Open Journal of Genetics
Vol.4 No.4(2014), Article
ID:48053,10
pages
DOI:10.4236/ojgen.2014.44030
Phylogenetic Position of North Sulawesi Tarsius sp. Based on Partial Cytochrome b Gene Sequences
Decky David Wemvrid Kamagi1, Aloysius Duran Corebima2, Mariana Rengkuan1
1Faculty of Mathematic and Natural Science, State University of Manado, Tondano, Indonesia
2Faculty of Natural Science and Mathematic, State University of Malang, Malang, Indonesia
Email: deckykamagi@yahoo.com, durancorebima@yahoo.com, ine_rengkuan@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 May 2014; revised 4 June 2014; accepted 3 July 2014
ABSTRACT
Cyt b gene of North Sulawesi Tarsius sp., T. tumpara, T. sangirensis and T. tarsier (T. spectrum) had been partially sequenced. The homologous sequence of three groups had been compared to describe the phylogenetic position among them, as well as several other species accessed from the Genbank. Total DNA extracted from the muscular tissue had been obtained through tail cut sampling using the innuPREP DNA micro kit, and amplified using a pair of universal primer, L14841 and H15149. The size of the cyt b gene sequence amplified was 307 bp long. Sequence aligned using CLUSTAL-X program and diversity analysis were done using version 5.2.2. MEGA5 program. Genetic distance had been calculated by Tamura 3 parameter method and phylogenetic tree had been built using Neighbor-Joining and Maximum Likelihood methods. Genetic distance based on cyt b gene nucleotide was found from 0 to 0.240 with an average of 0.080. The phylogenetic tree constructed by Neighbor Joining and Maximum Likelihood methods indicated that T. tarsier, T. sangirensis and T. tumpara were closely related with Tarsius tarsier-complex, and distantly related with Cephalopachus bancanus and Carlito syrichta. The genetic distance and the phylogenetic tree had been constructed on the base of partial cyt b gene sequence of T. tarsier, T. sangirensis, T. tumpara and 5 other tarsier species and their accession. Those results are consistent with taxonomy based on morphology and vocal acoustic form.
Keywords:Molecular Phylogeny, Tarsius sp., Sulawesi, Cyt b Gene, Partial Sequence

1. Introduction
The main island of Sulawesi and its surrounding islands located at Wallacea zone, possess abundant biodiversity. In the past, Sulawesi Island did not join with any other land [1] . Isolated condition in long period of time seemed to push evolution of many species so that Sulawesi Island had a high endemism level [2] . Therefore, the main priority in the management of natural resources in Sulawesi is conserving a resource of genetic diversity, because species conserved in the Sulawesi are not found in other places [3] . Sulawesi has become “top hotspots” for biodiversity conservation [4] . Sulawesi has about 529 endemic vertebrates or approximately 1.9% of endemic vertebrates in the world [2] , including 7 species of endemic monkeys [5] and 7 - 9 Tarsius species of Tarsius tarsier-complex [6] . Recently, they are threatened to distinct due to natural predators, illegal hunting, and habitat destructions by human intervention.
Tarsius taxonomy up to now remains a problem continuously debated. Originally, tarsier was a monotypic genus of Tarsiidae family [7] . Based on morphological characteristics, tarsier consists of two different groups, western tarsier and eastern tarsier [8] ; but based on genetic analysis and vocal acoustic form, tarsier is divided into three groups, western tarsier living in great Sunda Islands (Borneo and Sumatera), eastern tarsier living in Sulawesi and Phillippine tarsiers living in southern Phillippine [6] . Nowadays, the genera of Tarsiidae family had been revised to be three separate genera, Tarsius, Cephalopachus, and Carlito, each of which is allopatrically distributed in three different biogeographic regions, Sulawesi, great Sunda Islands (Borneo and Sumatera) and Mindanao [6] .
