Open Journal of Obstetrics and Gynecology
Vol. 2 No. 2 (2012) , Article ID: 20309 , 4 pages DOI:10.4236/ojog.2012.22022
Assessment of pressure generated by catamenial pads in genital and behind-the-knee sites in vivo*
![]()
The Procter & Gamble Company, Cincinnati, USA
Email: #farage.m@pg.com
Received 13 March 2012; revised 10 April 2012; accepted 21 April 2012
Keywords: Assessment of pressure; catamental PADS; BTK; Sensors
ABSTRACT
We conducted an in vivo pilot study to evaluate the pressure exerted by a feminine hygiene pad worn in the traditional way in the genital area and behind the knee, as measured using a novel pressure sensor device. The purpose was to determine whether pressure measured behind the knee correlated with genital pressure, thereby showing that behind-the-knee pressure measurements could be extrapolated to genital pressures recorded under clinical in-use conditions. Four female volunteers wore each of 3 currently marketed feminine hygiene pads and the sensors on the genital area and behind the knee while walking, sitting, and standing for approximately 2 minutes of each activity. Four participants completed the walking and sitting portions of the study and three completed the walking, sitting, and standing portions. The pressure data collected for all 3 pads in both the genital and BTK locations were similar inn both clinical tests. The preliminary results obtained in this clinical pilot study successfully confirm that pressure exerted from feminine hygiene pads in the BTK clinical test model is very similar to real product wear conditions.
1. INTRODUCTION
Products and substances that contact the skin have the potential to cause irritation, such as burning, itching, erythema, edema, and eschar formation resulting from mechanical contact with the skin, such as friction and pressure. Many consumer personal hygiene products, such as menstrual protection pads and diapers, are worn in close proximity to the skin and sometimes for prolonged periods; thus, these products have the potential for causing irritation while at the same time, providing the intended protection for the wearer.
We developed a test method for evaluating the skin effects of catamenial products eliminated some difficulties of in-use clinical testing yet still provide reliable results of the potential irritant effects [1]. The Behindthe-Knee (BTK) test method evaluates the inherent chemical irritation potential of materials or products, in addition to the potential for mechanical irritation. This test method has been proven to be a suitable and valid test for assessing mechanical irritation from prolonged skin contact and friction caused by movement, reliable in identifying irritating materials, and consistent in differentiating between irritating and non-irritating materials [1]. Subsequent studies of the BTK clinical method have further validated its utility in assessing the irritant potential of personal hygiene products [2-5]. The BTK test protocol has been described in previous publications. [2], and in the American Society for Testing Materials (ASTM) standard protocol [6].
The present study evaluated the pressure exerted by a feminine hygiene pad worn in the traditional way in the genital area, as well as a pad worn BTK, using a novel pressure sensor device. The pressure sensor is designed to detect pressure in prosthetic devices, clothing, and diapers, but has never been used directly on the skin in the genital and BTK sites.
2. MATERIALS AND METHODS
2.1. Study Design and Methodology
This was an unblinded, nonrandomized, longitudinal methods development study evaluating the pressure exerted by a feminine hygiene pad worn in the genital area, as well as BTK. The area behind the knees was screened for any cuts, scratches, rashes, sunburn, acne, abrasions, scar tissue, tattoos. The knee circumference was measured to determine the appropriate knee brace size in accordance with the manufacturer’s sizing guidelines.
The Tekscan™ sensor (Prosthetic Model 9801, Tekscan, Inc., South Boston, MA, USA) consists of two thin, flexible laminated polyester sheets (approximately 0.1 mm thick) that have electrically conductive electrodes deposited in varying patterns (Table 1). The inside surface of one sheet forms a row pattern while the inner surface of the other employs a column pattern. The spacing between the rows and columns varies according to sensor application and can be as small as ~0.5 mm. The sensor was trimmed and slit into 6 independent strips of 16 sensing cells each. The sensor technology includes thin, film-based sensors, data acquisition hardware, and software.
