Open Journal of Animal Sciences
Vol.3 No.3(2013), Article ID:34059,5 pages DOI:10.4236/ojas.2013.33026
Effect of heat stress on the maturation, fertilization and development rates of in vitro produced bovine embryos
![]()
1Cell Biology Laboratory, Research and Development Center for Animal Health, São Paulo Biological Institute, São Paulo, Brazil
2Department of Animal Reproduction, College of Medicine Veterinary and Animal Science, São Paulo University, São Paulo, Brazil; *Corresponding Author: ona@usp.br
Copyright © 2013 Mayra Fernanda Alves et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 16 April 2013; revised 10 May 2013; accepted 19 May 2013
Keywords: Bovine; Embryo; Heat Stress; in Vitro Fertilization
ABSTRACT
Heat stress is one of the main reasons for reproductive performance decrease in cattle, resulting in severe economic losses. The aim of this study was to evaluate the effect of heat stress during maturation, fertilization and development of in vitro produced bovine embryos. Cumulus oocyte complexes (COCs) were obtained by follicular puncture from slaughterhouse ovaries and after identification, were divided into four groups: control (CG), exposed 1 (EG1), exposed 2 (EG2), and exposed 3 (EG3). The oocytes of the group CG and CG3 were cultured at 38˚C and the oocytes of group EG1 and EG2 were cultured at 40˚C during the maturation period (24 hours at 5% CO2 in air). After the maturation period, oocytes of group CG, EG1, EG2, and EG3 were fecundated with frozen thawed semen. The oocytes of CG, EG2 and EG3 groups were cultured at 38˚C, and the group EG1 was cultured at 40˚C (18 hours at 5% CO2 in air). After that, the CG and EG2 groups were cultured in SOF at 38˚C and the groups EG1 and EG3 at 40˚C during embryonic development. The embryos were evaluated for cleavage, morula and blastocyst rates by optical microscopy. In control (CG) and EG3 groups, the oocytes showed uniform expansion of cumulus cells, classified as moderate to high, with brown color and uniform appearance of the ooplasm. In the oocytes exposed to 40˚C (EG1 and EG2) we observed a decrease in the expansion of cumulus cells, and the same showed rounded appearance and retraction of the ooplasm with dark coloration. The control group (CG) had 68.23% ± 2% of cleavage, 50.16% ± 2% morulas, and 43.28% ± 1% blastocysts. Whereas the EG2 had 31.46% ± 2% cleavage, 35.64% ± 2% morula, and no blastocysts development. The EG3 had 3.7% ± 2% cleavage, and no embryo production. These data suggest that in all stages of exposure to heat stress, the embryos and the gametes are susceptible, leading to a decrease in embryonic development.
1. INTRODUCTION
There is a change in behavior as well as neuroendocrine and physiological responses to keep homeostasis for temperature adaptation in which the animal is being submitted. Regarding reproduction, during heat stress, the gonadotropins and gonadal hormones are changed, impairing the reproductive cycle. Given these changes, it is common to see the occurrence of reduced fertility, low rates of estrus identification, conception decrease, abortion and embryonic mortality [1].
On the other hand, the reproductive capacity is changed also by the direct action of raising the internal temperature of the animal reproductive tract cells and tissues [2]. Occytes and embryos are the prime targets of the negative effects induced by heat stress, resulting in apoptosis [3]. These consequences cause a great loss to farmers [4].
According to Hansen and Aréchiga [5], embryos respond to maternal heat stress depending on the stage of development and the most critical periods for the embryo are among ovulation, the end of oocyte and the first days after fertilization. Regarding the embryonic development stage, the ability of an embryo responding to changes in his environment is limited during the first division, when much of the embryonic genome is still inactive. This period of low transcriptional activity creates a window, in which embryos are particularly sensitive to certain forms of stress [6].