Sulawesi tarsier grouped in one group called Tarsius tarsier-complex, comprises of 9 species: T. sangirensis, T. tumpara, T. wallacei, T. lariang, T. pumilus, T. fuscus, T. tarsier (T. spectrum), T. pelengensis and T. dentatus [6] [9] . Tarsius tarsier complex is a vague taxonomic group due to consisting of closely related species, so that it is less distinct to recognize the interspecific diversity based on the morphological variations. The taxonomic status of Sulawesi tarsier, especially of low land tarsier, has been long disputed due to interspecific similarity. The low land tarsier species has no significant difference in body size or body proportion [9] .
Study on genetic diversity and population biology had been carried out using mitochondrial DNA [10] [11] . Variation patterns of mtDNA can be used as species distinction as well as species investigation of those endangered species [12] . The mitochondrial DNA is often used as genetic marker for closely related interspecific and intraspecific genetic diversity study, since it evolves faster than nucleic genes so that it could give more variations to reconstruct the evolutionary history [13] . Mitochondrial DNA genes had been widely applied to estimate the phylogenetic relationship of primate [14] -[16] . These genes had also been used to restudy the phylogenetic relationship among closely related taxa [12] . The mitochondrial cyt b gene is known as a gene evolving faster, so it can have more variation and can be used for phylogenetic and biogeographic study [16] -[18] .
Whole mitochondrial genome studies had been conducted on T. syrichta [19] , T. bancanus [20] , T. wallacei [21] , T. lariang [22] and T. dentatus [23] , so that these results can be used as references to study several genetic markers of encoding or noncoding genes in the mitochondria. The cyt b gene contain discrete character groups (base position at codon) representing the mutation rate, so that it can be used as a phylogenetic marker [24] .
Partial sequencing of the cytochrome b gene had been used to uncover the phylogenetic position and the genetic relationship among several tarsier species and among other primates [25] [26] . The cyt b gene is one of the protein coding gene of the mtDNA. Its product is cytochrome b (cyt b) apoenzym identified as the central catalytic subunit of Q cycle. The cyt b gene of tarsier has a length of 1140 bp, known as coding region of the protein located at 14169 to 15308 mtDNA sequence, flanked by the tRNA-Glu gene and the tRNA-Thr gene [20] .
Several universal olygonucleotide primers had been developed for amplificating and sequencing cyt b gene of different animals [27] . Partial sequence of the cyt b gene had been applied to uncover the genetic diversity among T. sangirensis, T. tumpara and T. tarsier (T. spectrum or Manado tarsier) and several other Tarsius sp. obtained from the GenBank.
2. Material and Method
2.1. Sample Collection and Treatment
Sample specimen used was muscular tissue obtained by tail cut sampling. It was then stored at –20˚C, in alcohol of 95%. Because of difficulty of collecting samples, each species consisted only 2 sample specimens. The sampling site and the specimen treatment is shown in Table1
The data sequence of C. bancanus, C. syrichta, T. wallacei, T. dentatus, T. dentatus × lariang and T. lariang had been obtained from the GenBank. Tarsier species and their accession numbers taken from GenBank are shown in Table2
The primer used was universal primer L14841 and H15149 [27] . The specific location of the primers, L14841 (33 bp) and H15149 (34 bp) in the cyt b gene region was from the 63st nucleotide sequence downstream related to the forward primer and from the 435st nucleotide sequence upstream related to the reverse primer.
2.2. DNA Extraction and Cyt b Gene Amplification
Total DNA had been extracted using InnuPREP DNA micro Kit. Purity measurement of total DNA isolated of the sample resulted in a concentration of 46 - 187 µg per gram sample with λ260/λ280 ratio.
The PCR component and condition were optimized so that the cyt b gene could be amplified (Table 3 and Table 4).
2.3. Cyt b Gene Sequencing
The amplification product had been sent to First BASE, Laboratories Sdn. Bhd. Selangor, Malaysia to be sequenced. The equipment used was ABI PRISM 3730xl Genetic Analyzer. Biosystem USA.
