Open Journal of Immunology
Vol.2 No.4(2012), Article ID:26266,19 pages DOI:10.4236/oji.2012.24021
Structural and functional consequences of switching carboxy terminal domains in mouse CD200 receptors
![]()
Transplant Research Division, Toronto General Hospital, University Health Network, Toronto, Canada; *Corresponding Author: ismat.khatri@utoronto.ca
Received 20 September 2012; revised 20 October 2012; accepted 19 November 2012
Keywords: CD200 Binding; Dap12 Phosphorylation; Domain Switching; Glycosylation; Mabs to mR2
ABSTRACT
CD200:CD200R interactions regulate immune responses. Since CD200Rs show extensive homology in their extracellular region, generating anti-CD200R specific antibodies is a challenge. We report below on the generation of mAbs specific for murine (m)R1/R2 and evidence that mR2 is expressed on the cell surface in the absence of the adaptor protein Dap12. Despite homology between mR1 and mR4, the unexpected reduction in the molecular mass (i.e. 90 kDa vs 48 kDa) between the two receptors suggested that the TM and cytoplasmic region of mR4 regulated glycosylation. Substitution of the TM and cytoplasmic region of mR1 and mR2 with that of mR4 reduced glycosylation of the chimeric receptors mR1r4 and mR2r4 implying that these regions regulated the glycosylation of mCD200Rs. In activation experiments, phosphorylation of Dap12 following interaction with CD200 occurred on cells expressing mR2V5 but not mR4V5. Similar experiments with the chimeric receptors mR1r2 and mR1r4 also produced phosphorylation of Dap12. Our data suggest that the TM and cytoplasmic region of mCD200Rs dictate their state of glycosylation and provide further evidence that both mCD200R1 and mCD200R2 bind CD200 as ligand with functional consequences for downstream signaling.
1. INTRODUCTION
CD200 and CD200R are type-I transmembrane proteins of the Ig superfamily, that are highly conserved during evolution. Importantly, while the CD200 gene exists as a single copy, multiple copies of CD200R exist in the rodent and human genome [1-3] . Data from a number of independent groups clearly indicates a role for CD200 interaction with its receptor(s), CD200R (1-5 in mouse), in the regulation of a variety of inflammatory and immunological responses. General agreement exists concerning the effect of CD200:CD200R1 interactions, with direct suppression of macrophage/dendritic cell activation and suppression of inflammation and immune responses [1,3-8]. However, significant controversy remains concerning the ligand binding of alternate receptors CD200R2-R5 (mR2-mR5) and it has been suggested that the physiological ligand(s) for these receptors remain unknown [1,9,10]. We have suggested that CD200 does indeed signal mR2-mR5 directly, as determined both by altered binding to, and phosphorylation of Dap12 [11], and by monitoring biological responses occurring in vitro following addition of CD200Fc in the presence of a blocking R1 peptide, or using activating anti-(R2-R4) antibodies themselves [12,13].
Primary sequence comparison of mR1 with the alternate receptors mR2-mR4 showed that mR2, mR3 and mR4 shared 84%, 39% and 85% identity with mR1. The transmembrane (TM) region of mR2-mR4, contains a lysine residue known to be an important “docking site” for adaptor proteins. Each alternate receptor bears a short 15 amino acid cytoplasmic tail that is highly related in sequence but nevertheless not identical between the different receptors. In contrast, mR1 has a long 67 amino acid cytoplasmic tail, with 3 conserved tyrosine residues. One of these residues is contained within an NPXY sequence motif which upon ligand binding is phosphorylated and subsequently binds to Dok1 and Dok2 adaptor proteins. Thus signaling via alternate receptors involves a Dap12 pathway, which is not implicated following CD 200:mR1 interaction [14-16] .
Using C-terminally tagged mR4 and mR3 isoforms, several studies including our own [2,11] show that Dap12 associates with alternate receptors mR3 and mR4 and that association of Dap12 with mR4 is contingent upon engagement with CD200Fc. Such studies are lacking for receptor mR2.
One unusual feature of the CD200Rs is the high content of potential N-linked glycosylation sites, with mR1- mR4 containing 10, 6, 5 and 9 N glycans respectively. The change in apparent molecular mass of mR1 observed following removal of N-linked sugars by PNGase F treatment from ~90 kDa to ~25 kDa, indicates a content of around 70% by weight of carbohydrates. This is very high for N-linked carbohydrates and is more typical of O-linked sugars found in mucins [17]. To date, there has been no direct evidence for the involvement of N-linked oligosaccharides in the structure and function of CD200 receptors. However, both mutagenesis data [18] and a comparison to similar IgSF interactions [19], suggest that CD200-CD200R interactions are mediated by the protein not carbohydrate domains. It remains possible that the carbohydrate moiety is important in preventing non-specific protein-protein interactions thus preserving the membrane orientation of the CD200Rs. The possibility that multiple CD200Rs are co-expressed on individual cells constitutively or following immune induction, and the potential functional consequences this might have, remains unexplored. The potential of mR1 and alternate receptors mR2-mR4 to homo/hetero dimerize when coexpressed has also not been investigated.
Commercially available antibodies for mR1 have been used by investigators to study the surface expression of mR1 and also to study the interaction of mR1 with its ligand CD200. Since mR1 and mR2 share over 84% amino acid homology in their extracellular region, generating anti-mR2 specific antibodies has been a great challenge and commercial antibodies specific to mR2 are non-existent. Furthermore, the available anti-mR1 antibodies have not been thoroughly investigated for their ability to discriminate between mR1 and alternate CD200Rs.
In the present study, we have generated monoclonal antibodies to mR1 and mR2 and characterized them extensively for their specificity. The glycosylation state of the CD200Rs, and the effect of switching carboxy terminal domains between the receptors on CD200R structure and interaction with CD200 were also explored using anti-R1 and anti-R2 specific antibodies.
2. MATERIALS AND METHODS
2.1. Cloning and Expression of CD200R-Fc Fusion Proteins in CHOK Cells
The extracellular (i.e. V + C) regions of mCD200 receptors, mR1, mR2, mR3 and mR4 were isolated by PCR from cDNA purified from mouse bone marrow cells and the sequence confirmed. Fc fusion proteins were generated by inserting the extracellular region of the four mCD200 receptor isoforms into a Not1/Age1 digested pIRESneo3 expression vector encoding the Fc domain of mouse IgG2a (mutated in the CH2 domain to inhibit binding to FcRs).
Transient transfections were performed in CHOK/Hek 93 cells in serum free medium using lipofectamine 2000. 24 h post transfection, supernatants and cell lysates were analyzed by SDS/PAGE and western gels using antibody specific to mouse IgG2a. The mean concentration of each receptor fusion protein produced was in the range of 0.8 - 1 µg/ml, as measured using purified mCD200Fc as standard.
Stable cell lines of CHOK expressing high levels of mR1Fc, mR2Fc, mR3Fc, and mR4Fc were established from cells transfected as above. Supernatants collected from 24 h and 48 h cultures of transfected cells using G418 selection and cloning were subjected to Protein A column chromatography with protein purity confirmed by subsequent Comassie staining. Table 1 shows the predicted and measured molecular mass of the various CD200Rs and their respective fusion proteins.
2.2. Monoclonal Antibodies to CD200R-Fusion Proteins
Purified receptor-Fc fusion proteins were used by ImmunoPrecise Antibodies Ltd (Victoria BC, Canada) to generate monoclonal antibodies in rats. Multiple attempts to generate anti-mR3 and anti-mR4 monoclonal antibodies failed but we were successful in generating monoclonals specific to mR2. Purified mR1his protein was used to generate anti-mR1 monoclonals.
2.3. Screening of Anti-CD200R Monoclonals
The anti-mR1 monoclonal used throughout (2A10) was screened by ELISA, FACS and western blots. The detection antigen in ELISA was pure mR1his protein whereas FACS was carried out using Hek293 cells stably expressing mR1. In western blot experiments pure mR1his protein as well as cell lysates of mR1 cells were used for detection. 2A10 was also assessed for its ability to immunoprecipitate mR1 from cell lysates of mR1 transfected cells.
A sandwich ELISA was developed and used to screen for mR2 specific hybridomas. Purified anti-IgG2aFc antibody (Sigma Aldrich) was used as capture to bind pure mR2Fc, followed by incubation with the hybridoma supernatants expressing reactive anti-CD200R2. Hybridomas positive for mR2 were selected using anti-rat IgG HRP (Jackson Immunochemicals) as detection and TMB (Pierce Biochemicals) as substrate. Positive hybridomas were further selected for the absence of anti-Fc reactivity by repeating the ELISAs using mIgG2a as the capture antigen. Table 2 summarizes the properties of five antimR2 monoclonal antibodies judged by ELISA, FACS and westerns.
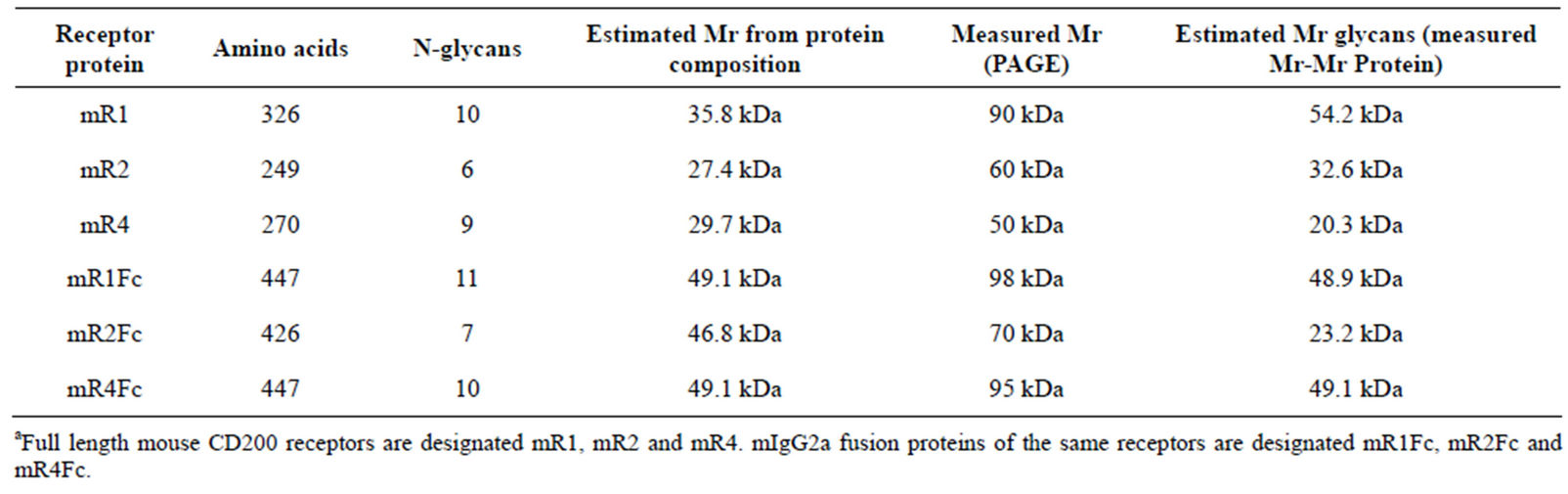
Table 1. Predicted molecular weight of mCD200 receptors and mCD200R-Fc fusion proteins (+/− N glycan chains)a.
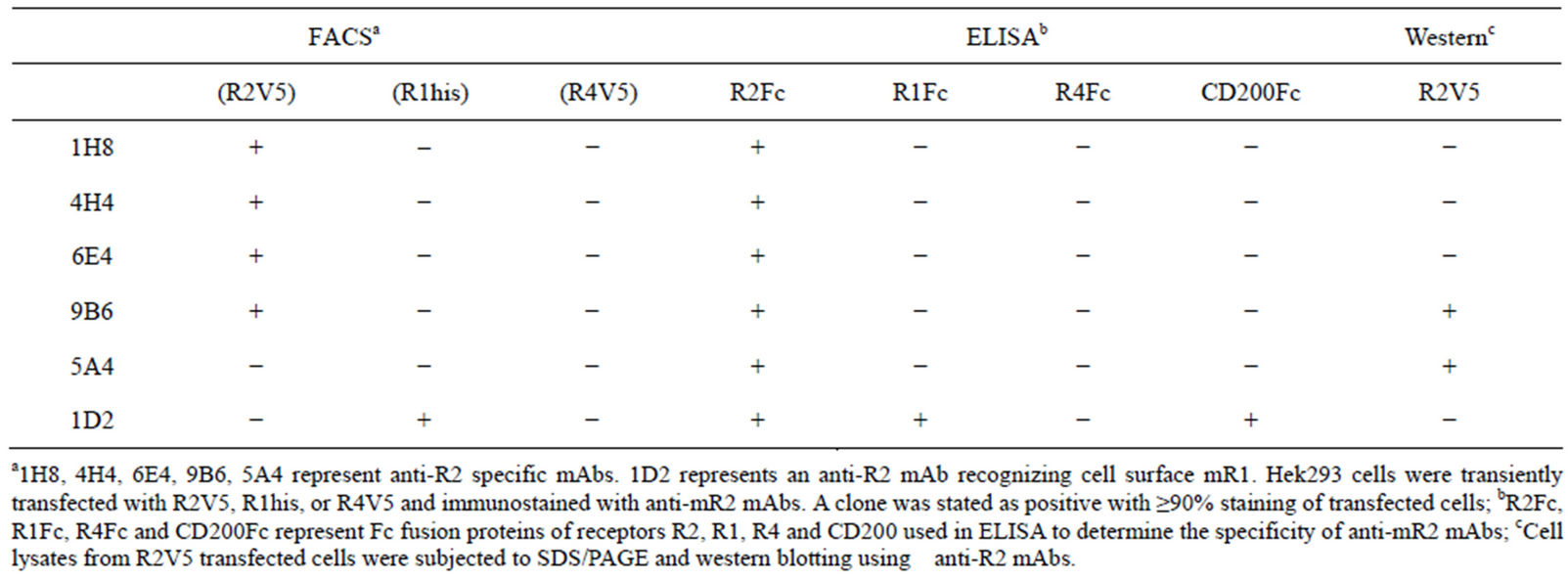
Table 2. Characterization of anti-R2 Monoclonals.