In general, the period of greatest vulnerability of the embryo in relation of heat stress for the establishment and maintenance of pregnancy is up to 4 - 8 cells. This affects the development to the blastocyst stage. However, when stress is focused at the morula stage no changes occur [7,8]. Pires et al. [7] demonstrated the gestational period which goes up to 7 days is the most sensitive to the effects of severe heat. Roman-Ponce et al. [9] pointed out that a uterine temperature above 40˚C is sufficient to stop any embryo development. However, the oocytes and embryos to heat stress can be explained by cellular injuries associated to heat shock, resulting from a multifactorial process that involves changes in the percentage of fatty acids in the lipid membrane of the oocyte, cumulus cells and the cell fluid as well as the inhibition of antioxidants such as gluthathione and certain proteins responsible for the thermo-tolerance [10-12].
According to Edwards and Hansen [10], the oocytes and embryos sensivity to heat stress are due to insufficient production of heat shock protein (HSP) and as glutathione. Ealy et al. [13] demonstrated that the most sensitive period after fertilization occurs until the second 93 days, because, from this moment, the embryo begins to acquire resistance against high temperatures. De Souza et al. [14] reported that the 2 cells embryos are not able to synthesize HSP70 in response to heat stress. Mouse 2 - 4 cells embryos only supported the induced thermotolerance in more advanced stages. The mice HSPO synthesis occurs prematurely in 8 cells stage due to full activation of the embryonic genome [13]. The resistance development of bovine embryos can follow this theory, since its genome is activated between 8 - 16 cells (third day of fertilization). On the other hand, Saeki et al. [15] found that, during the 1 cell stage, it already exists a transcription of messenger RNA for the synthesis of heat shock proteins.
The aim of this study was to evaluate the effect of heat stress during maturation, fertilization and development of in vitro produced bovine embryos.
2. MATERIAL AND METHODS
For this study, oocytes were divided into four groups: control group (CG); exposed group 1 (EG1), kept at 40˚C during the maturation, fertilization, and embryonic development; exposed group 2 (EG2), kept at 40˚C during the oocyte maturation; exposed group 3 (EG3), maintained at 40˚C during the stage of embryonic development. All groups were evaluated for morphology, fertilization and embryo development rates.
2.1. Chemicals and Reagents
Unless otherwise stated, all chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Tissue culture media 199 (TCM 199 Hepes and Bicarbonate-buffered), PBS, and fetal calf serum (FSC) were obtained from GibcoTM, Invitrogen Corporation (Grand Island, NY, USA).
2.2. In Vitro Maturation
Cattle ovaries were obtained from a local slaughterhouse (unknown breed), and transported to the laboratory in PBS with penicillin (100 units/mL)—streptomycin (100 μg/mL) at 38˚C. Cumulus oocyte complexes (COCs) were aspirated from 2 to 6 mm in diameter follicles, and selected only those had intact zona pellucid with uniform ooplasm surrounded by at least two layers of cumulus cells. All groups (CG; EG1; EG2; EG3) were matured (20 COCs/drop) in 100 μL of TCM-199, LH (6 μg/mL), FSH (8 μg/mL) (Sioux Biochemical, Sioux Center, IA, USA), and penicillin-streptomycin for 24 h at 38˚C (CG; EG3) and 40˚C (EG1; EG2), 5% CO2 in air [16].
2.3. In Vitro Fertilization and Embryo Development
Frozen-thawed spermatozoa (Bos indicus) were washed by a 45%/90% layered Percoll gradient centrifugation. Oocytes were co-incubated with 10 × 104 spermatozoa in the fertilization medium supplemented with 2 μg/mL of heparin and 20 μL of PHE solution (20 μM penicillamine, 10 μM hypotaurine, 1 μM adrenaline; Hasler et al., 1995). The control group (CG), EG2, and EG3 were cultured at 38˚C and and EG1 at 40˚C, 5% CO2 in air (v/v).
After 18 h, presumptive zygotes were washed, removing the cumulus cells by pipetting, and cultured with synthetic oviductal fluid (SOF; [17]) at 38˚C (CG, EG2) and at 40˚C (EG1, EG3). The number of eggs cleaving by day 4 of culture, reaching blastocysts stage at day 7 was used for statistical analyses. The morphological analyses were observed in Olympus microscope (Tokyo, Japan).
