Open Journal of Preventive Medicine
Vol.4 No.1(2014), Article ID:42149,10 pages DOI:10.4236/ojpm.2014.41005
An intervention to reduce psychosocial and biological indicators of stress in African American lupus patients: The balancing lupus experiences with stress strategies study
![]()
1Institute for Partnerships to Eliminate Health Disparities, Arnold School of Public Health, University of South Carolina, Columbia, USA; *Corresponding Author: willi425@mailbox.sc.edu
2Institutional Assessment and Compliance, University of South Carolina, Columbia, USA
3Division of Rheumatology and Immunology, Department of Medicine, Medical University of South Carolina, Charleston, USA
4 Medical Service Ralph H. Johnson VA Medical Center, Charleston, USA
Copyright © 2014 Edith M. Williams et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Edith M. Williams et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 24 September 2013; revised 8 December 2013; accepted 25 December 2013
KEYWORDS
Lupus; Stress; African-American; Self-Management
ABSTRACT
Objective: Very little is known about the impact of psychosocial stress on African American lupus patients. Due to the exposure of African Americans to a unique trajectory of stressors throughout life, it may be critical to understand the relationship between psychosocial stress and underlying biological mechanisms that influence disease activity and pathology in this high risk group. Methods: The Balancing Lupus Experiences with Stress Strategies (BLESS) study piloted the validated “Better Choices, Better Health” Chronic Disease Self-Management Program (CDSMP) in 30 African-American lupus patients participating in the SLE Clinic Database Project at the Medical University of South Carolina (MUSC). Measures of psychosocial and biological indicators of stress were collected in all of the patients in each of the study conditions before and after intervention activities, as well as four months’ post-intervention, to assess the effectiveness of the program in reducing perceived and biological indicators of stress. Results: Participation in the workshops had large effects upon depression (d = 1.63 and d = 1.68), social/role activities limitations (d =1.15), health distress (d = 1.13 and d = 0.78), fatigue (d = 1.03), pain (d = 0.96), and lupus self-efficacy (d = 0.85). Neither the differences in cortisol or DHEA levels preand post-intervention were found to be significantly different between intervention participants and controls. Conclusion: The intervention workshops acted to reduce perceived stress and improve quality of life. Our findings imply that comparable, if not more significant gains in relevant health indicators are possible in African American patients when provided the opportunity to participate in CDSMP’s.
1. INTRODUCTION
A number of studies have investigated chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations [1]. Acute stress responsivity (including stress reactivity and recovery of hypothalamic-pituitary-adrenal [HPA] axis, autonomic, and cardiovascular systems) as well as disturbances in immune regulation as a result of stress has been examined in healthy subjects [1-3]. It is believed that stress worsens the clinical symptomatology of patients with lupus. Recommendations to lupus patients to avoid stress are based on numerous studies that have demonstrated associations between daily stress and disease exacerbations [4-8]. Investigators have found that particularly daily stress with social relationships and social duties may be factors related to the course of disease activity [6]. Daily stress is related impairments in visual memory, fluency and attention in patients with SLE [5].
Even less is known about this phenomenon in African American lupus patients. In the United States, blacks have three-fold higher incidence and prevalence rates of SLE as well as cause-specific mortality rates, compared with whites [9-11]. It has been suggested that African Americans are exposed to a unique set of risk factors that lead to a pattern of cumulative disadvantage over time. High rates of unemployment, poverty, violent crime, incarceration, and homicide among African American adults reflect this accumulation of disadvantage at multiple transition points during their development and across the life course [12,13]. It is highly likely that early childhood exposure to segregated, economically impoverished neighborhoods created by institutionalized racism adversely affects child health and growth and sets the Black child on a low education and economic trajectory that increases the risk of poor physical and mental health in adulthood [14]. Additional stressors include deprivation of resources and facilities, differential exposure to health risks in the physical environment because of economically disadvantaged neighborhoods and poor quality housing, higher costs of goods and services in deprived areas, as well as roles of social networks and social capital, which often give rise to peer pressure against academic achievement and in support of crime and substance use [12,13,15,16].