2.4. Cloning and Expression of C-Terminally Tagged Full Length mCD200 Receptors in Hek293
Full length mCD200Rs derived from previously described full length cDNA clones were subcloned into the pIRESneo3 vector with addition of different epitope tags at the carboxy terminus [1]. mR1 was tagged with (his)6 and mR2 and mR4 both carried the V5 epitope tag. Mouse Dap12 protein was subcloned into the pIRESneo3 vector from an I.M.A.G.E. clone (6814478) containing the full length cDNA sequence.
Each tagged receptor (namely mR1his, mR2V5 and mR4V5) was transiently expressed in Hek293 cells and 24 h post transfection cell lysates were subjected to SDS/ PAGE and western blotting using anti-his or anti-V5 antibodies.
For immunoprecipitation studies cells were lysed in RIPA buffer containing protease inhibitor cocktail. Specific antibody and Protein A/G Agarose beads (Pierce Biochemicals) were added to clear the supernatant and the mixture incubated overnight with shaking at 4˚C. The antigen-antibody-Protein A/G complex was washed twice with RIPA, boiled in reduced sample buffer and subjected to SDS/PAGE and western blotting using the appropriate antibody.
Stable cell lines of Hek293 expressing mR2V5 were established following cloning and screening by western blot using anti-V5 antibody.
2.5. Carboxy Terminal Domain Switching between CD200 Receptors
The transmembrane (TM) and cytoplasmic (CT) region of mR1, mR2 and mR4 were generated by PCR using the full length CD200 receptors described above as template. Following BamHI and AgeI digestion, the fragments were ligated into a BamHI/AgeI digested pIRESneo3 expression vector encoding the extracellular region of the CD200 receptors. The receptor chimeras generated were given the nomenclature mRxry where “x” represents the source of the extracellular domain and “y” the transmembrane and cytoplasmic domain. mR1r2, mR1r4, mR2r1, mR2r4, and mR4r1 were transfected into Hek293/CHOK cells in serum free medium using lipofectamine. 24 h post transfection cell lysates were subjected to SDS/ PAGE and western blotting using appropriate antibody.
In some studies, tunicamycin at 50 - 100 ng/ml was added 5 h post transfection and maintained during the entire time of experiment to block N-linked glycosylation.
2.6. Binding and Activation of CD200 Receptor R1/R2 and Receptor Chimeras with mCD200Fc/Shed Soluble mCD200
Cells were transfected using lipofectamine 2000 with receptor chimeras as described. 24 h post transfection, cells were washed with cold PBS, and incubated with purified dimeric mCD200Fc (5 µg/ml) for 15 min at 37˚C, followed by staining with FITC/PE labeled anti-mouse IgG(Fab)2. Binding of mCD200Fc was assessed by flow cytometry. Mouse Fc fragment (purchased from Pierce Biochemicals) was used as a negative control.
For activation studies, supernatants from 48 h serum starved mCD200-expressing Hek293 cells (containing shed soluble CD200), confirmed by ELISA and westerns using antibody to CD200 [20] were incubated for 15min at 37˚C with cells expressing different CD200Rs or CD200 receptor chimeras with or without mouse Dap12. Cells were lysed in RIPA buffer containing 50 mM NaF, 1mM Na3VO4, and protease inhibitors. Cleared lysates were immunoprecipitated overnight at 4˚C with one of the following antibodies: 2A10; commercial anti-phosphotyrosine antibody (Millipore); rabbit polyclonal antibody to the tyrosine phosphorylated cytoplasmic region of mR1 (a gift from Dr. Karim Berrada, Ingenious Therapetics, NY, USA); or polyclonal antibody to mouse Dap12 (Alpha Diagnostics).
The immune complexes were recovered by incubation with ProteinA/G Agarose beads (Pierce Biochemicals) for 1 h at 4˚C. After washing twice in lysis buffer containing 1 mM Na3VO4, the immune complexes were dissociated by boiling in reduced SDS/PAGE sample buffer followed by SDS/PAGE and western blotting. Primary Abs were detected with HRP-conjugated secondary Abs (Jackson Immunochemicals) and chemiluminescence substrate.
3. RESULTS
3.1. Characterization of Anti-mR2 Monoclonals
A total of 17 anti-mR2 monoclonals were generated and characterized for their specificity. Table 2, summarizes the characteristics of five monoclonals specific for mR2 as judged by ELISA, flow cytometry and westerns. FACS data for Hek293 cells stably transfected with mR2V5 and immunostained with the different anti-mR2 monoclonals are shown in Figure 1(A). Anti-mR2 monoclonals namely 9B6/6E4, 1H8, and 4H4 recognized mR2 expressed on the cell surface with no immunoreactivity towards cells expressing mR1 or mR4V5 (Supplementary Figure 1). Hek293 cells transiently transfected with mR2 (lacking a C-terminal tag) gave essentially similar results with the four anti-mR2 specific antibodies (not shown). As judged by westerns, mR2 and mR2V5 protein was best detected in transfected cell lysates using the two anti-mR2 monoclonals 5A4 and 9B6 (Figure 1(B)). As noted, while 9B6 was able to detect both cell surface mR2 in FACS and mR2 in western gels (at molecular mass 60 kDa), monoclonal 5A4 could only recognize mR2 protein by westerns (see Table 2).
3.2. Characterization of Anti-mR1 Monoclonals
As shown in Figure 2(A), anti-mR1 2A10, the mAb secreted following immunization with mR1his was able to detect mR1 expressed on the cell surface of Hek293 cells and was superior in this regard to the commercial anti-mR1 antibody (AbD Serotec, MorphoSys). 2A10, unlike the commercial anti-mR1, was also able to detect mR1 protein by western blots whereas cell lysates of mR2V5 and mR4V5 transfected cells showed no immunoreactivity (Figure 2(B)). However, neither 2A10 nor the commercial anti-mR1 antibody was specific for cell surface mR1, since flow cytometric analyses showed that both antibodies also stained cells expressing mR2V5 (Supplementary Figures 2(A) and (B)) with insignificant immunoreactivity with mR4V5 transfected cells (Supplementary Figures 2(C) and (D)). We conclude that though anti-R2 specific monoclonals show specificity in western blots and FACS, unique anti-R1 specific monoclonals, as analyzed by FACS staining, have yet to be described.
3.3. Cloning and Expression of mCD200 Receptor-Fc Fusion Proteins in CHOK Cells
Fc fusion proteins for the four mouse CD200Rs were generated by linking the extracellular region of each receptor to the Fc region of mIgG2a in the expression vector pIRESneo3. The number of N-glycans and the predicted molecular weight of the full length receptors and their Fc fusion proteins are shown in Table 1. Receptor fusion proteins (mR1Fc-mR4Fc) were transiently expressed in CHOK and supernatants analyzed by SDS/PAGE and westerns using an anti-IgG2a antibody. As shown in Figure 3(A), the molecular weight of each receptor fusion protein was related to the number of N-glycans, with mR1Fc detected at molecular mass 98 kDa, mR4Fc at 95 kDa, mR2Fc at 70 kDa and mR3Fc at
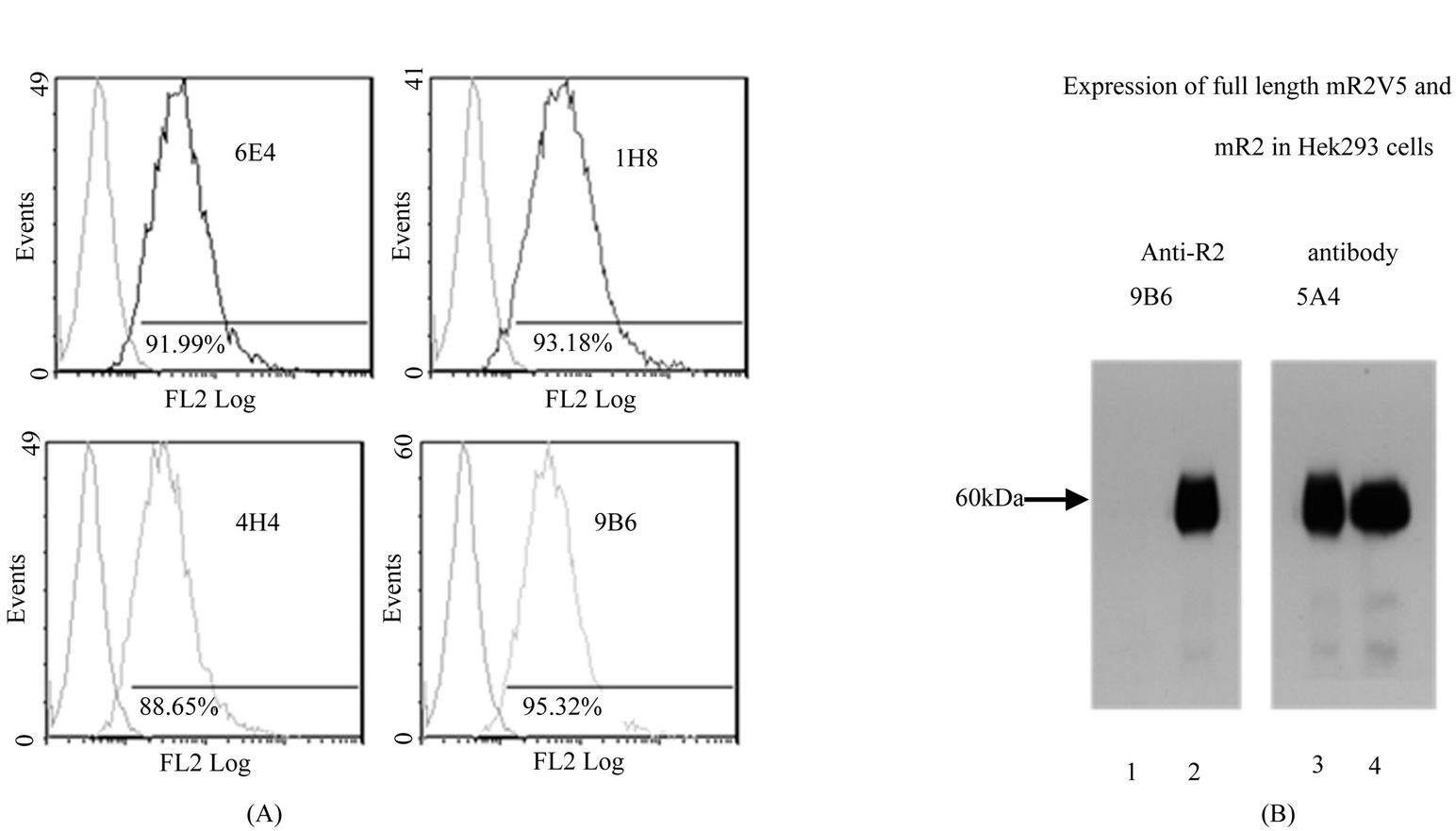
Figure 1. Characterization of anti-mR2 monoclonals. (A) Flow cytometry data for Hek293 cells stably transfected with mR2V5 were immunostained with anti-mR2 monoclonals 6E4, 1H8, 4H4 and 9B6; (B) Lysates (25 µg protein) of Hek293 cells transfected with mR2V5 (lanes 2 and 3), and mR2 (lane 4) were subjected to SDS/PAGE and western blotting using anti-R2 antibody 9B6 and 5A4. Lane 1 represents lysates of mock transfected cells.

Figure 2. Characterization of anti-mR1 monoclonals. (A) Flow cytometry data of Hek293 cells stably transfected with mR1 and immunostained with mR1 monoclonal 2A10 (0.1 and 0.5 g). C represents staining with (0.5 g) commercial anti-mR1 and 1D2 represents staining by a monoclonal antibody (0.5 µg) raised by injection of mR2Fc with so significant binding to mR2 by FACS and western; (B) Lysates (25 µg protein) of cells transfected with mR1 (a, b), with mR2V5 (c) with mR4V5 (d) and mock transfected Hek293 cells (e) were subjected to SDS/PAGE and western using anti-R1 monoclonal 2A10. Molecular weight markers are indicated.
Mr 65 kDa.
3.4. Characterization of Full Length CD200 Receptors mR1his, mR2V5 and mR4V5 Expressed in Hek293 Cells
Full length CD200 receptors with C-terminal tags namely mR1his, mR2V5 and mR4V5 were expressed in Hek293 cells. 24 h post transfection, cells were lysed in the presence of protease inhibitors and lysates subjected to SDS/PAGE and westerns usinSg anti-his or anti-V5 antibody. As shown in Figure 3(B), mR1his was detected at molecular mass 90 kDa and mR2V5 at 60 kDa, the sizes expected for glycosylated receptors (Figure 3(B), panels a and b). However, in mR4V5 transfected cells, despite the presence of protease inhibitors during cell lysis, the prominent band for mR4V5 appears to be at molecular mass of 26 kDa (Figure 3(B), panel c).