To measure the genital pressure, a sensor was applied by a medical professional vertically on either side of the labia majora using individual strips of tape, Tegaderm™ (3 M, St. Paul, MN, USA) (Figures 1(a) and (b)); prior to application, the genital area was cleaned using a wetted Bounty Paper Towel and then dried using a dry paper towel. A 6” × 8” piece of tape cut into strips was used to adhere each of the 6 columns of the sensor independently to the test sites. One section of the sensor contains 3 strips of 16 individual pressure units. Once the sensor was in place in the genital area, the participant applied one of three currently marketed feminine hygiene pads (Always Infinity®, Always® Maxi and Ultra Thin; Procter & Gamble Co., Cincinnati, Ohio, USA) to a pair of underwear (Fruit of the Loom™, Fruit of the Loom, Inc.). Denim jeans (provided by the subject) were worn over the underwear. The sensor was connected to a laptop computer to record output. While recording, the subject was asked to stand, walk, and sit as she normally would for approximately 2 minutes for each activity and for each pad tested. Once the genital measurements are completed, the sensor was removed and disposed of in a biohazard container.
To measure the BTK pressure, a new sensor was applied by study staff horizontally to the BTK area using individual strips of tape, similar to the procedure used for the genital area. As in the genital pressure assessment, with the sensor is in place, staff applied one of three feminine hygiene pads BTK held in place with the appropriately sized bandage (Ace™, 3 M, St. Paul, MN, USA). The sensor was connected to a laptop computer to record output. While recording, the participant was asked to stand, walk, and sit as she normally would for approximately 2 minutes/each activity. The timing of the commencement of the different activities was recorded and the process repeated for each pad tested. The same sensor was used for all pads worn in the BTK area. Once the BTK measurements were completed, the sensor was removed and disposed.
2.2. Study Participants
Qualified volunteer participants (women) were enrolled from a local hospital’s volunteer panelist database. The trial was conducted during a single visit for each participant. At that visit, participants’ eligibility to participate was determined according to inclusion and exclusion criteria and written informed consent was obtained. The exclusions were: 1) Pregnancy (self-reported); 2) Cuts, scratches, rashes, sunburn, acne, abrasions, scar tissue,
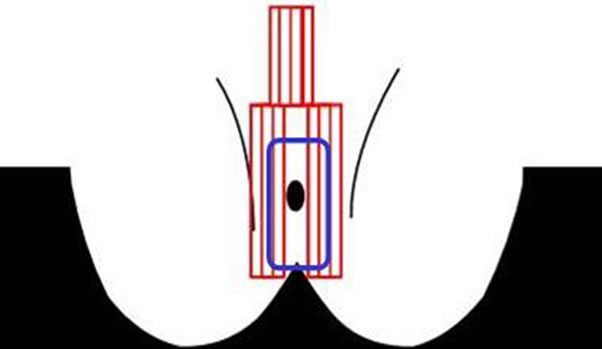 (a)
(a)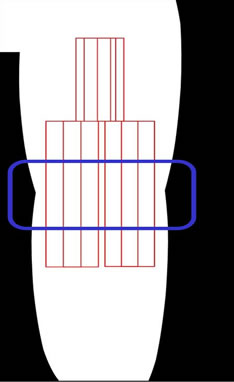 (b)
(b)
Figure 1. (a) Genital sensor position; inside rectangle indicates feminine hygiene pad position; (b) Behind-The-Knee (BTK) sensor position; inside rectangle indicates horizontal position of feminine hygiene pad over sensor.
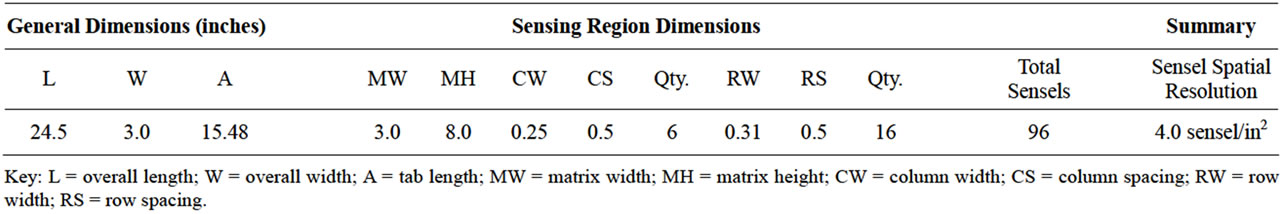
Table 1. Dimensions for the tekscan™ model 9801 sensor.
