → Sr + O2(6)
For LaSr0, the Figure 5(b) shows four losses of mass corresponding to four reactions.
The first, below 300˚C indicates the drainage of water.
The formation of La2O3 is obtained after two stages “Reactions (7) and (8)” of the de-hydroxylation of La(OH)3. Between 350˚C and 400˚C a loss of mass, about 7.76%, is attributed to the appearance of lanthanum oxy-hydroxides, and that of La2O3 worms 500˚C. Finally between 650˚C and 800˚C, a loss of mass of 3.18% could be due to the de-carboxylation of La2O2CO3according to the “Reaction (9)”. This compound comes from the easy carbonation of lanthanum oxide and of the tri-hydroxide La(OH)3 with the free air [8].
La(OH)3 → LaO(OH) + H2O(7)
2LaO(OH) → La2O3 + H2O (8)
La2O2CO3 → La2O3 + CO2 (9)
3.3.2. TG and DTA of LaSrX Samples
Measurements TG and DTA of the samples LaSrX (X = 5, 10, 15 and 20) are reported on the Figures 5(c)-(f). The TG shows four stages of losses of mass. First is due to the removal of water towards 100˚C. The last one towards 900˚C is very weak and does not exceed the 0.25% for the four samples. As for the two other significant changes of mass one notes two different behaviours. Indeed, the DTA shows two simple peaks worms 380˚C and the other towards 560˚C for the samples LaSr5 and LaSr10; on the other hand for LaSr15 and LaSr20 the second peak presents two distinct shoulders. For the first couple of samples it had two reactions and four reactions for the second couple of samples.
Samples LaSr5 and LaSr10
Towards 560˚C, the change of mass is neither representative of La(OH)3 (Figure 5(b)) or that of SrCO3 (Figure 5(a)).
LaO(OH) exit of the de-hydroxylation of La(OH)3 reacts with SrCO3 to form a mixed oxide according to “Reaction (10)”.
2LaO(OH) + SrCO3 → SrLa2O4 + CO2 + H2O(10)
Samples LaSr15 and LaSr20
Three peaks DTA, represented on the Figures 5(e) and (f), which makes the superposition of three pics, it can be to explain by the two phases shifts of strontium carbonate and by the departure of CO coming from the decomposition of SrCO3 according to the “Reaction (5)”. Nevertheless, the formation of the mixed compound SrLa2O4 Reaction (10) is not excluded. This reaction would be supported by an acid catalysis of the ions La3+. The quantity of this mixed oxide would be weak and equivalent to that obtained during the treatment of LaSr5 and LaSr10.
Lastly, it should be noted that contrary to the samples LaSr5 and LaSr10, quantity of water which is eliminated from the samples LaSr15 and LaSr20 towards 100˚C is relatively more significant. This water accounts for 1.5% (Dm/m) of the treated mass.
4. Conclusions
A series of samples, LaSrX where X is the atomic percentage in strontium was prepared by hydrolysis of La2O3 and SrCO3 in neutral medium. These samples was calcined under air at 450˚C and 1150˚C, then characterized by BET specific surface area, XRD analysis and SEM microscopy. The reactivity of lanthanum oxide and SrCO3 was followed by TG and DTA.
After calcination at 450˚C, the addition of strontium is without effect on surfaces of lanthanum oxide. The XRD patterns shows data for crystalline phases of La2O3, La(OH)3, LaO(OH) and La2O2CO3 and of strontium carbonate. There are no interaction between lanthanum oxide and strontium carbonate.
After calcinations at 1150˚C, the addition of 5% and 10% of strontium make slightly increase the specific surface of lanthanum oxide, at this temperature the sintering is important, 70% of values of the surfaces are decreases compared to those calcined at 450˚C. The XRD spectra shows that LaSrX samples are formed by two oxides: a mixed compounds with structure of SrLa2O4 and La2O3. This result are confirmed by SEM micrography.
The TG curves of pure La2O3 and SrCO3 indicate that, SrCO3 decompose at 620˚C approximately and the weight losses of La2O3 result for the drainage of water and the partial de-hydroxylation of La(OH)3 with formation of LaO(OH). The reactivity of LaSrX showed three endothermic weight losses; elimination of water, a partial de-hydroxylation of La(OH)3 and formation of La2O2CO3 and La2(CO3)3. In the range of temperature of 500˚C - 580˚C the DTA indicates, a first allotropic transition from the SrO2 and its decomposition on CO and SrO2 for LaSr15 and LaSr20.