2.4. Sequence Alignment and Data Analysis
The cyt b gene sequence data of the North Sulawesi Tarsius sp. and those accessed from the GenBank had been aligned using CLUSTAL-X programme [28] . Bayesian Information Criterion (BIC) had been used to consider the best substitution pattern. The genetic distance had been analyzed using the TN 93 + G (Tamura-Nei) and T92 + I (Tamura 3-parameter) methods. The phylogenetic tree had been constructed based on Tarsius sp. cyt b gene using two different approaches, Neighbor-Joining (NJ) [29] and Maximum Likelihood (ML) method [30] where C. bancanus and C. syrichta were treated as outgroup.
Table 3. Optimization component of PCR.
Table 4. Condition optimation of PCR.
Note: Before sequencing, the PCR product was purified and electrophorized in agarosa gel 1.5%.
3. Results
3.1. Extraction and Amplification of cyt b Gene
Total DNA of six samples of North Sulawesi Tarsius sp. had been isolated and amplified. The results of electrophoresis on 1.5% agarose gel showed that cyt b gene amplified was at about 400 bp (Figure 1).
3.2. Sequence Characteristic
Multiple alignment of cyt b gene sequence of 307 bp long derived from T. sangirensis, T. tarsier, T. tumpara and those from homologous cyt b gene sequence of several tarsier species taken from GenBank indicates that invariable sites character as much as 72.97%, informative parsymony sites as much as 27.03% and variable sites as much as 27.03% (Table 5).
Nucleotide composition of the partial gene cyt b sequence of each tarsier species exhibits variations indicated by frequency difference of each bases among species. Average base frequency of T = 32.80%, C = 22.67%, A = 28.63% dan G = 15.90%. Analysis of several parameters of partial cyt b gene sequence (Table 6) found that nucleotide diversity (Pi) = 0.0698, total mutation = 97, ts/tv ratio (R) = 4.978 and ts/tv (k) ratio between purine bases = 5.253 and between pyrimidine bases = 13.418, respectively.
3.3. Genetic Distance
Genetic distance measured using the Tamura 3 parameters indicate that the value varies from 0 to 0.240 (complete matrix data are not included). The genetic distance of 0 is shown by sample pairing of the same species. Genetic distance is shown by pairing of C. bancanus and other species, i.d. from 0.181 to 0.240, as well as pairing of C. syrichta and other tarsier species, with values from 0.181 to 0.200. Pairing among Sulawesi Tarsius sp. have the values 0 to 0.095. Overall mean distance is 0.080. These data indicate that those Sulawesi tarsiers are classified as closely related taxa and relatively distant related to the Borneo tarsier C. bancanus as well as to the Philippine tarsier C. syrichta.
3.4. Phylogenetic Tree
Figure 2 and Figure 3 are phylogenetic trees based on nucleotides of partial cyt b gene constructed by method
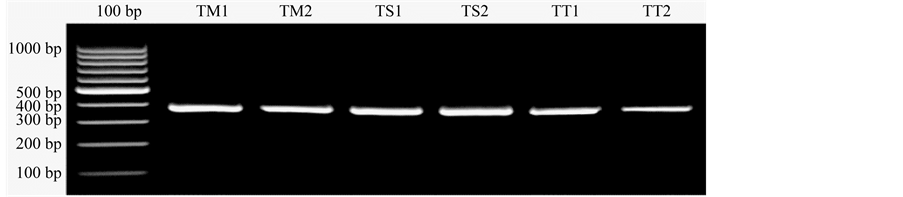
Figure 1. Cyt b gene amplification of North Sulawesi Tarsius sp. sample on 1.5% agarose gel. Lanes: 100 bp as a marker, TM1 and TM2 = T. tarsier, TS1 and TS2 = T. sangirensis, TT1 and TT2 = T. tumpara.
Table 5. Summary of cyt b gene sequence diversity.
Table 6. Analysis results of cyt b gene partial sequencing.