tattoos, or any other skin abnormality at the test sites that could potentially interfere with interpretation of test results; 3) Known irritancy or discomfort in the area behind the knee or in the lower leg that would prevent the subject from wearing a knee brace for 1 hour each day during their study participation (e.g., substantial leg ache after standing); 4) Any type of malignancy, any heart and/or circulatory disease, defect or condition (including blood clots) (self-reported); 5) Leg varicosities which would interfere with the subject wearing a knee brace for up to 1 hour each day during their trial participation; 6) Knee circumference measurement > 21 inches; 7) Known allergies to tape(s) and/or adhesives; 8) History of any recent and/or chronic edema or arthritis in the lower extremities.
3. RESULTS
Four participants completed the walking and sitting portions of the study and of those participants, only three completed a standing portion. The average pressures for the genital and BTK sites for each pad are shown in Table 2. The pressure data collected for all 3 pads in both the genital and BTK locations resulted in similar results (Figures 2-4). The Always® Ultra Thin, Always Infinity®, and Always® Maxi pads showed corresponding decreasing pressures, respectively, for the different activities. There were no adverse events.
4. DISCUSSION
The BTK clinical test method has been established as a valid and reliable tool to assess both the inherent chemical irritation potential of materials and products and the potential for mechanical irritation. This test method has been proven to be a suitable and valid test for assessing mechanical irritation from prolonged contact with the skin and friction caused by movement, reliable in identifying irritating materials, and consistent in differentiating between irritating and non-irritating materials [1]. Furthermore, the skin behind the knee—although of different composition than the genital mucosa—has been shown to be a valuable and reliable alternative to clinical in-use testing of catamenial products directly on the genital area [4]. This is an especially important and valuable benefit of BTK test site, because conducting clinical in-use test of menstrual hygiene pads can be logistically difficult. In addition, in-use testing often must coincide with the participants’ menstrual cycles, which can delay availability of results for 4 - 5 weeks. Because genital skin irritation is graded by visual assessment, in-use clinical testing can be intrusive to the participants, and each participant can test only one product at a time [4].
In continuing efforts to further validate the BTK method, this study sought to determine whether this
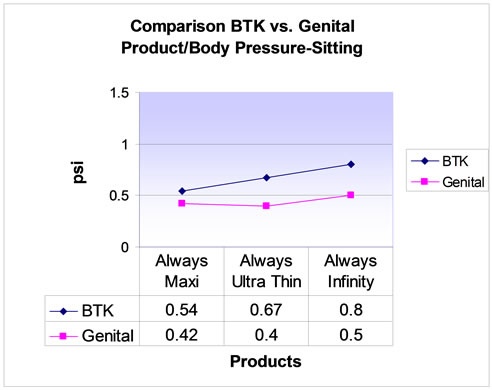
Figure 2. Comparison of Behind-The-Knee (BTK) vs genital product/body pressure: sitting.
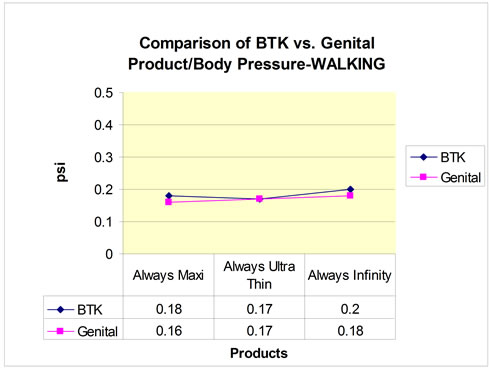
Figure 3. Comparison of Behind-The-Knee (BTK) vs genital product/body pressure: walking.
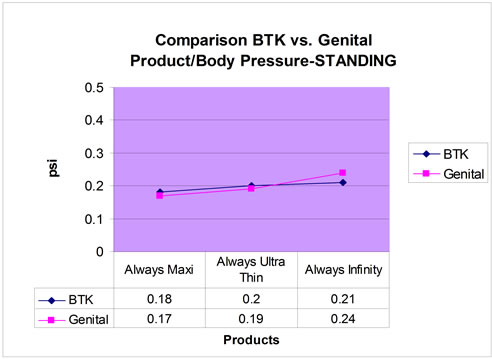
Figure 4. Comparison of Behind-The-Knee (BTK) vs genital product/body pressure: standing.
method would prove valuable in evaluating pressure (in psi) caused by feminine hygiene pads on the genital area during sitting, standing, and walking. The pressure sensor used was designed to detect pressure to assist in prosthetic fit assessment, handgrips, clothing fit, diaper, and pressure garments. This pilot study is the first to use the sensor applied directly to genital and BTK skin for pressure detection from a feminine hygiene pad and to determine the BTK site’s accuracy in mirroring the results obtained directly from the genital area.