3. STATISTICAL ANALYSES
Each experiment was repeated five times and data from each experiment were pooled. Approximately 90 - 100 oocytes were evaluated in each replicate. Data were analyzed using least square means to determine the effect of each treatment on embryonic cleavage and development to the blastocysts stage (SAS Institute Inc., 1989- 1996). The significance level for all tests was P < 0.005.
4. RESULTS
After 18 h, the control group showed symmetrical cleavage and uniform ooplasm. The EG1 group, which was exposed at 40˚C during maturation, fertilization and embryo development, had retracted ooplasm with granular appearance. In the EG2, exposed at 40˚C during in vitro maturation, observed asymmetric cleavages, and retracted ooplasm with granular appearance. Whereas the EG3 group, exposed at 40˚C the embryo development, showed degenerated embryos with retracted ooplasm (Figure 1).
After the day 7, embryos from the control group (CG) were in morula and blastocysts stages. The EG1 and EG3 groups did not show morula and blastocysts, but the EG2 had some morulas and no blastocysts.
The Figure 2 shows the cleavage, morula and blastocysts rate from all groups. The control group (CG) had 68.23% ± 2% of cleavage, 50.16% ± 2% morulas, and 43.28% ± 1% blastocysts. Whereas the EG2 had 31.46% ± 2% cleavage, 35.64% ± 2% morula, and no blastocysts development. The EG3 had 3.7% ± 2% cleavage, and no embryo production.
5. DISCUSSION
The interaction between animal and environment should be considered when seeking greater efficiency in the livestock exploration, due to the different responses of the animal to the peculiarities of the surrounding en-
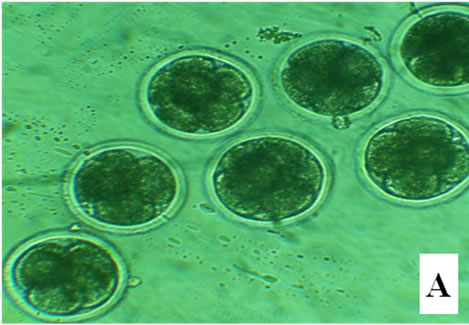
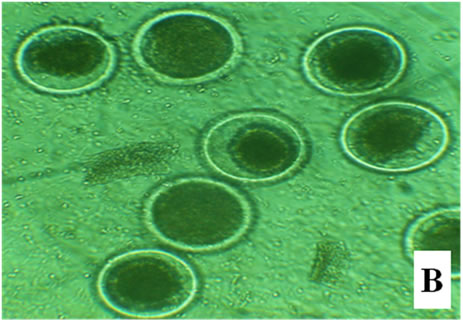
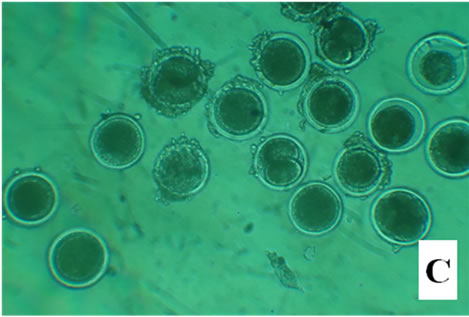
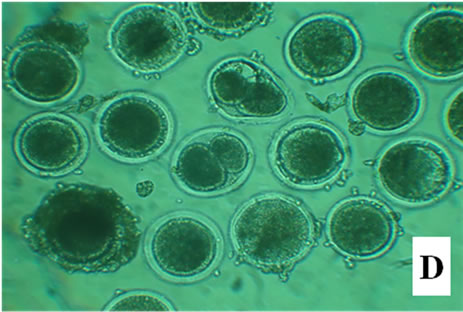
Figure 1. Bovine embryos from groups CG, EG1, EG2, and EG3 after 18 h pos insemination. The control group showed symmetrical cleavage and uniform ooplasm. The EG1 group, which was exposed at 40˚C during maturation, fertilization and embryo development, had retracted ooplasm with granular appearance. In the EG2, exposed at 40˚C during in vitro maturation, observed asymmetric cleavages, and retracted ooplasm with granular appearance. Whereas the EG3 group, exposed at 40˚C the embryo development, showed degenerated embryos with retracted ooplasm (Figure 1).