Cell lysates of mR4V5 transfected cells fractionated into membrane and cytosolic fractions are shown in Figure 3(C). The molecular mass of mR4V5 in the membrane fraction was 48 kDa (Figure 3(C), lane 7) whereas the cytosolic fraction showed a 26kDa product similar to that seen in cell lysates (Figure 3(B), lane 8). Thus, even though mR4 and mR1 share high homology in their extracellular region and contain similar numbers of potential N-glycan sites, mR4V5 appears to be far less glycosylated. Replacing the TM and cytoplasmic region of mR4 with an Fc fragment restored the molecular mass of mR4Fc to the size expected from the number of N-glycans (i.e. 95 kDa, Figure 3(A)), suggesting that the carboxy terminal region of the CD200Rs can regulate the difference in the degree of glycosylation in mR1 vs mR4.
3.5. N-linked Glycan Differences between mR2V5 and mR4V5
To investigate differences in the degree of glycosylation in mR2V5 and mR4V5, Hek293 cells were transfected with mR2V5 or mR4V5 in the presence/absence of tunicamycin and cell lysates harvested 24 h post transfection. As shown in Figure 4, the presence of tunicamycin produced a significant drop in the molecular mass of mR2V5 from 60 kDa to 29 kDa, the expected molecular mass of the non-glycosylated mR2. Expression of mR4V5 in the presence of tunicamycin produced no change in the molecular weight of the cell lysate product detected by anti-V5 antibody. We conclude that the 26 kDa mR4V5 likely represents a non-glycosylated molecule.
In membrane fractions of transfected cells, mR4V5 is present at a molecular mass of 48 kDa (Figure 3(C), lane 7) and in the presence of tunicamycin, the molecular mass dropped to 26 kDa (Figure 3(C), lane 9). The 26 kDa product observed in the cytosolic fraction that remained unchanged following treatment with tunicamycin (Figure 3(C), lanes 8 and 10) likely represents the cytoplasmic non-glycosylated form of mR4V5.
Expression of mR1his in the presence of tunicamycin produced two bands at 52 kDa and 34 kDa (Figure 4(C)), which may reflect the presence of both N and O-linked glycan chains. Given that mR1 and mR4 share over 85% amino acid identity in their extracellular V + C region, with all N-glycan sites conserved, the differences in the observed molecular mass (90 kDa vs 48 kDa) suggests that the carboxy terminal region of CD200Rs regulates the differences in the degree of glycosylation in mR1 vs mR4.
3.6. Lack of Heterodimerization after Co-Expression of mR1 and mR2 in Hek293 Cells
Hek293 cells stably expressing mR1 were transiently transfected with mR2V5 and 24 h post transfection, the surface expression of mR2V5 in cells expressing mR1 was confirmed using anti-mR2 specific antibody 4H4 (Supplementary Figure 3(A)). RIPA cell lysates of the transfected cells were analyzed by western blotting with anti-V5 antibody and anti-mR1 (2A10) as shown in (Supplementary Figure 3(B)), to confirm co-expression of the molecules. When dual transfected cells were immunoprecipitated with anti-V5 and analyzed on westerns using 2A10 and 5A4, only the latter mAb (Supplementary Figure 3(C), lane 1) gave a protein band, with no bands detected by 2A10 (Supplementary Figure 3(D), lane 3). These data suggest that despite co-expression on the same cell, receptors mR1 and mR2 do not heterodimerize. When cells were immunoprecipitated with 2A10, with the immunoprecipitates analyzed by western blotting using anti-V5 antibody, this conclusion was confirmed with no band detected on westerns by 5A4 (data not shown).
3.7. Effect of Carboxy Terminal Domain Switching between CD200 Receptors
Using molecular cloning techniques, the TM and cytoplasmic region of mR1 was replaced with the corresponding regions of mR2. The newly generated chimeric receptor mR1r2 when expressed in Hek293, resulted in a protein with molecular mass of 45 kDa, unlike mR1 with observed molecular mass 90 kDa (Figure 5(A)). In the presence of tunicamycin, the molecular mass of mR1r2 was further reduced to 30 kDa, the size expected for mR1r2 protein (Figure 5(B)). Replacing the TM and cytoplasmic region of mR1 with the corresponding region of mR4 gave essentially the same results. The newly generated chimeric receptor mR1r4 when expressed in Hek293 appeared at the same molecular mass as mR1r2 (i.e. 45 kDa). When expressed in the presence of tunicamycin the molecular mass of mR1r4 was reduced to 30kDa, the size expected for mR1r4 protein (Figure 5(C)). Thus the TM and cytoplasmic region of mR1 when replaced with the corresponding regions of either mR2 or mR4, reduced the glycosylation of mR1 by ~50%. Lower molecular weight bands in Figure 5(A) likely represent non-specific bands.
Replacing the TM and cytoplasmic region of mR2 with the corresponding region of mR1 did not produce a significant drop in the molecular mass of the chimeric receptor mR2r1 (Figure 6(A)). We interpret this as indicating that the TM and cytoplasmic region of mR1 did not influence the glycosylation state of mR2, even
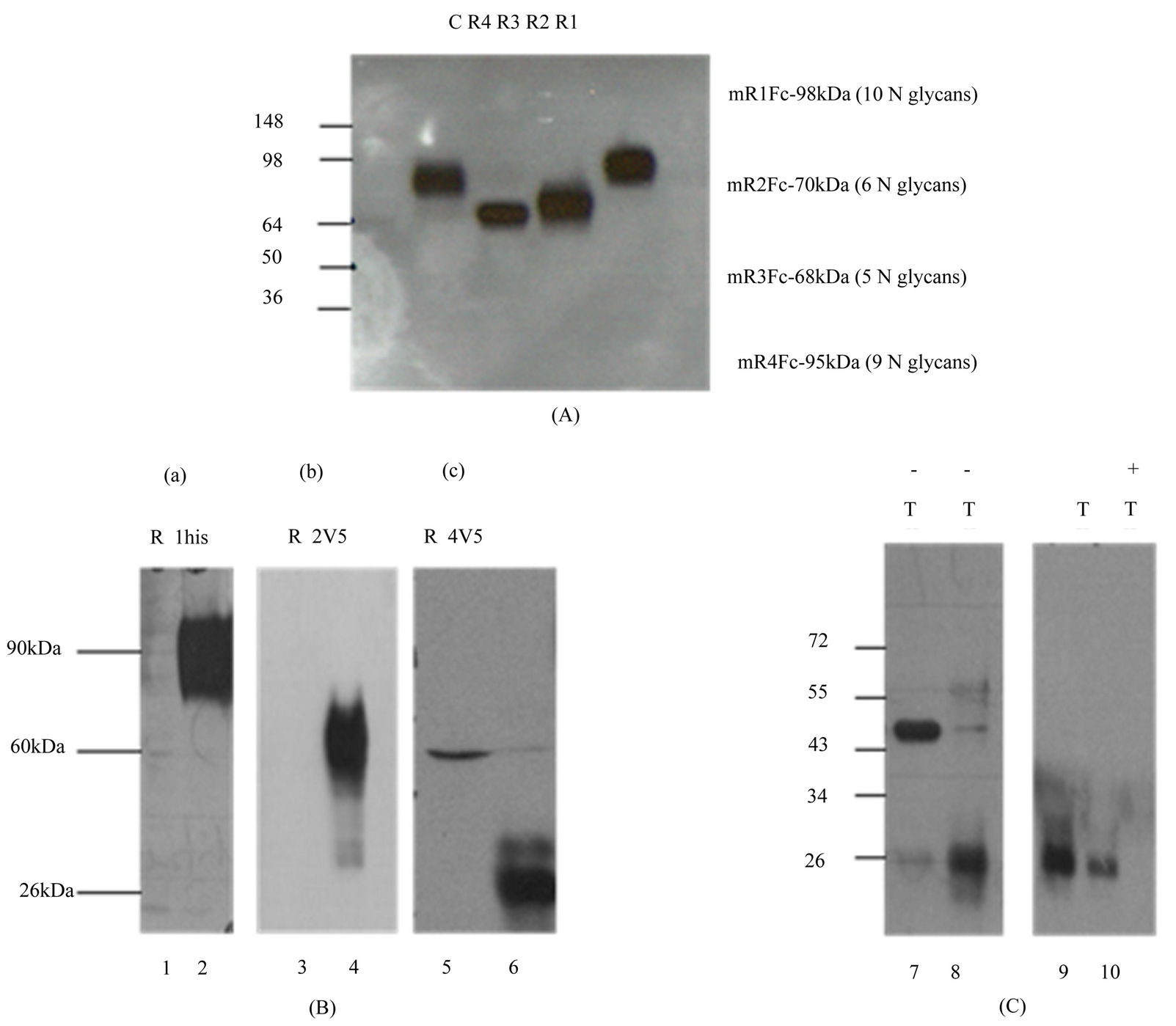
Figure 3. Characterization of mCD200R-Fc fusion/full length mCD200 receptor. proteins (A) Supernatants from Hek293 cells transfected with CD200R-Fc fusion proteins namely mR1Fc (R1), mR2Fc (R2), mR3Fc (R3) and mR4Fc (R4) were subjected to SDS/PAGE and western blotting using anti-mouse IgG2a antibody. (C) represents mock transfected cells. Molecular weight markers are indicated; (B) Lysates (25 µg protein) of Hek293 cells transfected with mR1his (panel a, lane 2), mR2V5 (panel b,lane 4) or mR4V5 (panel c, lane 6) subjected to SDS/PAGE and western blotting using anti-his antibody (a) or anti-V5 antibody (b & c). Lanes 1, 3 and 5 represent lysates of mock transfected cells; (C) Hek293 cells transfected with mR4V5 (lanes 7 - 10) and separated into membrane (lanes 7, 9) and cytosolic (lanes 8,10) fractions were subjected to SDS/PAGE and western blotting using anti-V5 antibody. −/+ T represents transfections in the absence and presence of tunicamycin. Molecular weights markers are indicated.
though the corresponding region of mR2 did reduce the glycosylation of mR1 by about 50%. In contrast, replacing the TM and cytoplasmic region of mR2 with the corresponding regions of mR4, resulted in a significant drop in the molecular mass of the chimeric receptor mR2r4 (Figure 6(B)). Compared to mR2 with ~molecular mass 60 kDa, the chimeric receptor mR2r4 was detected at 40 kDa as judged by western blots using 5A4 antibody. Expressing mR2r4 in the presence of tunicamycin resulted in an unexpected loss of the epitope detected by 5A4 on westerns (Figure 6(B)) although this was not seen with mR2V5 transfected cells grown in the presence of tunicamycin (see Figure 4(A)).
Similar to mR1r1 (=mR1) and mR2r2 (=mR2), chimeric receptors mR1r2, mR1r4, mR2r1 were expressed at the cell surface as judged by flow cytometry analyses using antibody 2A10 (Supplementary Figure 4(A)) and also bound mCD200Fc (Supplementary Figure 4(B)). Since the chimeric receptors are transiently expressed, their binding to mCD200Fc could only be assessed qualitatively.
Despite the fact that mR2r1 transfected cells main
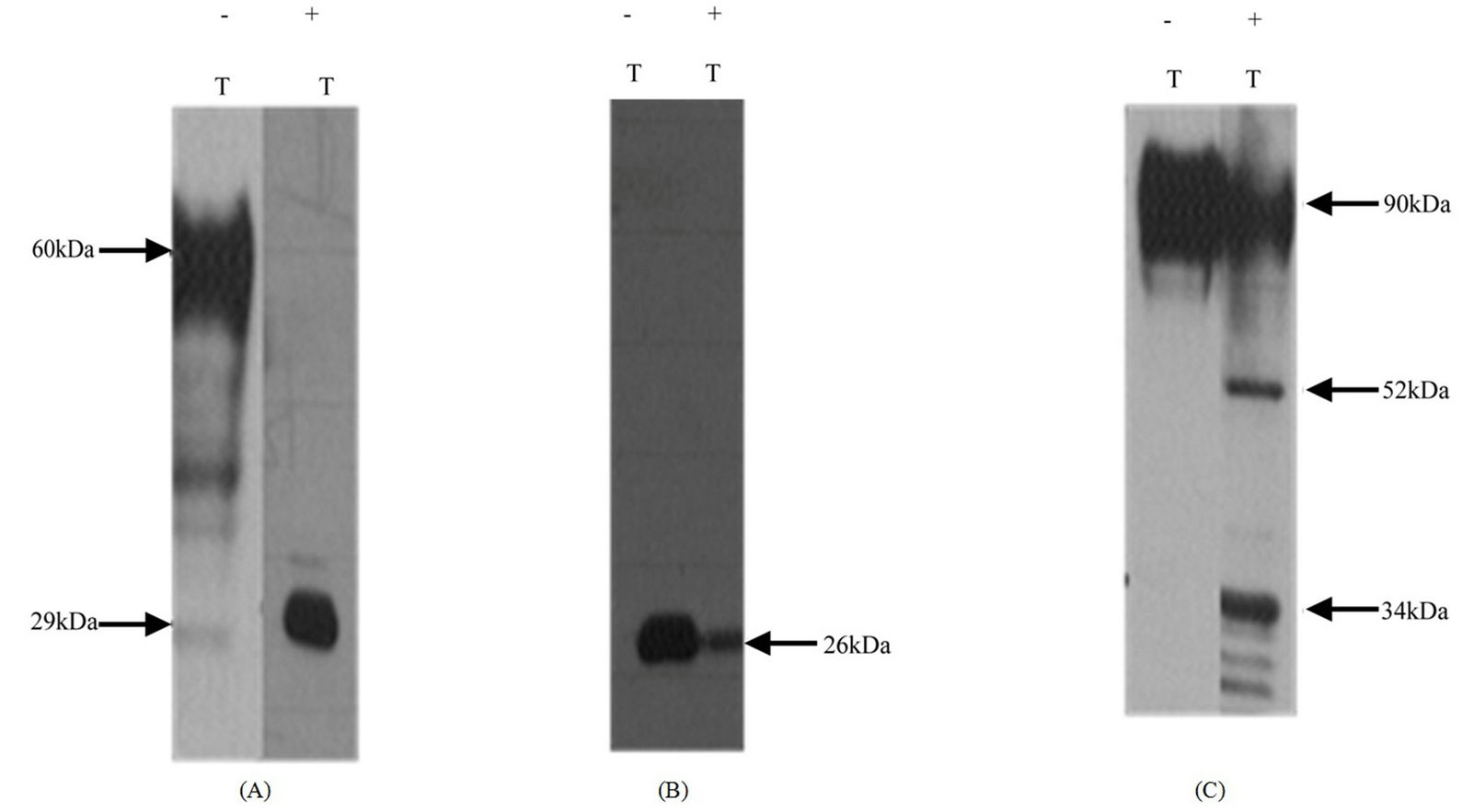
Figure 4. N-linked glycan differences between mR2V5 and mR4V5. Hek293 cells transfected with mR2V5 (A), mR4V5 (B) and mR1his (C) were incubated with medium containing 100 ng/ml tunicamycin for 24 h at 37˚C. Protease inhibitor containing cell lysates were subjected to SDS/PAGE and westerns using anti-V5 antibody (a and b) and antibody 2A10 (C). −/+ T represent absence or presence of tunicamycin in the medium.