Note: Analysis of base frequency only involved 306 bp, adjusted to the reading frame encoding amino acids, started from second nucleotide of each sequence amplified.
based on distance of Neighbor-Joining (NJ) and Maximum Likelihood (ML). Both NJ and ML phylogenetic trees constructed based on the nucleotide sequences showed same tree topologies. Both NJ and ML phylogenetic tree put North Sulawesi tarsier, T. sangirensis, T. tumpara, and T. tarsier relatively at the same position at the tree.
In NJ phylogenetic tree, both T. sangirensis and T. tumpara form a monophyletic clade and separate from the others Tarsius tarsier-complex (99% BR). On the other hand, T. tarsier forms a monophyletic clade and occupies position in Tarsius tarsier-complex clade (94% BR). In ML phylogenetic tree both T. sangirensis, T. tumpara also form a clade separated from the other T. tarsier-complex (98% BR). T. tarsier is located on Tarsius tarsiercomplex clade. In general, both the NJ and ML trees showed similarities in grouping populations, in which T. sangirensis, T. tumpara, T. tarsier and other species are clustered in a larger clade called Tarsier tarsier-complex.
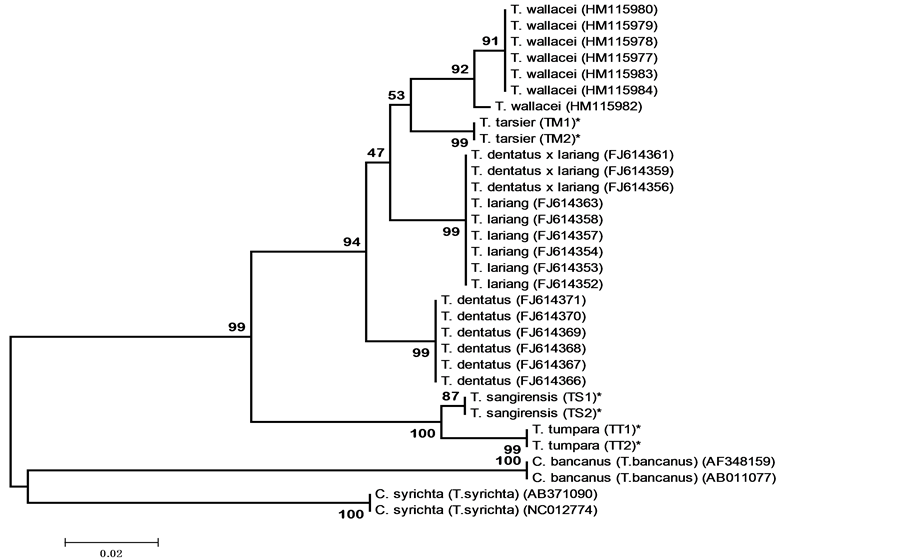
Figure 2. Phylogenetic tree based on nucleotide sequences had been constructed by Neighbor-Joining method. NJ-1000 BR, substitution models: Tamura-Nei model, (TN93 + G). Numbers at the branches are bootstrap values. Note: species marked with an asterisk are species sampled.
4. Discussion
Prior classification of tarsier consisted of C. bancanus (T. bancanus), C. syrichta (T. syrichta) and T. tarsier (T. spectrum) [7] . These three species are closely related [31] . Recently, genera and species of the Tarsiidae family originally known as a monotypic genus, right now this family consists of three genera [9] . These three genera are Chepalopachus, inhabiting the biogeographic region of Borneo and Sumatra, Carlito known as the Philippine tarsier and Tarsius known as east tarsier or Sulawesi tarsier or Tarsius tarsier-complex. Striking morphological characteristic differences of all three genera are mainly in the form of teeth, long legs and arms, tail end hair (tail-tuft) and mammary gland. Most Tarsius forms social group and has a duet song; Carlito does not form social groups in the wild but could socialize in captivity, as well as has no duet song, and Chepalophacus does not form social groups in the wild, as well as can not socialize even in captivity and has no duet song [9] .