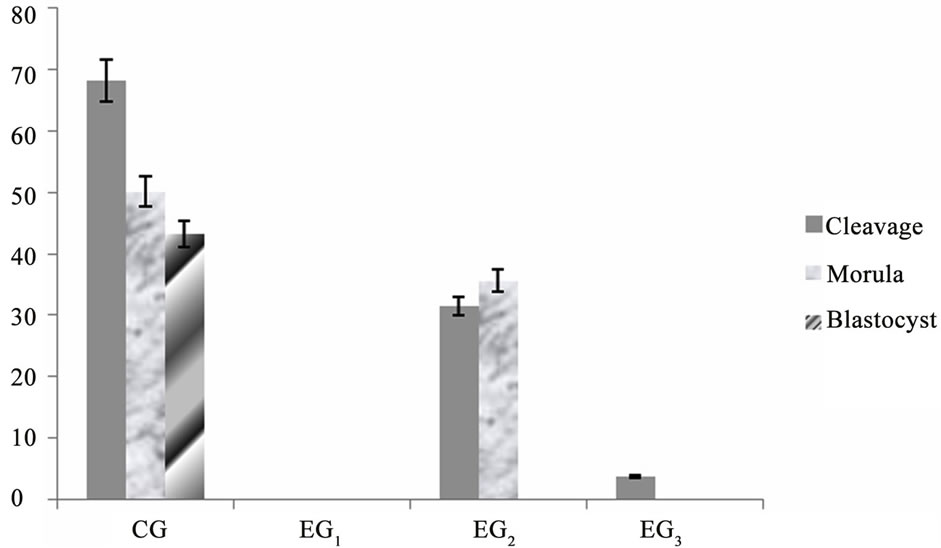
Figure 2. Mean percentage ± S.E.M. of oocytes cleaved, morulas, and blastocysts. The oocytes of the group CG and CG3 were cultured at 38˚C and the oocytes of group EG1 and EG2 at 40˚C during the maturation period (24 hours at 5% CO2 in air). After the maturation period, oocytes of CG, EG2 and EG3 groups were fecundated at 38˚C and the group EG1 at 40˚C (18 hours at 5% CO2 in air). The CG and EG2 groups were cultured in SOF at 38˚C and the groups EG1 and EG3 at 40˚C during embryonic development (P < 0.005).
vironment. Thus the correct identification of the factors that influence the productive life of the animal, like heat stress, result of climate change over the past year, may allow adjustments in production management, enabling them to sustainability and economic viability. Therefore, the need for a deeper and more detailed study of the damages caused by the increase of environmental temperature on animal reproduction, led us to this research in order to study the effect of heat stress during maturation, fertilization and development of in vitro produced bovine embryos.
In the present study, the control group (CG) showed 68.23% ± 2% of cleavage, 50.16% ± 2% morulas, and 43.28% ± 1% blastocysts. Whereas the oocytes exposed at 40˚C during maturation, fertilization, and embryo development (EG1) interfered with oocyte viability, inhibiting the process of fertilization and cleavage in 100%. However, in the EG2 (exposed at 40˚C during maturation), observed 31.46% ± 2% cleavage, 35.64% ± 2% morula, and no blastocysts development.
Edwards and Hansen [10] observed that exposure of oocytes to heat stress of 40˚C and 41˚C for 12 hours blocked or reduced development to blastocysts stage (30 and 40% for oocytes cultured at 40˚C and 41˚C, respectively). Another study by the same authors, showed similar results with 35% and 18% for oocytes cultured at 39˚C and 41˚C, respectively [18].