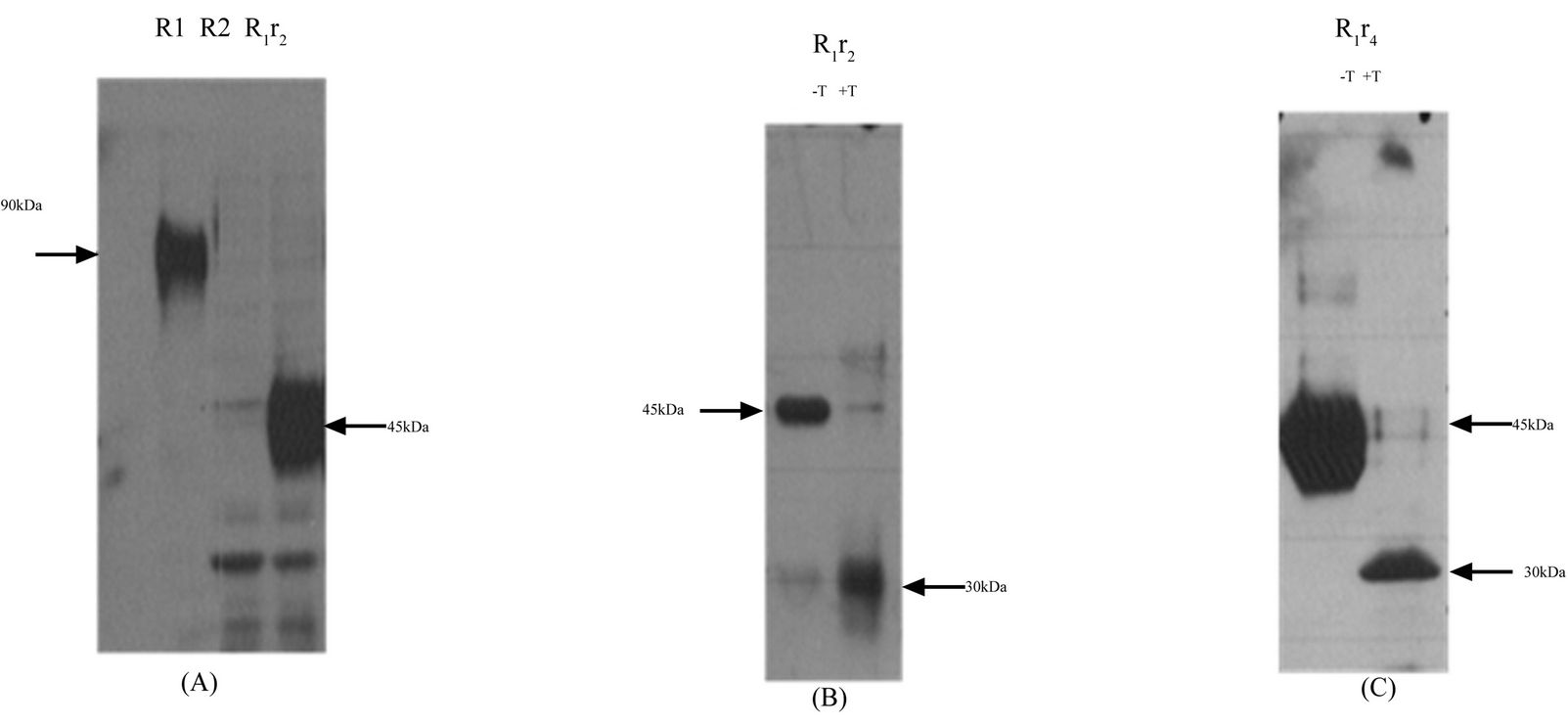
Figure 5. Effect of carboxy terminal domain switching between CD200 receptors mR1r2 and mR1r4. (A) Chimeric receptor mR1r2 was transiently expressed in Hek293 cells, 24 h post transfection cell lysates were subjected to SDS/PAGE and western blotting using anti-R1 antibody 2A10; Cell lysates of Hek293 cells transiently transfected with mR1 (lacking a his C-terminal tag), served as positive control, and lysates of mR2 (lacking a V5 C-terminal tag) served as negative control; (B) Chimeric receptor mR1r2 expressed in the presence (+T) or absence (−T) of tunicamycin with lysates subjected to SDS/PAGE and western blotting using anti-R1 antibody 2A10; (C) Chimeric receptor mR1r4 expressed in the presence (+T) or absence (−T) of tunicamycin with lysates subjected to SDS/PAGE and western blotting using anti-R1 antibody 2A10. Cell lysates for western blotting were used at concentration of 25 - 30 µg protein.
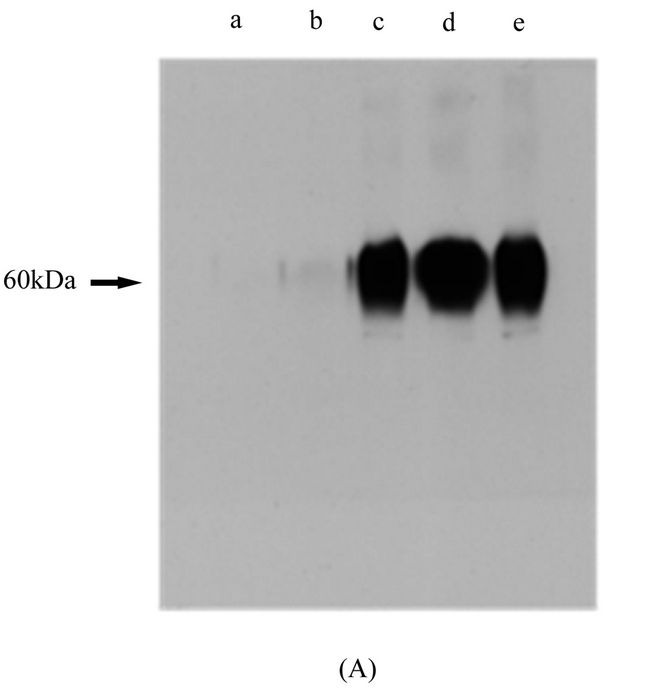
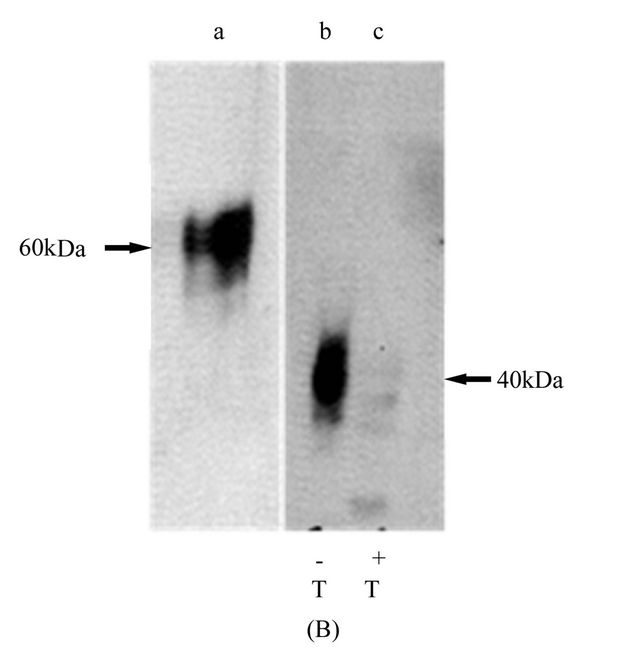
Figure 6. Effect of carboxy terminal domain switching between CD200 receptors mR2r1 and mR2r4. (A) Chimeric receptor mR2r1 was transiently expressed in Hek293 cells (lane c). 24 h post transfection cell lysates were subjected to SDS/PAGE and western blotting using anti-R2 antibody 5A4. Cell lysates of Hek293 cells transiently transfected with mR2 (lacking C-terminal tag) (lane d) and mR2V5 (lane e) served as appropriate control. Mock transfected cells and mR1 transfected cells are shown in lanes a and b respectively; (B) Chimeric receptor mR2r4 was transiently expressed in Hek293 cells, and 24 h post transfection cell lysates were subjected to SDS/PAGE and western blotting with anti-R2 antibody 5A4. Lanes b and c represent mR2r4 transfected cells in the presence (+T) and absence (−T) of tunicamycin. Lane a represents lysates of mR2 transfected cells. Cell lysates for western blotting were used at concentration of 25 - 30 µg protein.
tained their immunoreactivity with the R2 specific monoclonals (Supplementary Figure 4(C) panel a), cells transfected with chimeric receptor mR2r4 could not be detected by flow cytometry using R2 specific antibodies (Supplementary Figure 4(C) panel b). Furthermore, mR2r4 transfected cells failed to bind mCD200Fc (data not shown). Expression of R4 (tagged with V5 i.e. R4V5) could only be detected by westerns using anti-V5 antibody in cell lysates of mR4V5 transfected cells (Figure 3(C)). In the absence of anti-mR4 specific antibodies, we have been unable to characterize any cell surface expression of mR4 and appraise its binding to mCD200.
3.8. Functional Activation of Chimeric Receptors R2r1, R1r2 and R1r4 after Stimulation by mCD200
Hek293 cells transfected with R2r1 or R1 were stimulated at 37˚C for 15 min with soluble CD200 released into the supernatant of serum starved mCD200 expressing cells [20] . Cell lysates prepared in RIPA buffer containing protease as well as phosphatase inhibitors were immunoprecipitated using antibody against the phosphorylated cytoplasmic tail of mR1 or the commercial anti-phosphotyrosine antibody followed by western using antibody 2A10 (Figure 7). Similar to data observed with mR1, stimulation of mR2r1 with mCD200 resulted in tyrosine phosphorylation of the cytoplasmic domain (r1) of this chimeric receptor. Thus replacing the TM and CT region of R2 with that of R1 results in a receptor (R2r1) that binds CD200 and functionally mimics CD200R1 (with phosphorylation of the cytoplasmic tail) following CD200 binding.
Hek293 cells were also co-transfected with mDap12 and mR2V5, mR4V5, mR1r2 or mR1r4 and stimulated with soluble mCD200, with immunoprecipitates analyzed by westerns. Lysates of cells transfected with mR2V5 and mR4V5 were immunoprecipitated with anti-V5 antibody whereas lysates from mR1r2 and mR1r4 transfected cells were immunoprecipitated with anti-phosphotyrosine antibody. All immunoprecipitates were analyzed by westerns using anti-dap12 antibody. As shown in Figure 8(A) (panel e), lysates from (mR1r2 + Dap12) and (mR1r4 + Dap12) transfected cells analyzed by westerns using antibody 2A10 showed the expected product size of 45 kDa for mR1r2 and mR1r4.
All 4 receptors namely mR4V5, mR2V5, mR1r2 and mR1r4 associated with Dap12 when cotransfected. However, Dap12 phosphorylation occured only after stimulation and mCD200 binding to either mR2V5 (Figure 8(A), panel b) or the chimeric receptors mR1r2 and mR1r4 (Figure 8(A), panels c and d). No Dap12 phosphorylation was seen in mR4V5 transfected cells following stimulation (Figure 8(A)). Thus having switched the carboxy terminal region, both mR1r2 and mR1r4 chimeric receptors bind CD200 but thereafter function like mCD200R2 with phosphorylation of the adaptor protein Dap12.