Some species of Tarsius tarsier complex had separated from the other Sulawesi tarsier, because these species inhabit separate biogeographic region of Sulawesi mainland (See Figure 4). Both species of T. sangirensis and T. tumpara inhabit the archipelago biogeographic region known as the Sangihe archipelago. T. tumpara inhabits Siau island but T. sangirensis inhabits Sangihe island. Two islands are located at a distance of ± 60 km and restricted by the deep sea. T. sangirensis and T. tumpara are not allied with the Philippine tarsier C. syrichta, although their biogeographic regions are close to each other. In other hand C. syrichta is allied with C. bancanus, although their biogeographic regions are relatevely far apart. T. tumpara is subtly different from T. sangirensis, but both are significantly different from other tarsier of the Tarsius tarsier complex. Morphological features related to rare tail hair of T. sangirensis and T. tumpara resemble the features of Philippine tarsier C. syrichta.
Related to the phylogenetic posisition of T. sangirensis, T. tumpara and T. tarsier this research result supports the hypothesis that T. sangirensis, T. tumpara are the sister takson and allied with Tarsius tarsier-complex [32] . Grouping T. sangirensis, T. tumpara together and grouping of T. tarsier to the large clusters of T. wallacei, T. dentatus and T. lariang are in accordance with distribution of Sulawesi tarsiers.
Related to the distribution of Sulawesi tarsiers, there were some distribution forms like Manado form, Libou form, Sejoli form, Tinombo form, Kamamora form, and Togian form [2] . The distribution of Sulawesi tarsiers had occured in part by events of Pleistocene vikarian and partly had been influenced by tectonic activities

Figure 3. Phylogenetic tree based on nucleotide sequence had been reconstructed by Maximum Likelihood (ML), Bootstrap 1000. Substitution Model: Tamura 3-parameter model (T92 + I). Branch Swap Filter: Very Strong. Numbers at the branches are bootstrap values. Note: species marked with an asterisk are species sampled.
occured before the Pleistocene era [3] .
Distribution of Sulawesi tarsier is closely related to that of vocalization forms (duet song). Distribution of vocalization forms of tarsiers of the northern and central parts of Sulawesi are in accordance with each species locality [33] . There was a presupposition saying that the vocal forms of tarsiers were different among the tarsier population in the south and southeast Sulawesi, as well as in offshore islands of Selayar, Buton and Kabane region [34] . The striking difference is related to their acoustic features; tarsier populations of south, southeast, and offshore islands of Sulawesi are classified as different species. Tarsier species having different acoustic duet song features are Tarsius dianae (T. dentatus), T. lariang, Togian tarsier, T. pelengensis.
This research result reinforces the fact that T. tarsier, T. sangirensis and T. tumpara are three distinctive species genetically, bioacousticly, morphologically as well as they have different distribution. This explanation is consistent with the hypothesis that speciation of tarsiers had occured as a result of the spread of proto-Sulawesi island, followed by various subsequent fragmentations [23] . The phylogenetic positions of the North Sulawesi tarsiers uncovered by this research are based only on 307 nt sequence of the cyt b gene, but these results are in accordance with the several publications reported [9] [33] [34] . To obtain more reliable results, the results of this
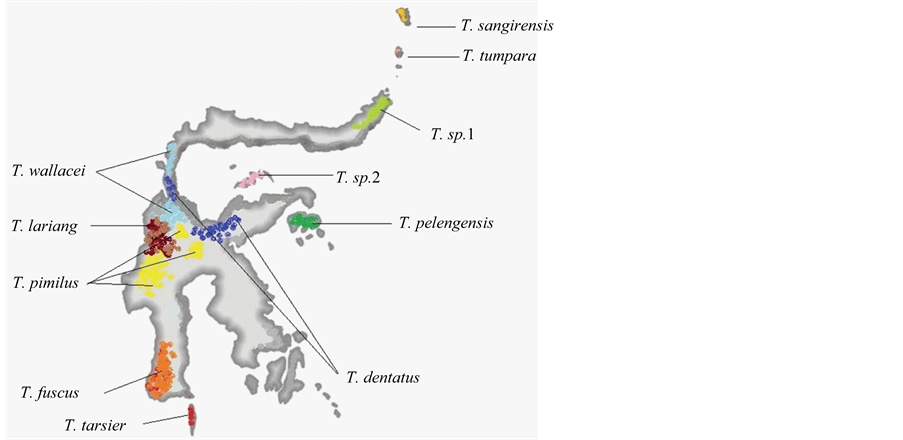
Figure 4. Distribution Map of Tarsier Found in Sulawesi. This figure modified from Groves and Shekelle, 2010.