Ju et al. [19] observed that the heat stress in oocytes for one hour at 40˚C or 42˚C had no deleterious effects on blastocysts formed after the IVF. In another study, the blastocysts rate decreased when the heat shock is sustained for 12 hours at 41˚C, being exacerbated as the exposure time increased [18]. When the temperature increased to 43˚C, the developmental competence of treated oocytes was severely reduced following an exposure of 45 minutes [19]. It was observed that the temperature and exposure duration are limiting factors for in vitro embryo production [19,20].
These results support the hypothesis that oocytes exposed to heat stress and suffered cell damage may interfere with subsequent fertilization and embryo development, and that the longer the duration of exposure to high temperatures, the greater the damage. However, it was observed that the EG1 from this study, after 18 hours exposed at 40˚C, presented sperm with no mobility, cloggy and no tail. This shows that the increase in temperature adversely affects the survival of the spermatozoa and in the fertilization.
According to Hansen et al. [21], exposure of spermatozoa to the high temperatures in the uterus or oviduct of females under heat stress may compromise the ability of sperm survival and fertilization. Damage to spermatozoa can occur due to the production of free radicals, however, no one knows the time required for the production of free radicals increase [22]. These data suggest that the heat stress, besides induced changes in the quality of oocytes, can cause damage to sperm, leading to a decrease in the rate of fertilization and embryo development.
Regarding EG3 group, exposed at 40˚C during embryo culture, was observed only 3.7% of cleavage, without the development of morulas and blastocysts. Similar results were described in an experiment conducted by Al-Katanani and Hansen [12] where the proportion of embryos at the stage of two cells which have developed to the blastocysts stage, after exposure to heat stress treatment at 42˚C for 80 minutes and 41˚C for 12 hours, was 0% against 39.5% in the control group.
Edwards and Hansen [18] also compared the resistance of 2 cells embryos, 4 - 8 cells and morulas to elevated temperatures. The heat stress at 41˚C for 12 hours decreased the blastocysts development in 2 cells embryos and in 4 - 8 cells embryos exposed, but not when more lies were exposed. The same was observed when Bos taurus and Bos indicus 2 cells embryos were exposed for more than 3 hours [20,23].
These findings may be due to the fact that the first cleavages correspond to the critical phase, in which genomic activation occurs through the maternal control to embryonic. During this period, there seems to be susceptibility to the heat stress, leading to failure in embryonic genome activation and blastocysts development [24]. Since the embryonic genome is not fully activated until the 8 - 16 cells phase, it is possible that some of the genes which confer cellular resistance to heat shock has not yet been expressed in 2 - 4 cells step [25].
According to Krininger III et al. [23], the presumable explanation for the discrepancies in the in vitro data is the difference between phases of embryonic development in a time when the heat shock was applied, or the intensity and duration of the same, which can explain the low cleavage rate in the EG3 group (3.7%) cultured at 40˚C during embryo development, against the EG2 (31.26%) exposed at 40˚C during maturation.
Some studies demonstrated that the 2 cells bovine embryos, when subjected to heat stress at 40˚C for 3 hours, developed to the blastocysts stage normally [20,26]. The same authors observed that embryo development decreased when subjected to heat stress at 41˚C and 42˚C for a long period of time (12 hours). These effects were also reported in porcine embryos by Ju and Tseng [27]. For these authors, it seems clear that the effects of conditions produced by high temperatures on the viability and developmental competence of oocytes and embryos depend on the temperature and duration of exposure to the heat stress.
Studies carried out by Bényei and Barros [28] demonstrated that exposure to stress in dairy cows during summer or in a hyperthermic environmental chamber (42˚C) during 7 days reduced the viability of embryos collected and increased the incidence of degenerated embryos and late development. In this study we had the same observations in EG2 and EG3 groups, in other words, formation of asymmetric divisions, embryos in the process of degeneration and developmental delay. Thus this study showed that all stages of exposure to heat stress the embryos and gametes were susceptible, leading to a decrease in embryonic development.