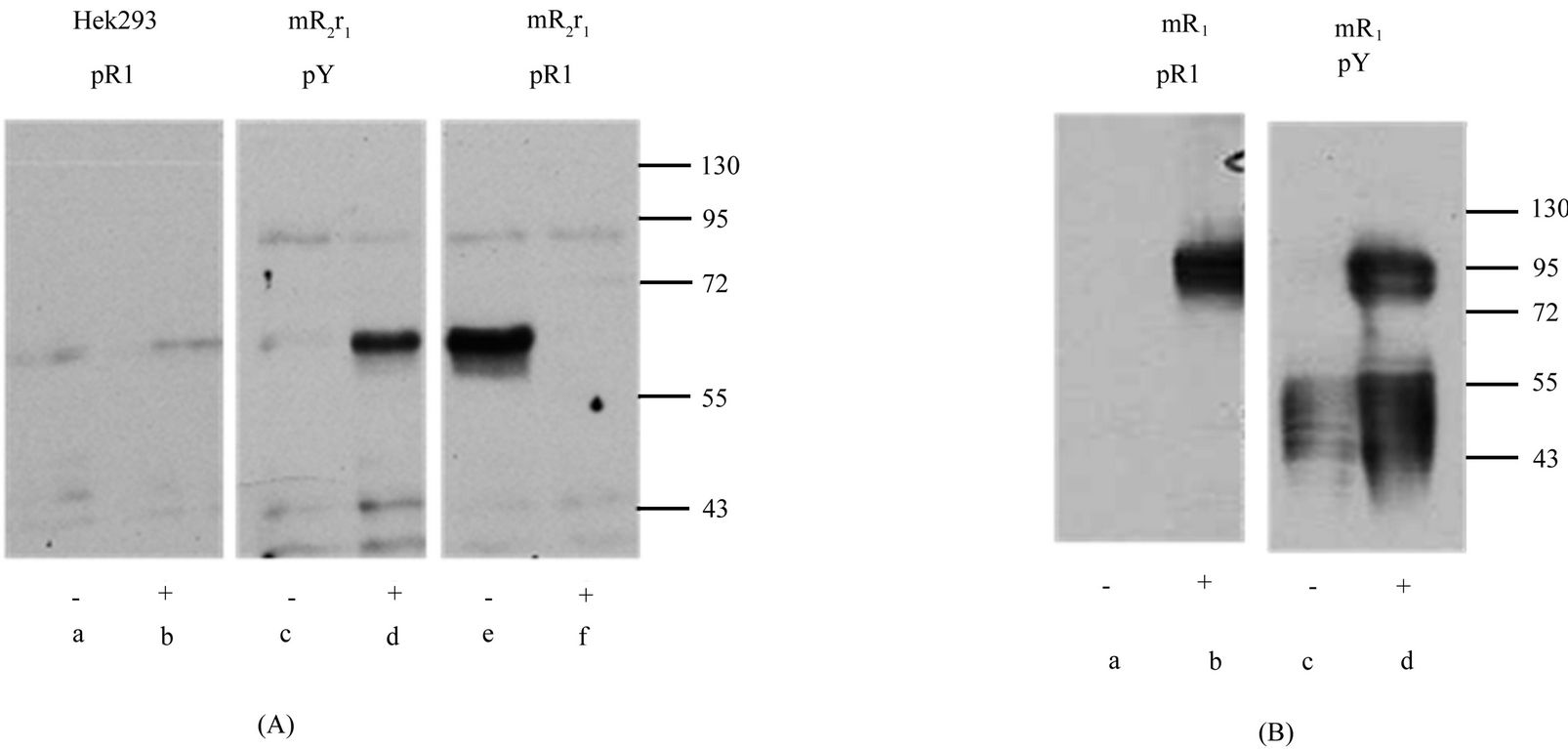
Figure 7. Functional activation of chimeric receptor mR2r1. Chimeric receptor mR2r1 (panel A) and receptor mR1 (panel B) were transiently transfected in Hek293 cells. 24 h post transfection, cells were incubated for 15 min, at 37˚C with (+) or without (−) supernatants from serum starved mouse CD200 expressing cells. Cell lysates were immunoprecipitated with antibody to tyrosine phosphorylated mR1 cytoplasmic region (pR1) or commercial anti-phosphotyrosine antibody (pY) followed by western blotting using antibody 2A10 (panel B) or 5A4 (panel a). As controls, Hek293 cells were subjected to the same treatment as mR2r1.
3.9. Evidence for Expression and Phoshorylation of Chimeric Receptor mR4r1
As noted above, in the absence of anti-R4-specific mAbs, we have been unable to detect unequivocal expression of mR4 on the cell surface. Hek293 cells transfected with the chimeric receptor mR4r1 were stimulated with sodium pervanadate and subsequently immunoprecipitated with anti-phosphotyrosine antibody, followed by western gel analysis using antibody against the phosphorylated cytoplasmic tail of R1 as shown in Figure 8(b). Incubation of cells transfected with mR1r1 under similar conditions served as a positive control in these experiments. In preliminary studies we were unable to immunoprecipitate a specific phosphorylated product from mR4r1 transfected cells following incubation with mCD200 (data not shown), unlike the stimulation shown in Figure 7 with mR2r1 transfected cells. One interpretation of these data is that the CD200R4 receptor is not expressed functionally at the cell surface.
Interestingly, substitution of the TM and cytoplasmic region of mR4 with the corresponding region of mR1 resulted in a product that was fully glycosylated with molecular mass of 85 kDa relative to mR1 which was detected at ~90 kDa. Thus replacing the TM and CT region of mR4 with the corresponding regions of mR1 or with the Fc region of IgG2a, resulted in a highly glycosylated form of mR4 with its size in accordance with the number of N-glycans in the extracellular region.
4. DISCUSSION
In the present study we have successfully generated monoclonals specific to mR2 and using these reagents have shown that mR2 is expressed on the cell surface. Cell surface expression of mR2 does not require the coexpression of adaptor molecule Dap12 in either Hek293 or CHOK cells. Furthermore, the presence of a C-terminal V5 tag on mR2 (i.e. mR2V5) did not affect its cell surface expression and immunoreactivity with the five anti-mR2 monoclonals described, two of which were able to detect mR2 and mR2V5 in cell lysates by westerns. In contrast, an antibody specific to mR1 has not, to our knowledge, been similarly defined. Rigorous tests of both our own anti-mR1 monoclonal (2A10) and a commercial anti-mR1 antibody (Serotec) showed these also recognized cell surface mR2 (and mR2V5). Unlike the commercial anti-CD200R1 reagent, 2A10 also bound to mR1 on western gels and in immunoprecipitation experiments.
Using antibodies specific to mR2, we have failed to detect heterodimers from cells expressing both mR1 and mR2. This makes it unlikely that multiple heterodimeric CD200Rs interact on individual cells following expression of more than one CD200R in any given cell.
5/17 monoclonals derived from immunization with mR2Fc were specific for mR2. Anti-mR2 monoclonals
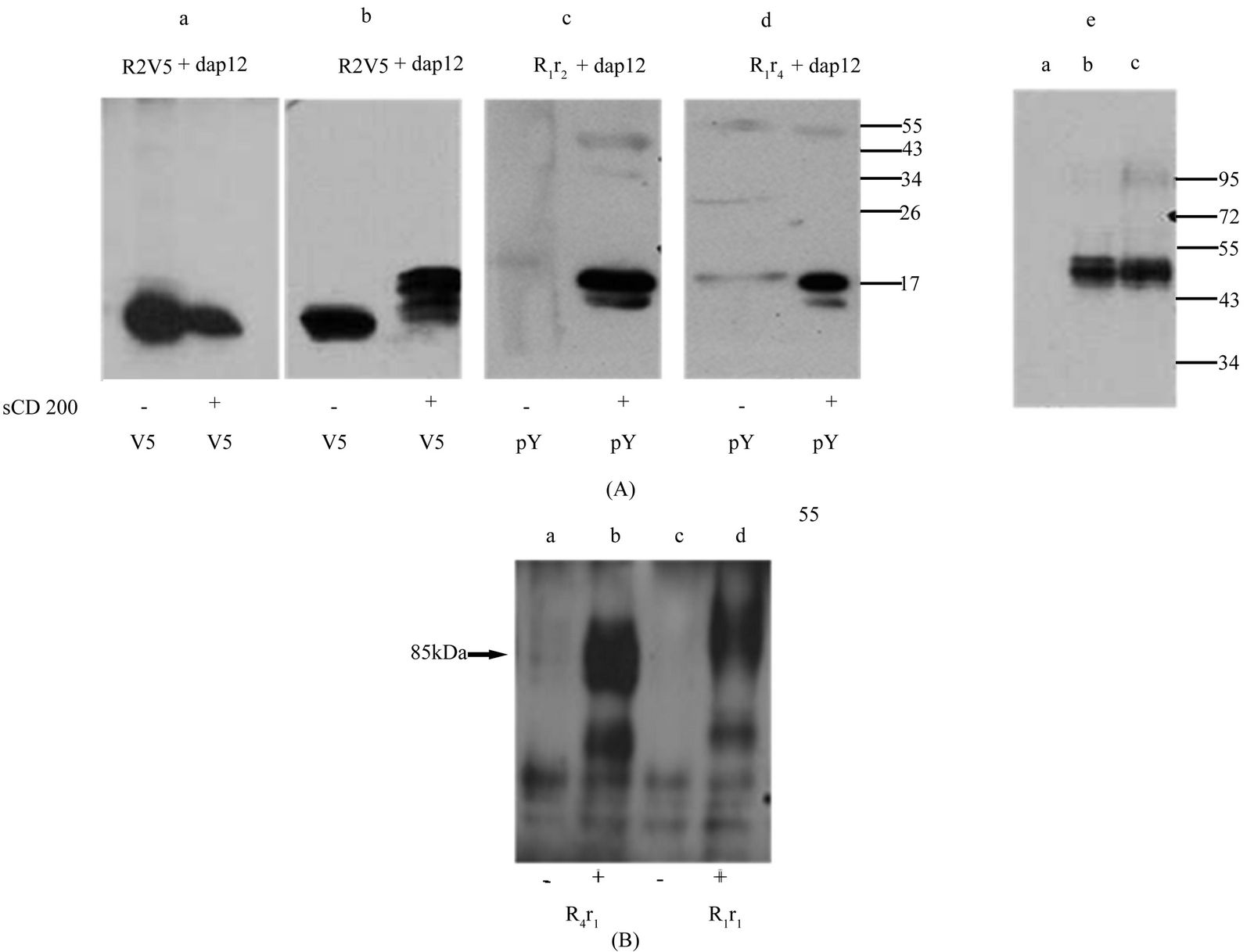
Figure 8. Functional activation of chimeric receptor mR1r2 and mR1r4. (A) Hek293 cells were transiently co-transfected with mdap12 and receptors mR4V5, mR2V5, chimeric receptors mR1r2 / mR1r4. 24 h post transfection, cells were stimulated by incubating for 15 min, at 37˚C with (+) or without (−) supernatants from serum starved mouse CD200 expressing cells. Panel a and b: Cell lysates, were immunoprecipitated with anti-V5 and western blotted using antibody to mDap12. Panel c and d: Cell lysates were immunoprecipitated with anti-phosphotyrosine antibody (anti-pY) and western blotted using antibody to mDap12. Panel e: Hek293 cell lysates (a), [mR1r2 + Dap12] lysates (b) and [mR1r4 +Dap12] lysates (c) were subjected to SDS/PAGE and western blotting using antibody 2A10; (B) Data shows a western gel from cell lysates of mR4r1 (lanes a, b) and mR1r1 (lanes c,d) transfected cells immunoprecipitated with anti-phosphotyrosine antibody followed by western using antibody against the phosphosphorylated cytoplasmic tail of mR1. +/− represents incubation of transfected cells with or without pervanadate for 10min at 37˚C.
1D2 and 6C10 were also able to recognize cell surface mR1, which is not surprising since mR1 and mR2 show ~84% sequence homology in their extracellular region. We have to date, as noted earlier, been unable to generate anti-R4 specific reagents using the approach successful in generating anti R1/R2 mAbs.
The presence of N-linked glycans on rat CD200R1 has been demonstrated by Wright et al. [3] with a drop in molecular mass from 90 to 25 kDa following PNGaseF treatment of rat R1, indicating a content of ~70% by weight of carbohydrate. In the studies reported above we confirmed that the extracellular region of all 4 mouse CD200Rs fused to Fc are glycosylated and the molecular weights of the molecules were dictated by the number of N-glycans with mR1Fc at molecular mass 98 kDa, mR4Fc at 95 kDa, mR2Fc at 70 kDa and mR3Fc at 65 kDa.
Interestingly, western blots of cell lysates from mR4V5 transfected cells showed a significantly lower molecular mass product compared to mR1 (48 kDa vs 90 kDa) and experiments using tunicamycin also suggested that glycosylation in mR4V5 was reduced by more than 50% compared to mR1. Since mR1 and mR4 share the greatest homology in their extracellular region and contain similar number of N-glycans, this unexpected reduction in the molecular weight of mR4V5 was taken to infer that the TM and CT region of mR4 may regulate the degree of glycosylation. We therefore explored the effect of switching carboxy terminal domains between the receptors on CD200R glycosylation and interaction with CD200 using anti-R1 and anti-R2 specific antibodies.
Substitution of the TM and CT region of mR1 and mR2 with that of mR4 significantly reduced glycosylation of the chimeric receptors mR1r4 and mR2r4 (Figure 5(c) and Figure 6(b)). Replacing the TM and CT region of mR1 with that of mR2 also caused a similar drop in molecular mass of the chimeric receptor mR1r2 (Figure 5(a)). This in itself was not surprising given that the TM and CT regions of mR2 and mR4 are conserved except for a few amino acids. In contrast to these data, however, substitution of the TM and CT region of mR2 with that of mR1 did not result in reduced glycosylation of the chimeric receptor mR2r1, inferring that the reduction of glycosylation was an effect regulated by the TM and CT region of mR4/mR2 but not of mR1. Due to the lack of anti-mR4 antibodies, the chimeric receptor mR4r1 could not be studied in these experiments. However, in separate experiments, using an antibody specific to the phosphorylated cytoplasmic tail of mR1, we were able to show that when the TM and CT region of mR4 were replaced with the corresponding regions of mR1, the chimeric receptor mR4r1 (following activation with vanadate) was indeed glycosylated and appeared at the expected size of 85 kDa (Figure 8(b)).