study need to be examined again by cyt b gene sequence analysis as a whole, or to be examined using other applications of genetic markers.
5. Conclusions
Based on the cyt b gene partial sequence of North Sulawesi Tarsius sp., this result of research uncovers that T. tarsier, T. sangirensis and T. tumpara are closely related to Tarsius tarsier—complex, and relatively open related to C. bancanus and C. syrichta. This was supported by the genetic distance of each species uncouvered at NJ phylogenetic tree as well as at ML phylogenetic tree.
The positions of North Sulawesi tarsiers at the phylogenetic trees constructed based on the partial sequence of cyt b gene are in accordance with the classification based on morphology, distribution according to biogeographic region, as well as on distribution according to vocalization forms.
Acknowledgements
Great appreciation is given to Mr. S. Sahambangung (Talawit, Siau Sitaro), Mr. Utu (Kapeta, Siau Sitaro), Mr. Icon (Ahapatung, Sangihe) and Mr. D. Rotty (Matungkas, North Minahasa), who had helped to collect samples. This appreciation is also given to Mr. B. Kolondam who had helped the laboratory work.
References
- Hall, R. (2001) Cenezoic Recostructions of SE Asia and the SW Pacific: Changing Patterns of Land and Sea. In: Metcale, I., Smith, J.M.B., Morwood, M. and Davidson, I.D., Eds., Faunal and Floral migration in SE Asia-Australia, A. A. Balkema (Swets and Zietlinger Publishers), Lisse, 35-36.
- Shekelle, M. and Leksono, S.M. (2004) Strategi Konservasi di Pulau Sulawesi dengan Menggunakan Tarsius Sebagai Flagship Spesies. Biota, IX, 1-10.
- Wilson, K.A., McBride, M.F., Bodel, M. and Possingham, H.P. (2006) Prioritizing Global Conservation Efforts. Nature, 440, 337-340. http://dx.doi.org/10.1038/nature04366
- Myers, N., Mittermeier, R.A. and Mittermeier, C.G. (2000) Biodiversity Hotspots for Conservation Priorities. Nature, 403, 853-858. http://dx.doi.org/10.1038/35002501
- Evans, B.J., Supriatna, J., Andayani, J.N., Setiadi, M.I., Cannatella, D.C. and Melnick, D.J. (2003) Monkeys and Toads Define Areas of Endemism on Sulawesi. Evolution, 57,1436-1443. http://dx.doi.org/10.1111/j.0014-3820.2003.tb00350.x
- Hill, W.C.O. (1955) Primates: Comparative Anatomy and Taxonomy. II. Haplorhini: Tarsiidea. Edinburgh University Press, Edinburgh.