6. ACKNOWLEDGEMENTS
The authors thank the staff at the Fenix slaughterhouse, to FAPESP (2010/01077-9), Vitrocell/Embriolife®, and to CNPq.
REFERENCES
- Pires, M.F.A., Ferreira, A.M. and Coelho, S.G. (1999) Estresse calórico em Bovinos de Leite. Caderno técnico de Veterinária e Zootecnia, 29, 23-37.
- Encarnação, R.O. (1992) Estresse e produção animal. Ph.D. Dissertation, EMBRAPA-CNPGC, Campo Grande.
- Santos Junior, E.R. (2010) Efeito do estresse térmico na maturação in vitro de oócitos caprinos e ovinos em protocolo de produção de embriões. Ph.D. Dissertation, Universidade Federal Rural de Pernambuco, Recife.
- Ferro, F.R.A., Cavalcanti Neto, C.C., Toledo Filho, M.R., Ferri, S.T.S. and Montaldo, Y.C. (2010) Efeito do estresse calórico no desempenho reprodutivo de vacas leiteiras. Revista Verde, 5, 1-25.
- Hansen, P.J. and Aréchiga, C.F. (1999) Strategies for managing reproduction in the heat-stressed dairy cow. Journal of Animal Science, 77, 36-50.
- Paula-Lopes, F.F. and Hansen, P.J. (2002) Heat-shock induced apoptosis in preimplantation bovine embryos is a developmentally-regulated phenomenon. Biology of Reproduction, 66, 1169-1177.
- Pires, M.F.A., Ferreira, A.M. and Coelho, S.G. (1999) Estresse calórico em Bovinos de Leite. Caderno técnico de Veterinária e Zootecnia, 29, 23-37.
- Vianna, F.P. (2002) Influência do estresse térmico na atividade reprodutiva de fêmeas bovinas. Ph.D. Dissertation, Universidade Estadual de São Paulo, Botucatu.
- Roman-Ponce, H., Thatcher, W.W., Caton, D., Barron, D.H. and Wilcox, C.J. (1978) Thermal stress effects on uterine blood flow in dairy cows. Journal of Animal Science, 46, 175-180.
- Edwards, J.L. and Hansen, P.J. (1996) Elevated temperature increases heat shock protein 70 synthesis in bovine two-cell embryos and compromises function of maturing oocytes. Biology of Reproduction, 55, 340-346. doi:10.1095/biolreprod55.2.341
- Zeron, Y., Ocheretny, A., Kedar, O., Borochov, A., Sklan, D. and Arav, A. (2001) Seasonal changes in bovine fertility: Relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction, 121, 447-454. doi:10.1530/rep.0.1210447
- Al-Katanani, Y.M. and Hansen, P.J. (2002) Induced thermotolerance in bovine two cells embryos and the role of heat schock protein 70 in embryonic development. Molecular Reproduction and Development, 62, 174-180. doi:10.1002/mrd.10122
- Ealy, A.D., Drost, M. and Hansen, P.J. (1993) Developmental changes in embryonic resistance to adverse effects of maternal heat stress in cows. Journal of Dairy Science, 76, 2899-2905. doi:10.3168/jds.S0022-0302(93)77629-8
- De Souza, P.A., Watson, A.J., Schultz, G.A. and Bilodeau-Goessels, S. (1998) Oogenetic and zygotic gene expression directing early bovine embryogenesis: A review. Molecular Reproduction and Development, 51, 112-121. doi:10.1002/(SICI)1098-2795(199809)51:1<112::AID-MRD14>3.0.CO;2-9
- Saeki, K., Matsumoto, K., Kaneko, T., Hosol, Y., Kato, H. and Iritani, A. (1999) Onset of RNA syntesis in early bovine embryos detected by reverse transcripitionpolymerase chain reaction following introduction of exogenous gene into their pronuclei. Theriogenology, 51, 192. doi:10.1016/S0093-691X(99)91751-X