N-glycosylation of eukaryotic membrane proteins is a co-translational event that occurs in the lumen of the endoplasmic reticulum. This process is catalyzed by a membrane associated oligosaccharyl transferase (OST) complex that transfers a preformed oligosaccharide (Glc3Man9GlcNAc2) to an Asn side chain acceptor located within the sequence (Asn-X-Ser/Thr-). Although the presence of a consensus glycosylation site within the amino acid sequence of a membrane protein is suggestive of a glycoprotein, not all consensus sites are necessarily utilized. Sites must be disposed to the luminal side of the endoplasmic reticulum membrane to be glycosylated, and not all luminal sites may be suitable acceptors. A role for N-glycosylation has been implicated in protein folding, regulation of cell surface expression and/or the half life of many proteins [21], though in many membrane proteins, N-linked oligosaccharides are not required for protein function [22-24] .
It has been shown that acceptor sites for N-glycosylation must be spaced a minimum distance away from the membrane surface to be N-glycosylated [25], a constraint imposed by the relative proximity of the active site of the membrane-associated OST, and the residues near the ends of the transmembrane (TM) segments [26]. For more complex polytopic membrane proteins, N-glycosylation requires loops of a minimum size with the acceptor site spaced a minimum distance from the adjacent TM segments [27,28]. In a survey of 115 human single-span Type 1 membrane proteins a minimum distance of 10 residues from the bilayer was found to be necessary for successful N-glycosylation of acceptor sites preceding a membrane-spanning segment [29]. In a survey of proteins containing multiple extracytosolic N-glycosylation consensus sites, N-glycosylated extracytosolic loops were observed to have a minimum size of 33 residues [29], with smaller extracytosolic loops (<30 residues) inefficient acceptors of oligosaccharide and thus poorly Nglycosylated despite the presence of consensus N glycolsylation sites [30] .
We observed a reduction in the glycosylation of the chimeric receptors mR1r2, mR1r4 and mR2r4, which may reflect a similar restriction in accessibility of acceptor sites on the nascent polypeptide to the oligosaccharyl tranferase in the lumen of the endoplasmic reticulum. Relative to mR4, mR1 has a much longer cytoplasmic tail (63 amino acids in mR1 vs 9 amino acids for mR2) and we hypothesize that the extended cytoplasmic tail in mR1 may allow more flexibility in the ER for the nascent polypeptide to be efficiently glycosylated. Note then that the (presumed) glycosylation restraint imposed on the polypeptide chain of mR4 is relieved entirely by replacing it with the TM and CT region of mR1 or by replacing the TM and CT region with murine Fc.
In our activation experiments, we were able to show phosphorylation of mDap12 following incubation of cells expressing mR2V5 with mCD200. These results confirmed that mR2V5 was expressed on the cell surface and its binding to mCD200 led to phosphorylation of Dap12 a conclusion reached in earlier studies [11]. In similar experiments, we showed that the chimeric receptors mR1r2 and mR1r4 also bind the adaptor protein Dap12 and mimic mR2V5 functionally following stimulation with mCD200 (Figure 8(a)). Furthermore, using the antibody 2A10, we showed that the chimeric receptor mR2r1 mimics mR1 functionally following stimulation with mCD200. These data further support the suggestion we made earlier that in fact the alternate CD200Rs (besides CD200R1) do indeed bind CD200 in a functionally important manner [1,11,13]. In contrast to these observations, cells expressing mR4V5 bound Dap12, but Dap12 was not phosphorylated following stimulation with mCD200. Since CD200 has been shown to bind mR4 [10], this failure to observe mDap12 phosphorylation following mCD200 stimulation is taken to imply that mR4V5 does not appear on the cell surface and that it is likely a non-functional molecule. The failure of mR4r1 to bind mCD200 in our experiments may similarly indicate that mR4r1 is also not expressed on the cell surface. Alternatively, the chimeric receptor may have undergone a conformational change at the ligand binding site.
Studies by Wright et al. and Hatherley et al. [2,9] using independent reagents to mR1, mR3 and mR4, showed expression of all three receptors on cell surface by flow cytometry following viral transduction in BaF3 cells stably expressing mDap12. The failure of this group also to demonstrate mR4 binding to a CD200Fc fusion protein may again reflect the absence of cell surface expressed mR4. Our characterization of R4 has relied on detection of mR4V5 using a anti-V5 antibody. The possibility that the V5 tag interferes with mR4 glycosylation/surface expression is less of a concern given that mR2V5 was glycosylated and expressed on the cell surface and was not different in its properties from untagged mR2. However, we cannot entirely exclude this hypothesis.
Cell surface expression of the chimeric CD200 receptors mR1r2, mR1r4, and mR2r1 was detected by FACS using antibody 2A10, whereas binding to mR2r4 was poor. No binding was observed with mR4r1 or mR4V5 transfected cells. Cumulatively, these results suggest that the 2A10 epitope in the extracellular region of the receptor is conserved between mR1 and mR2 and is maintained in the receptor chimeras. Since the epitope is lost in mR2r4 and mR4V5, both of which molecules have significantly reduced glycosylation, it may be that the 2A10 detects a conformational epitope dependent on glycosylation for its expression. Importantly the chimeric receptors mR1r2, mR1r4 and mR2r1 also maintained their binding to mCD- 200Fc. As judged by flow cytometry, mR2r1 but not mR2r4 showed immunoreactivity with 2A10 as well as with all R2 specific monoclonals. In contrast, the chimeric receptor mR2r4 behaves like mR4V5 in that it does not appear on the cell surface; it does not bind mCD- 200Fc; and its expression can only be detected by westerns.
In summary, our data indicate that the TM and CT region of mCD200 receptors dictate their state of glycosylation which can further affect their structure and conformation. Despite extensive homology between the different CD200Rs, the distinctive TM and CT region in these receptors may thus contribute significant heterogeneity with respect to ligand binding. We have also provided further evidence to support the hypothesis that both CD200R1 and CD200R2 bind CD200 as ligand with functional consequences for downstream signaling. Furthermore, with the CD200Rs shared amino acid identity and conserved N-glycosylation sites, raising antiCD200R specific monoclonals will always remain a challenge.
5. ACKNOWLEDGEMENTS
This work was supported by grants from the Canadian Institute for Health Research, MOP-221217
REFERENCES
- Gorczynski, R., Chen, Z., Kai, Y., Lee, L., Wong, S. and Marsden, P.A. (2004) CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. Journal of Immunology, 172, 7744-7749.
- Wright, G.J., Cherwinski, H., Foster-Cuevas, M., Brooke, G., Puklavec, M.J., Bigler, M., Song, Y., Jenmalm, M., Gorman, D., McClanahan, T., Liu, M.R., Brown, M.H., Sedgwick, J.D., Phillips, J.H. and Barclay, A.N. (2003) Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. Journal of Immunology, 171, 3034-3046.
- Wright, G.J., Puklavec, M.J., Willis, A.C., Hoek, R.M., Sedgwick, J.D., Brown, M.H. and Barclay, A.N. (2000) Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity, 13, 233-242. doi:10.1016/S1074-7613(00)00023-6
- Broderick, C., Hoek, R.M., Forrester, J.V., Liversidge, J., Sedgwick, J.D. and Dick, A.D. (2002) Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. American Journal of Pathology, 161, 1669-1677. doi:10.1016/S0002-9440(10)64444-6
- Gorczynski, R.M., Yu, K. and Clark, D. (2000) Receptor engagement on cells expressing a ligand for the toleranceinducing molecule OX2 induces an immunoregulatory population that inhibits alloreactivity in vitro and in vivo. Journal of Immunology, 165, 4854-4860.
- Hoek, R.M., Ruuls, S.R., Murphy, C.A., Wright, G.J., Goddard, R., Zurawski, S.M., Blom, B., Homola, M.E., Streit, W.J., Brown, M.H., Barclay, A.N. and Sedgwick, J.D. (2000) Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science, 290, 1768-1771. doi:10.1126/science.290.5497.1768
- Jenmalm, M.C., Cherwinski, H., Bowman, E.P., Phillips, J.H. and Sedgwick, J.D. (2006) Regulation of myeloid cell function through the CD200 receptor. Journal of Immunology, 176, 191-199.
- Snelgrove, R.J., Goulding, J., Didierlaurent, A.M., Lyonga, D., Vekaria, S., Edwards, L., Gwyer, E., Sedgwick, J.D., Barclay, A.N. and Hussell, T. (2008) A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nature Immunology, 9, 1074- 1083. doi:10.1038/ni.1637
- Hatherley, D., Cherwinski, H.M., Moshref, M. and Barclay, A.N. (2005) Recombinant CD200 protein does not bind activating proteins closely related to CD200 receptor. Journal of Immunology, 175, 2469-2474.
- Jiang, L. and Barclay, A.N. (2009) New assay to detect low-affinity interactions and characterization of leukocyte receptors for collagen including leukocyte-associated Iglike receptor-1 (LAIR-1). European Journal of Immunology, 39, 1167-1175. doi:10.1002/eji.200839188
- Boudakov, I.Z.P. and Gorczynski, R. (2006) Mechanisms involved in suppression induced by CD200:CD200R interaction. Recent Research Developments in Immunology, 7, 9-24.
- Gorczynski, R.M., Chen, Z., Kai, Y., Wong, S. and Lee, L. (2004) Induction of tolerance-inducing antigen-presenting cells in bone marrow cultures in vitro using monoclonal antibodies to CD200R. Transplantation, 77, 1138-1144. doi:10.1097/01.TP.0000121773.18476.1C
- Gorczynski, R.M., Lee, L. and Boudakov, I. (2005) Augmented induction of CD4+CD25+ Treg using monoclonal antibodies to CD200R. Transplantation, 79, 1180-1183. doi:10.1097/01.TP.0000152118.51622.F9
- Mihrshahi, R., Barclay, A.N. and Brown, M.H. (2009) Essential roles for Dok2 and RasGAP in CD200 receptormediated regulation of human myeloid cells. Journal of Immunology, 183, 4879-4886. doi:10.4049/jimmunol.0901531
- Voehringer, D., Rosen, D.B., Lanier, L.L. and Locksley, R.M. (2004) CD200 receptor family members represent novel DAP12-associated activating receptors on basophils and mast cells. The Journal of Biological Chemistry, 279, 54117-54123. doi:10.1074/jbc.M406997200
- Zhang, S., Cherwinski, H., Sedgwick, J.D. and Phillips, J.H. (2004) Molecular mechanisms of CD200 inhibition of mast cell activation. Journal of Immunology, 173, 6786- 6793.
- Gum Jr., J.R. (1992) Mucin genes and the proteins they encode: Structure, diversity, and regulation. American Journal of Respiratory and Critical Care Medicine, 7, 557-564.
- Preston, S., Wright, G.J., Starr, K., Barclay, A.N. and Brown, M.H. (1997) The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. European Journal of Immunology, 27, 1911-1918. doi:10.1002/eji.1830270814
- van der Merwe, P.A. and Barclay, A.N. (1994) Transient intercellular adhesion: The importance of weak proteinprotein interactions. Trends in Biochemical Sciences, 19, 354-358. doi:10.1016/0968-0004(94)90109-0
- Gorczynski, R.M., Chen, Z., Diao, J., Khatri, I., Wong, K., Yu, K. and Behnke, J. (2010) Breast cancer cell CD200 expression regulates immune response to EMT6 tumor cells in mice. Breast Cancer Research Treatment, 123, 405-415. doi:10.1007/s10549-009-0667-8
- Paulson, J.C. (1989) Glycoproteins: What are the sugar chains for? Trends in Biochemical Science, 14, 272-276. doi:10.1016/0968-0004(89)90062-5
- Casey, J.R., Pirraglia, C.A. and Reithmeier, R.A. (1992) Enzymatic deglycosylation of human Band 3, the anion transport protein of the erythrocyte membrane. Effect on protein structure and transport properties. The Journal of Biological Chemistry, 267, 11940-11948.
- Gregory, R.J., Rich, D.P., Cheng, S.H., Souza, D.W., Paul, S., Manavalan, P., Anderson, M.P., Welsh, M.J. and Smith, A.E. (1991) Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Molecular Cell Biology, 11, 3886-3893.
- Hryshko, L.V., Nicoll, D.A., Weiss, J.N. and Philipson, K.D. (1993) Biosynthesis and initial processing of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. Biochimica et Biophysica Acta (BBA), 1151, 35-42. doi:10.1016/0005-2736(93)90068-B
- Nilsson, I.M. and von Heijne, G. (1993) Determination of the distance between the oligosaccharyl transferase active site and the endoplasmic reticulum membrane. The Journal of Biological Chemistry, 268, 5798-5801.
- Killian, J.A. and von Heijne, G. (2000) How proteins adapt to a membrane-water interface. Trends in Biochemical Sciences, 25, 429-434. doi:10.1016/S0968-0004(00)01626-1
- Popov, M., Li, J. and Reithmeier, R.A. (1999) Transmembrane folding of the human erythrocyte anion exchanger (AE1, Band 3) determined by scanning and insertional N-glycosylation mutagenesis. Biochemical Journal, 339, 269-279. doi:10.1042/0264-6021:3390269
- Popov, M., Tam, L.Y., Li, J. and Reithmeier, R.A. (1997) Mapping the ends of transmembrane segments in a polytopic membrane protein. Scanning N-glycosylation mutagenesis of extracytosolic loops in the anion exchanger, band 3. The Journal of Biological Chemistry, 272, 18325- 18332. doi:10.1074/jbc.272.29.18325
- Landolt-Marticorena, C. and Reithmeier, R.A. (1994) Asparagine-linked oligosaccharides are localized to single extracytosolic segments in multi-span membrane glycolproteins. Biochemical Journal, 302, 253-260.