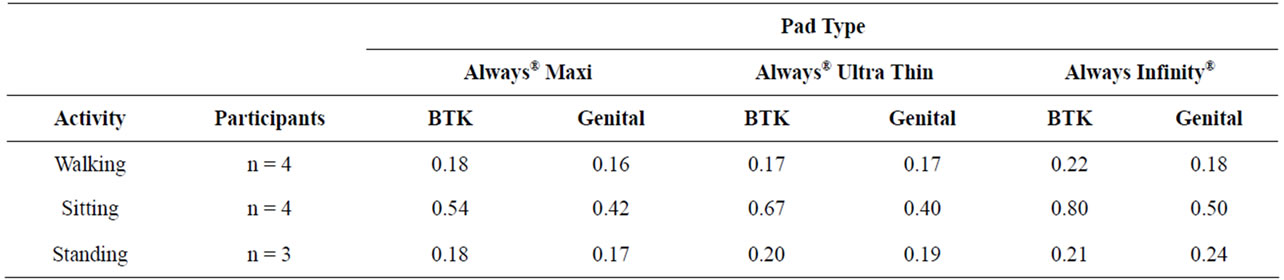
Table 2. Average pressures in psi for walking, sitting, and standing.
The results of this study indicate that the BTK test site can be utilized as an alternative test site to the genital area to determine pressure resulting from feminine hygiene pads. Although this study did not attempt to evaluate/grade any potential irritation resulting from the pressure of the pads, the BTK test has been established previously to be a valid and reliable site to evaluate irritative effects of materials and substances that can be extrapolated to the genital mucosa.
In conclusion, the preliminary results obtained in this clinical pilot study successfully confirm that pressure exerted from feminine hygiene pads in the BTK clinical test model is very similar to real product wear conditions. In addition, it continues to confirm the usefulness and robustness of the BTK test as a clinical test model for urogenital skin effects and replacement of more cumbersome, long and expensive in-use clinical studies.
5. ACKNOWLEDGEMENTS
We thank Ms. Melanie Hansmann, Ms. Rebecca Patterson, and Ms. Sharon Ferguson for the conduct of this study and Ms. J. Tremaine for technical help.
REFERENCES
- Farage, M.A., Gilpin, D.A., Enane, N.A. and Baldwin, S. (2001) Development of a new test for mechanical irritation: Behind the knee as a test site. Skin Research and Technology, 7, 193-203. doi:10.1034/j.1600-0846.2001.70309.x
- Farage, M.A., Meyer, S. and Walter, D. (2004) Development of a sensitive test method to evaluate mechanical irritation potential on mucosal skin. Skin Research and Technology, 10, 85-95. doi:10.1111/j.1600-0846.2004.00055.x
- Farage, M.A., Santana, M.V. and Henley, E. (2005) Correlation sensory effects with irritation. Cutaneous and Ocular Toxicology, 24, 45-52. doi:10.1081/CUS-200046189
- Farage, M.A. (2006) The behind-the-knee test: An efficient model for evaluating mechanical and chemical irritation. Skin Research Technology, 12, 73-82. doi:10.1111/j.0909-752X.2006.00184.x
- Farage, M.A. (2006) A behind-the-scenes look at the safety assessment of feminine hygiene pads. Annals of the New York Academy of Sciences, 1092, 66-77. doi:10.1196/annals.1365.006
- ASTM Standard F2808 (2010) Standard test method for performing Behind-The-Knee (BTK) test for evaluating skin irritation response to products and materials that come into repeated or extended contact with skin. ASTM International, West Conshohocken. www.astm.org doi:10.1520/F2808-1
NOTES
*Conflict of interest: none.
#Corresponding author.