- Gonçalves, R.F., Chapman, D.A., Bertolla, R.P., Eder, I., and Killian, G.J. (2008) Pre treatment of cattle semen or oocytes with purified milk osteopontin affects in vitro fertilization and embryo development. Animal Reproduction Science, 108, 375-383. doi:10.1016/j.anireprosci.2007.09.006
- Van Wagtendonk-de Leeuw, A.M., Mullaart, E., de Ross, A.P., Merton, J.S., den Daas, J.H., Kemp, B. and de Ruigh, L. (2000) Effects of different reproduction techniques: AI, MOET, or IVP, on health and welfare of bovine offspring. Theriogenolog, 53, 575-597. doi:10.1016/S0093-691X(99)00259-9
- Edwards, J.L. and Hansen, P.J. (1997) Differential responses of bovine oocytes and preimplantation embryos to heat shock. Molecular Reproduction and Development, 46, 138-145. doi:10.1002/(SICI)1098-2795(199702)46:2<138::AID-MRD4>3.0.CO;2-R
- Ju, J.C., Parks, J.E. and Yang, X. (1999) Thermotolerance of IVM-derived bovine oocytes and embryos after short term heat stress. Molecular Reproduction and Development, 53, 336-340. doi:10.1002/(SICI)1098-2795(199907)53:3<336::AID-MRD9>3.0.CO;2-M
- Ealy, A.D., Howell, J.L., Monterroso, V.H., Arechiga, C.F. and Hansen, P.J. (1995) Developmental changes in sensitivity of bovine embryos to heat shock and use of antioxidants as thermoprotectants. Journal of Animal Science, 73, 1401-1407.
- Hansen, P.J., Rivera, R.M., Paula Lopes, F.F., Al-Katanani, Y.M., Krininger III, C.E. and Chase Jr., C.C. (2001) Adverse impact of heat stress on embryo production: Causes and strategies for mitigation. Theriogenology, 51, 91-103. doi:10.1016/S0093-691X(00)00448-9
- Monterroso, V.H., Drury, K.C., Baly, A.D., Edwards, J.L. and Hansen, P.J. (1995) Effect of heat shock on function of frozen/thawed bull spermatozoa. Theriogenology, 44, 947-961. doi:10.1016/0093-691X(95)00282-D
- Krininger III, C.E., Block, A.J., Al-Katanani, Y.M., Rivera, R.M., Case Jr., C.C. and Hansen, P.J. (2003) Differences between Brahman and Holstein cows in response to estrus synchronization, superovulation and resistance of embryos to heat shock. Animal Reproduction Science, 78, 13-24. doi:10.1016/S0378-4320(03)00045-9
- Schultz, R.M. (2002) The molecular foundations of the maternal to zygotic transition in the preimplantation embryo. Human Reproduction Update, 8, 323-331. doi:10.1093/humupd/8.4.323
- Memili, E. and First, N.L. (2000) Zygotic and embryonic gene expression in cow: A review of timing and mechanisms of early gene expression as compared with other species. Zygote, 8, 87-96. doi:10.1017/S0967199400000861
- Tseng, J.K., Chen, C.H., Chou, P.C., Yeh, S.P. and Ju, J.C. (2004) Influences of follicular size on parthenogenetic activation and in vitro heat shock on the cytoskeleton in cattle oocytes. Reproduction of Domestic Animals, 39, 146-153. doi:10.1111/j.1439-0531.2004.00493.x
- Ju, J.C. and Tseng, J.K. (2004) Nuclear and cytoskeletal alterations of in vitro matured porcine oocytes under hyperthermia. Molecular Reproduction and Development, 68, 125-133. doi:10.1002/mrd.20054
- Bényei, B. and Barros, C.W.C. (2000) Variações fisioló- gicas de parâmetros reprodutivos em vacas de raça Holandesa importadas da Hungria para o Nordeste brasileiro. Brazilian Journal of Veterinary Research and Animal Science, 37, 3. doi:10.1590/S1413-95962000000300008