- Preston, G.M., Jung, J.S., Guggino, W.B. and Agre, P. (1994) Membrane topology of aquaporin CHIP. Analysis of functional epitope-scanning mutants by vectorial proteolysis. The Journal of Biological Chemistry, 269, 1668- 1673.
ABBREVIATIONS
CT: Cytoplasmic;
FACS: Flow cytometry analyses;
Fc: Fc domain of mouse IgG2a;
mR1: Mouse CD200 receptor 1;
mR2: Mouse CD200 receptor 2;
mR4: Mouse CD200 receptor 4;
mDap12: Mouse adaptor protein Dap12;
TM: Trans membrane;
mR1r1: Mouse CD200 receptor 1;
mR2r2: Mouse CD200 receptor 2.
SUPPLEMENTARY
(A)
(B)
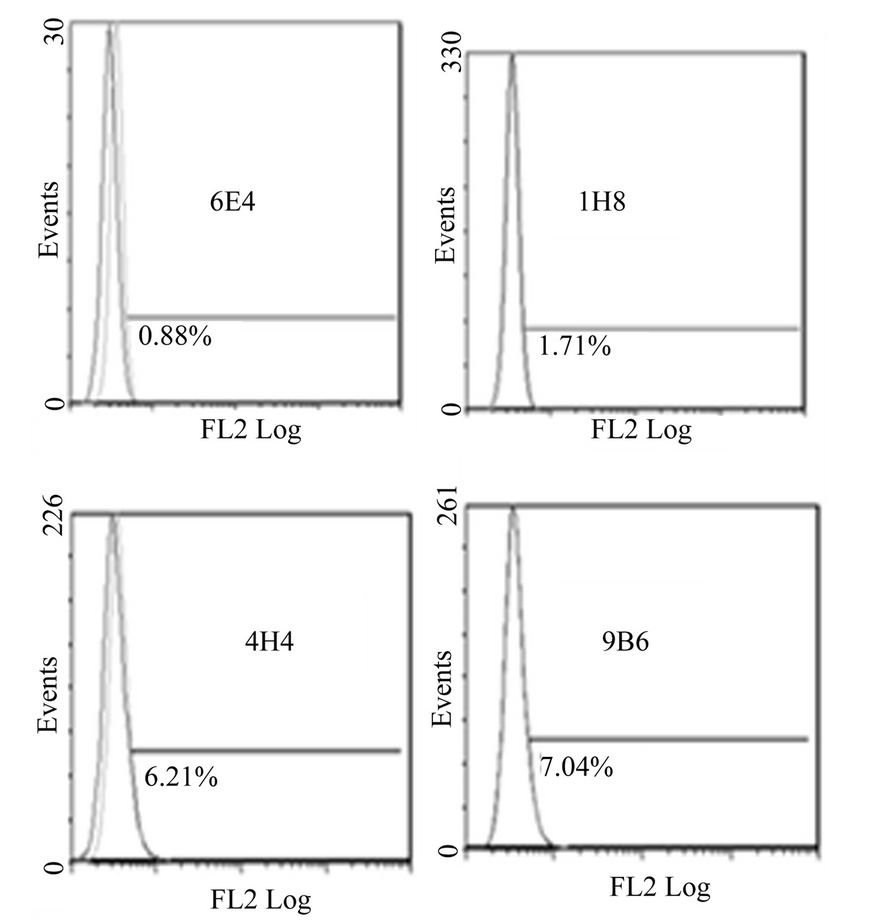
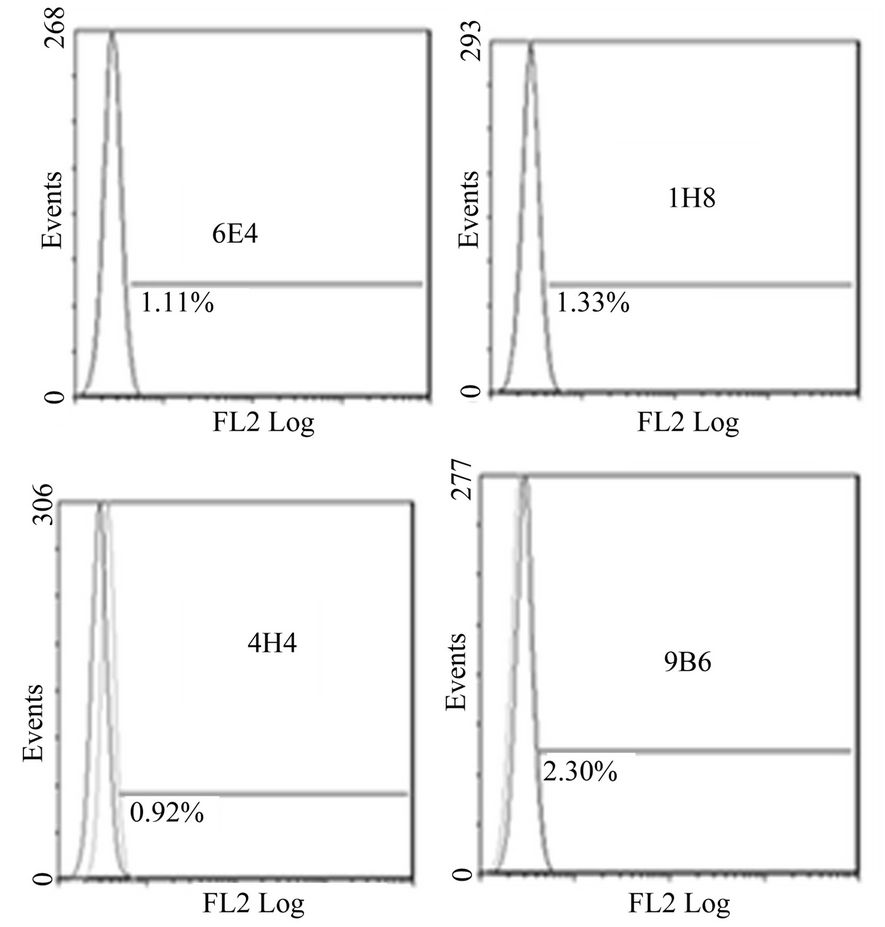
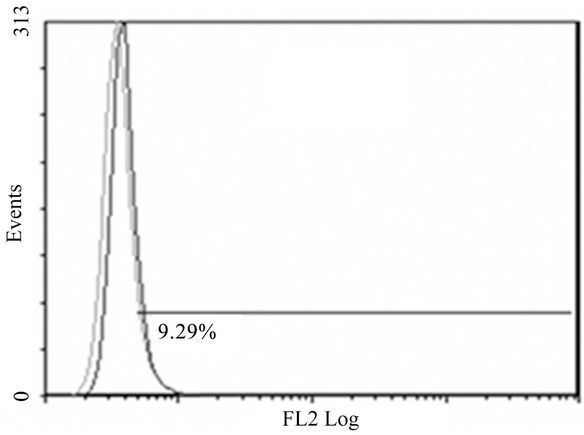
Figure 1. Anti-mR2 monoclonals do not recognize cells transfected with mR1his or mR4V5. Flow cytometery data for Hek293 cells stably transfected with mR1his (A) or mR4V5 (B). Cells were immunostained with anti-R2 monoclonals 6E4, 1H8, 4H4 and 9B6.
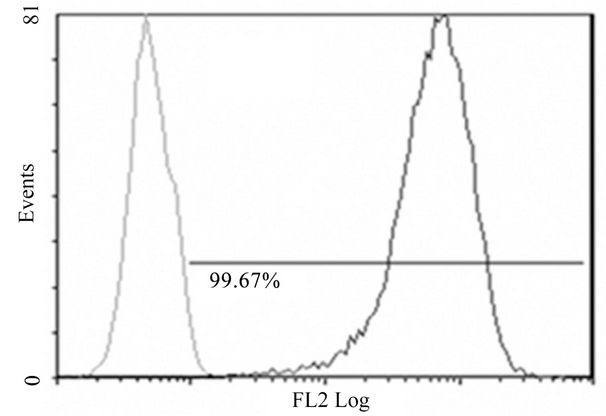
(A)
(B)
(C)
(D)
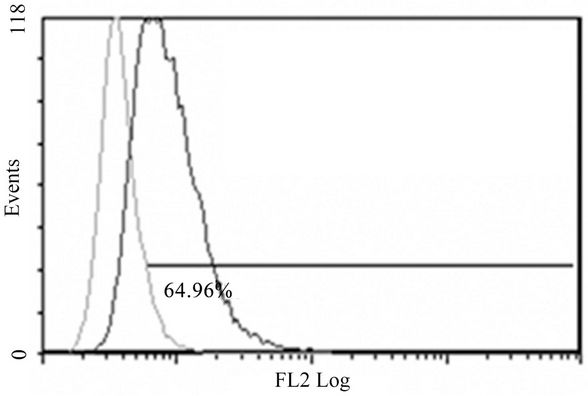
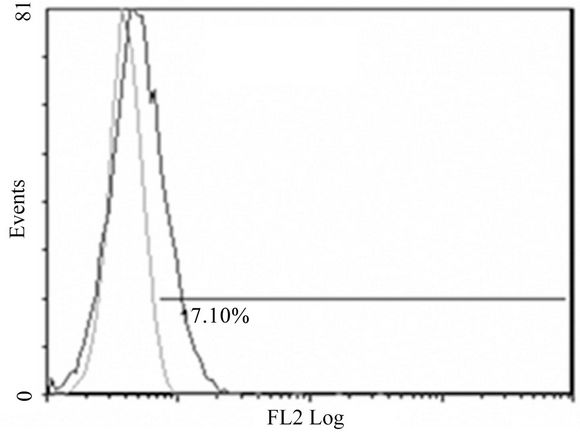
Figure 2. Anti-mR1 monoclonals recognize cells transfected with mR2V5 but not mR4V5. Flow cytometry data of Hek293 cells stably transfected with mR2V5 (A) and (B) or transiently transfected with mR4V5 (C) and (D) were immunostained with anti-R1 monoclonal 2A10 (A) and (C), or commercial ant-R1 antibody (B) and (D).
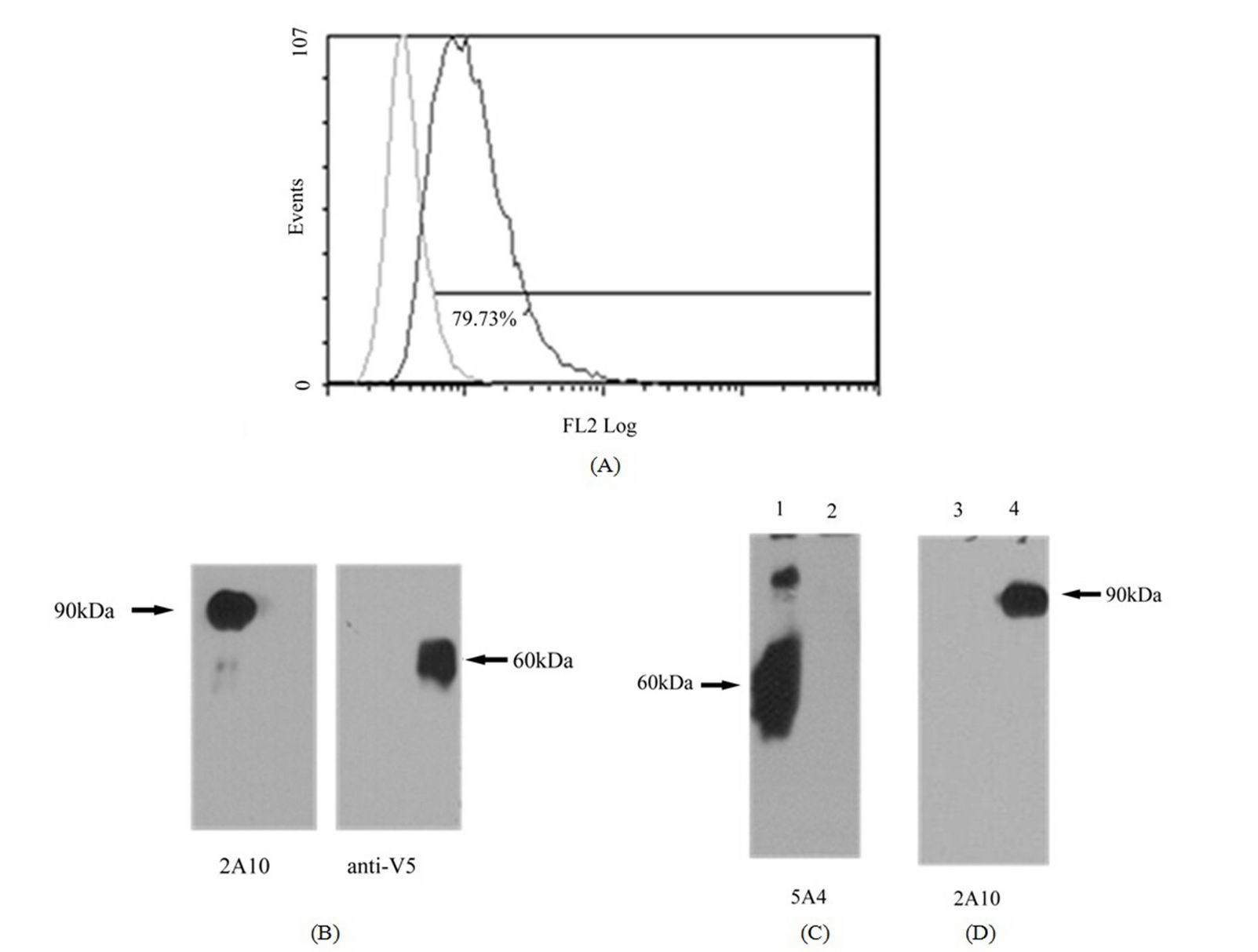
Figure 3. Lack of heterodimerization between mR1 and mR2V5. Stably transfected mR1 cells were transiently transfected with mR2V5 and 24 h post transfection stained with anti-R2 specific antibody 4H4 in FACS to confirm the expression of mR2V5 at the cell surface (A). Dual transfected cells were lysed and subjected to SDS/PAGE & western blotting using anti-R1 (2A10)/anti-V5 antibody (B), or were immunoprecipitated with anti-V5 antibody (C & D). Immunoprecipitates were analyzed using anti-R2 monoclonal antibody 5A4 (C, lane 1) or anti-mR1 monoclonal 2A10 (d lane 3). (lane 2 (C) represents immunoprecipitates from mock transfected cells, whereas (lane 4 (D) represents mR1 cell lysates (as control).