- Brandon-Jones, D., Eudey, A.A., Geissmann, T., Groves, C.P., Melnick, D.J., Morales, J.C., Shekelle, M. and Stewart, C.B. (2004) Asian Primate Classification. International Journal of Primatology, 25, 97-164.http://dx.doi.org/10.1023/B:IJOP.0000014647.18720.32
- Groves, C. and Shekelle, M. (2010) Genera and Spesies of Tarsiidae. International Journal of Primatology, 31, 1071-1082. http://dx.doi.org/10.1007/s10764-010-9443-1
- Shekelle, M., Meier, R., Wahyu, I., Wirdateti and Ting, N. (2010) Molecular Phylogenetics and Chronometrics of Tarsiidae Based on 12S mtDNA Haplotypes: Evidence for Miocene Origins of Crown Tarsiers and Numerous Species within the Sulawesian Clade. International Journal of Primatology, 31, 1083-1106.http://dx.doi.org/10.1007/s10764-010-9457-8
- Avise, J.C., Lansman, R.A. and Shade, R.O. (1979) Use Endonuclease to Measure Mitochondrial DNA Sequence Relatedness in Natural Population. I. Population Structure and Evolution in Genus Peromyscus. Genetics, 92, 279-295.
- Brown, W.M., Prager, E.M., Wang, A. and Wilson, A.C. (1982) Mitochondrial DNA Sequences of Primates: Tempo and Mode of Evolution. Journal of Molecular Evolution, 18, 225-239. http://dx.doi.org/10.1007/BF01734101
- Moritz, C., Dowling, T.E. and Brown, W.M. (1987) Evolution of Animal Mitochondrial DNA. Annual Review of Ecology and Systematics, 18, 269-292. http://dx.doi.org/10.1146/annurev.es.18.110187.001413
- Zubaidah, S. (2011) Integrasi Pendekatan Morfologi dan Molekuler DNA (Deoksibonucleic Acid) Dalam Taksonomi. Pidato Pengukuhan Guru Besar Dalam Bidang Genetika Pada FMIPA, Universitas Negeri Malang.
- Master, J.C., Boniotto, M., Crovella, S., Roos, C., Pozzi, L. and Delpero, M. (2007) Phylogenetic Relationships among the Lorisoidea as Indicates by Craniodental Morphology and Mitochondrial Sequence Data. American Journal of Primatology, 69, 6-15. http://dx.doi.org/10.1002/ajp.20322
- Randi, E. (1996) A Mitochondrial Cytochrome Phylogeny of the Alectoris partridges. Journal of Molecular Phylogenetics and Evolution, 6, 34-41.
- Roos, C., Nadler, T. and Walter, L. (2008) Mitochondrial Phylogeny, Taxonomy and Biogeography of Silver Langur Spesies Group (Trachypithecus cristatus). Molecular Phylogenetics and Evolution, 47, 629-636.http://dx.doi.org/10.1016/j.ympev.2008.03.006
- Lim, L.S., Ang, K.C., Mahani, M.C., Shahrom, A.W. and Md-Zain, B.M. (2010) Mitochondrial DNA Polymorphism and Phylogenetic Relationships of Proto Malays in Peninsular Malaysia. Journal of Biological Sciences, 10, 71-83. http://dx.doi.org/10.3923/jbs.2010.71.83
- Karanth, K.P., Lalji, S., Collura, R.V. and Beth, S.C. (2008) Molecular Phylogeny and Biogeography of Langurs and Leaf Monkey of South Asia. Molecular Phylogenetics and Evolution, 46, 683-694. http://dx.doi.org/10.1016/j.ympev.2007.11.026
- Matsui, A., Rakotondraparany, F., Munechika, I., Hasegawa, M. and Horai, S. (2009) Molecular Phylogeny and Evolution of Prosimians Based on Complete Sequences of Mitocondrial DNAs. Gene, 441, 53-66.