REFERENCES
- M. C. J. Bradford and M. A. Vannice, “CO2 Reforming of CH4,” Catalysis Reviews: Science and Engineering, Vol. 41, No. 1, 1999, pp. 1-42. doi:10.1081/CR-100101948
- T. Le Van, C. Louis, M. Kermarec, M. Che and J. M. Tatibouët, “Temperature and Conversion Dependence of Selectivities in Oxidative Coupling of Methane on La2O3 Catalysts,” Catalysis Today, Vol. 13, No. 2-3, 1992, pp. 321-328. doi:10.1016/0920-5861(92)80156-H
- A. Trovarelli, C. De Leitenburg and G. Dolcetti, “Design Better Cerium-Based Oxidation Catalysts,” Chemtech, Vol. 27, No. 6, 1997, pp. 32-37.
- S. J. Huang, A. B. Walters and M. A. Vannice, “NO Reduction with H2 or CO over La2O3 and Sr-Promoted La2O3,” Journal of Catalysis, Vol. 173, No. 1, 1998, pp. 229-237. doi:10.1006/jcat.1997.1911
- M. Traykova, N. Davidova, J.-S. Tsaih and A. H. Weiss, “Oxidative Coupling of Methane—The Transition from Reaction to Transport Control over La2O3/MgO Catalyst,” Applied Catalysis A: General, Vol. 169, No. 2, 1998, pp. 237-247.
- M. Ghelamallah, S. Kacimi and R. I. Fertout, “Incorporation of Barium in Titanium Oxide and Lanthanum Oxide,” Materials Letters, Vol. 59, No. 6, 2005, pp. 714-718. doi:10.1016/j.matlet.2004.06.072
- M. D. Mitchell and M. A. Vannice, “Adsorption and Catalytic Behavior of Palladium Dispersed on Rare Earth Oxides,” Industrial and Engineering Chemistry Fundamentals, Vol. 23, No. 1, 1984, pp. 88-96. doi:10.1021/i100013a016
- A. Galenda, M. M. Natile, L. Nodari and A. Glisenti, “La0.8Sr0.2Ga0.8Fe0.2O3−δ: Influence of the Preparation Procedure on Reactivity toward Methanol and Ethanol,” Applied Catalysis B: Environmental, Vol. 97, No. 3-4, 2010, pp. 307-322. doi:10.1016/j.apcatb.2010.04.004
- T. J. Toops, A. B. Walters and M. A. Vannice, “The Effect of CO2 and H2O on the Kinetics of NO Reduction by CH4 over a La2O3/γ-Al2O3 Catalyst,” Journal of Catalysis, Vol. 214, No. 2, 2003, pp. 292-307. doi:10.1016/S0021-9517(02)00092-1
- L. M. Cornaglia, J. Munera, S. Irusta and E. A. Lombardo, “Raman Studies of Rh and Pt on La2O3 Catalysts Used in a Membrane Reactor for Hydrogen Production,” Applied Catalysis A: General, Vol. 263, No. 1, 2004, pp. 91-101. doi:10.1016/j.apcata.2003.12.003
- K. Matsuzawa, T. Mizusaki, S. Mitsushima, N. Kamiya and K. Ota, “The Effect of La Oxide Additive on the Solubility of NiO in Molten Carbonates,” Journal of Power Sources, Vol. 140, No. 2, 2005, pp. 258-263. doi:10.1016/j.jpowsour.2004.08.041
- T. J. Toops, A. B. Walters and M. A. Vannice “The Effect of CO2 and H2O on the Kinetics of NO Reduction by CH4 over Sr-Promoted La2O3,” Catalysis Letters, Vol. 82, No. 1-2, 2002, pp.45-57. doi:10.1023/A:1020583806660
- B. A. A. Balboul, “The Solid State Reaction between Lanthanum Oxide and Strontium Carbonate,” Thermochimica Acta, Vol. 445, No. 1, 2006, pp. 78-81. doi:10.1016/j.tca.2006.03.005
- ICDD Diffraction Databases, 1994-1998. International Center for Diffraction Data (CDRom), Newtown Square.
- R. C Weast, “Handbook of Chemistry and Physics,” 66th Edition, CRC, Boca Raton, 1985-1986, p. B-148.
NOTES
*Corresponding author.