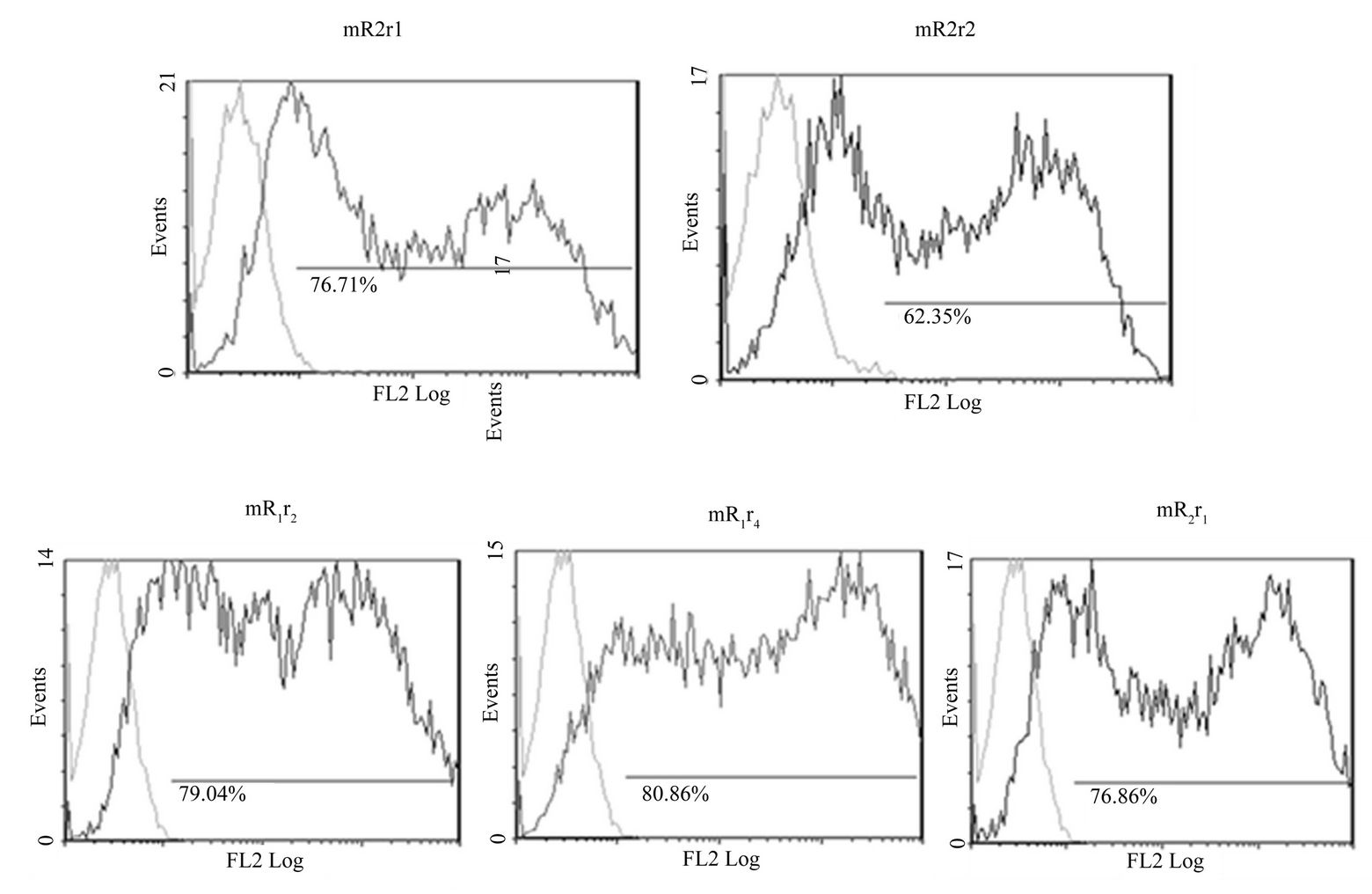
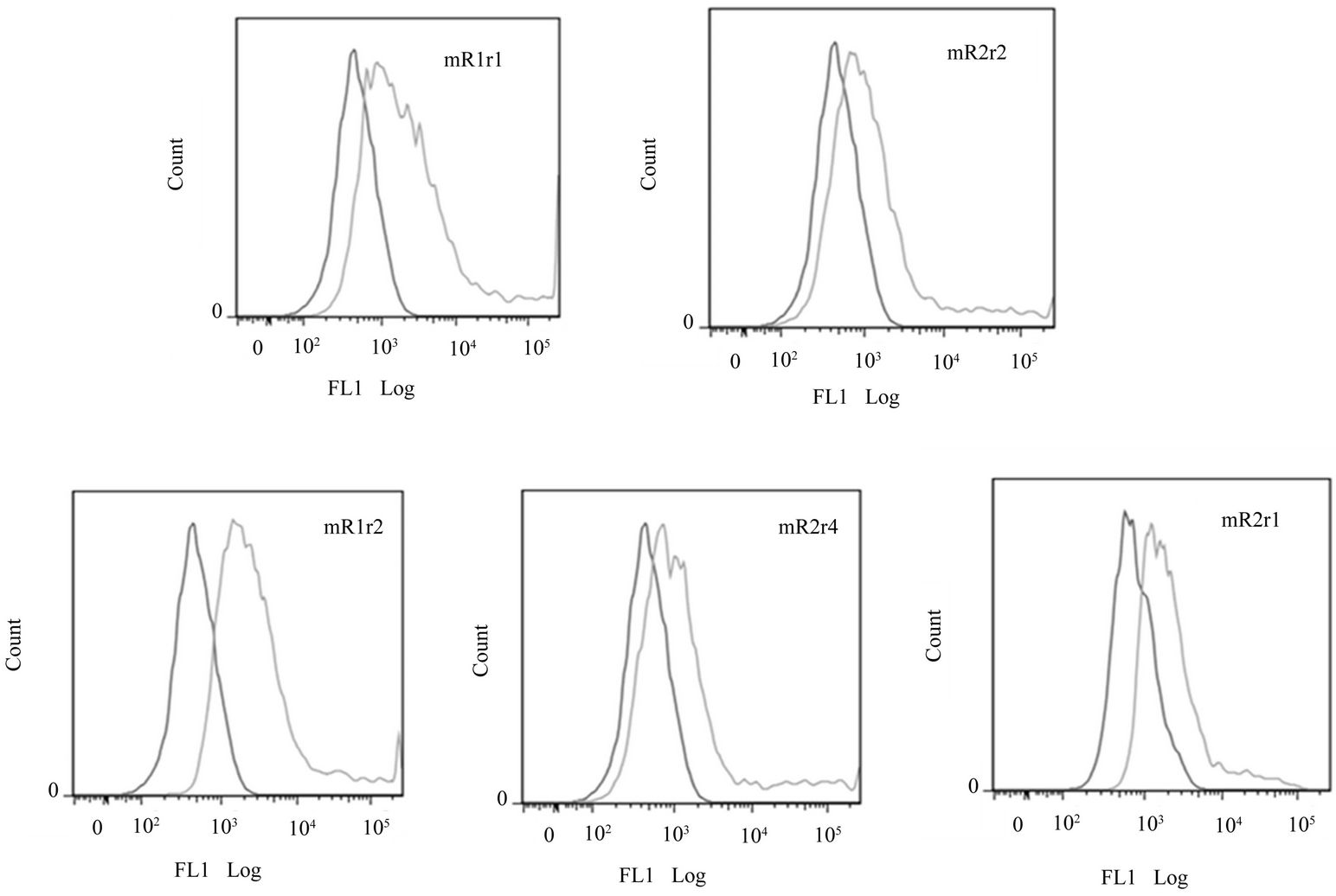
(A)
(B)
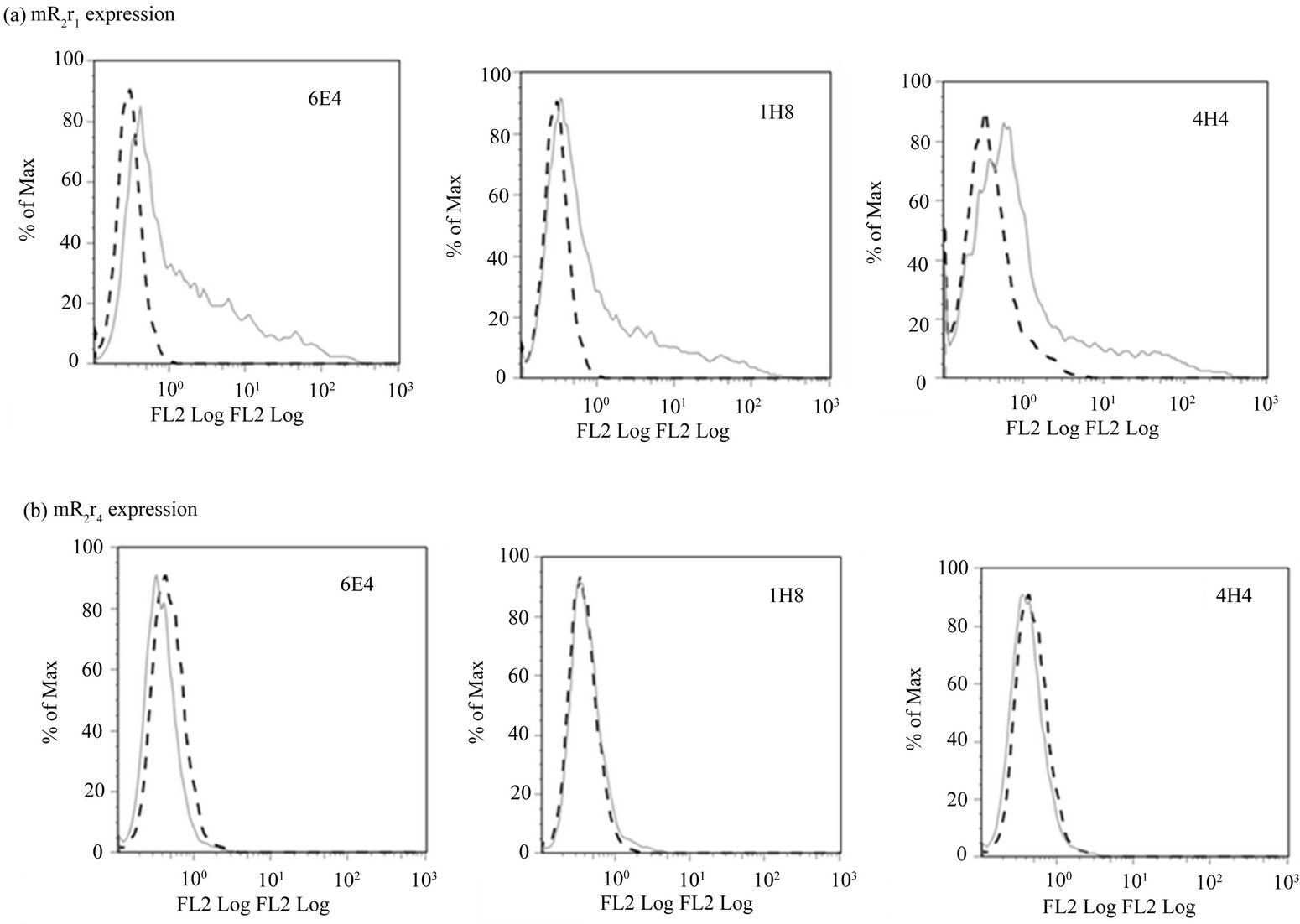
(C)
Figure 4. Expression of chimeric receptors in Hek293 and their binding to MCD200Fc. (A) Flow cytometry data of transiently transfected mR1r1, mR2r2, mR1r2, mR1r4 and mR2r1 cells immunostained with anti-R1 antibody 2A10 (panel a); (B) Binding of mCD200Fc to transiently transfected mR1r1, mR2r2, mR1r2, mR1r4 and mR2r1 cells was assessed by incubating transfected cells with 5 µg/ml mCD200Fc for 15 mins at 37˚C, followed by staining with FITC labeled anti-mouse IgG (Fab)2 fragment. Binding of mouse Fc (5 µg/ml) to transiently transfected cells was used as the negative control in these experiments; (C) Flow cytometry data of Hek293 cells transiently transfected with mR2r1 and immunostained with anti-R2 monoclonals antibodies 6E4, 1H8 and 4H4 (panel a). Flow cytometry data of Hek293 cells transiently transfected with mR2r4 and immunostained with anti-R2 monoclonal antibodies 6E4, 1H8 and 4H4 (panel b).