- Schmitz, J., Ohme, M. and Zischler, H. (2002) The Complete Mitochondrial Sequence of Tarsius bancanus: Evidence for an Extensive Nucleotide Compositional Plasticity of Primate Mitochondrial DNA. Molecular Biology and Evolution, 19, 544-553. http://dx.doi.org/10.1093/oxfordjournals.molbev.a004110
- Merker, S., Driller, C., Dahruddin, H., Wirdateti, Sinaga, W., Farajallah, D.P. and Shekelle, M. (2010) Tarsius wallacei: A New Tarsier Species from Central Sulawesi Occupies a Discontinuous Range. International Journal of Primatology, 31, 1107-1122. http://dx.doi.org/10.1007/s10764-010-9452-0
- Driller, C., Perwitasari-Farajallah, D., Zischler, H. and Merker, S. (2009) The Social System of Lariang Tarsiers (Tarsius lariang) as Revealed by Genetic Analysis. International Journal of Primatology, 30, 267-281. http://dx.doi.org/10.1007/s10764-009-9341-6
- Merker, S., Driller, C., Perwitasari-Farajallah, D., Pamungkas, J. and Zischler, H. (2009) Elucidating Geological and Biological Processes Underlying the Diversification of Sulawesi Tarsiers. Proceedings of the National Academy of Sciences of the United States of America, 106, 8459-8464. http://dx.doi.org/10.1073/pnas.0900319106
- Farias, I.P., Orti, G., Sampaio, I., Schneider, H. and Meyer, A. (2001) The Cytochrome b Gene as a Phylogenetic Marker: The Limits of Resolution for Analyzing Relationships among Cichlid Fishes. Journal of Molecular Evolution, 53, 89-103. http://dx.doi.org/10.1007/s002390010197 PMid:11479680
- Widayanti, R., Solihin, D.D., Sajuthi, D. and Dyah Perwitasari, R.R. (2004) Kajian Penanda Genetik Gen Cytochrome B Pada Tarsius sp. Jurnal Sain Veteriner, 24, 1-8.
- Md-Zain, B.M., Lee, S.J., Lakim, M., Ampeng, A. and Mahani, M.C. (2010) Phylogenetic Position of Tarsius Bancanus Based on Partial Cytochrome b DNA Sequences. Journal of Biological Science, 10, 348-354. http://dx.doi.org/10.3923/jbs.2010.348.354
- Kocher, T.D., Thomas, W.K., Meyer, A., Edwards, S.V., Paabo, S., Villablanca, F.X. and Wilson, A.C. (1989) Dynamics of Mitokondrial DNA Evolution in Animals: Amplification and Sequencing with Conserved Primers. Proceedings of the National Academy of Sciences of the United States of America, 86, 6196-6200. http://dx.doi.org/10.1073/pnas.86.16.6196
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmough, F. and Higgins, D.G. (1997) The Clustal-X Window Interface: Flexible Strategies for Multiple Sequences Alignment Aided by Quality Analysis Tools. Nucleic Acids Research, 25, 4876-4882. http://dx.doi.org/10.1093/nar/25.24.4876
- Saitou, N. and Nei, M. (1987) The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Molecular Biology and Evolution, 4, 406-425.
- Felsenstein, J. (1981) Evolutionary Trees from dna Sequences: A Maximum Likelihood Approach. Journal of Molecular Evolution, 17, 368-376. http://dx.doi.org/10.1007/BF01734359
- Musser, G.G. and Dagosto, M. (1987) The Identity of Tarsius Pumilus, a Pygmy Species Endemic to the Montane Mossy Forests of Central Sulawesi. American Museum Novitates, 2867, 1-53.
- Shekelle, M., Groves, C., Merker, S. and Supriatna, J. (2008) Tarsius tumpara: A New Tarsier Spesies from Siau Island, North Sulawesi. Primata Conservation, 23, 55-64. http://dx.doi.org/10.1896/052.023.0106
- Shekelle, M. (2008) Distribution of Tarsiers Acoustic Forms, North and Central Sulawesi: With Notes on The Primary Taxonomy of Sulawesi’s Tarsiers. Primates of The Oriental Night, CBCS-UI, Faculty of Mathematics and Science, University of Indonesia, Depok, 35-50.
- Burton, J.A. and Nietsch, A. (2010) Geographical Variation in Duet Songs of Sulawesi Tarsiers: Evidence for New Cryptic Species in South and Southeast Sulawesi. International Journal of Primatology, Springer Science + Business Media, LLC 2010.