A large body of evidence supports health-promoting programs in stress management as successful in helping people improve their health practices and related health conditions [17]. Based on reviews of scientific literature, investigators have suggested that therapeutic interventions should be proposed to reduce psychological distress to improve quality of life and possibly moderate the evolution of chronic and unpredictable diseases like SLE [4]. Cognitive-behavioral stress management (CBSM) programs effectively reduce cortisol responses to acute psychosocial stress [18], and such techniques have also resulted in short-term improvement in pain, psychological function, and perceived physical function among persons with SLE [19]. Programs designed to reduce stress levels of chronically ill patients have also included support therapy, lifestyle interventions incorporating elements of yoga or other similar disciplines, and minisessions on depression, adaptive coping strategies, and body image [19-21]. Two programs that have been shown to be successful in improving conditions in patients with arthritis are the Arthritis Self-Management Program (ASMP) and the generic Chronic Disease SelfManagement Program (CDSMP). Each program incorporates six weeks of peer led sessions ranging in diseasespecific and more general self-help content. Both programs have demonstrated significant improvements in health distress, self-reported global health, and activity limitation, with trends toward improvement in self efficacy and mental stress management [22].
There are multiple potential mechanisms by which everyday and lifetime stress may adversely affect disease pathology in lupus patients. While existing stress management programs have demonstrated improvements in biological markers of stress, psychological function, and physical function, interventions have not demonstrated reductions in endpoints specific to African American lupus patients. Due to the exposure of African Americans to a unique trajectory of stressors throughout the life course, it may be critical to understand the relationship between psychosocial stress and underlying biological mechanisms that influence disease activity and pathology in this high risk group. Therefore, we piloted a validated stress management program in African American lupus patients and incorporated valid measures of psychosocial and neuroendocrine responses to stress to assess its effectiveness in reducing perceived and biological indicators of stress and improving quality of life in African American lupus patients.
2. PATIENTS AND METHODS
2.1. Patients and Entry Criteria
Patients invited to participate in the Balancing Lupus Experiences with Stress Strategies (BLESS) study were African American systemic lupus erythematosus (SLE) patients attending rheumatology clinics at the Medical University of South Carolina (MUSC). All SLE patients met at least four components of the 1997 ACR revised criteria for SLE [23], were 18 years of age or older, and had not previously participated in a self management program. The total number of individual patients with SLE followed by clinicians at MUSC was 1121 between 2009 and 2012. The total number of new patients with SLE seen by clinicians at MUSC between 2011 and 2012 was 176, of which 61% were African-American and 88% were female. Patients invited to participate in the proposed study were lupus patients participating in a longitudinal observational web-based SLE Database at MUSC. There were 402 patients with lupus enrolled in the Database during enrollment in this study. All patients met at least four American College of Rheumatology (ACR) criteria. Patients in the Database were characterized longitudinally for disease activity and quality of life. The vast majority of subjects have had serum/urine/DNA/ RNA specimens collected and stored at −80˚C. As part of the informed consent process, participants agreed to future re-contact regarding other research studies. MUSC’s SLE cohort is geographically diverse, representing more than 60 South Carolina and North Carolina counties. Of the 402 patients with lupus, 336 were African-American, and 218 of whomwere Gullah African-American from the Sea Islands of South Carolina and Georgia. Additionally, as part of the associated SLE in Gullah Health (SLEIGH), 166 unrelated ageand gender-matched Gullah controls and 216 family-member Gullah controls were enrolled.
2.2. Recruitment and Randomization Procedures
Eligible patients from within this cohort were invited to participate by a mailed letter that described the study and in person, during regular clinic visits. Interested patients were randomly assigned to the intervention or usual medical care alone. Prior to study participation, subjects completed informed consent documents approved by the University of South Carolina (USC) and Medical University of South Carolina (MUSC) Institutional Review Boards.
2.2.1. Experimental Group
Intervention activities consisted of six weekly sessions of the “Better Choice, Better Health” Chronic Disease Self-Management Program (CDSMP), developed by Stanford University and also offered in a variety of community settings (e.g., senior centers, churches, hospitals) [24,25]. People with different chronic health problems attend together and support one another in making positive changes in their health. Workshops were facilitated by two trained leaders, one or both of whom were non-health professionals with a chronic disease themselves. Subjects covered included: 1) techniques to deal with problems such as frustration, fatigue, pain and isolation, 2) appropriate exercise for maintaining and improving strength, flexibility, and endurance, 3) appropriate use of medications, 4) communicating effectively with family, friends, and health professionals, 5) nutrition, and, 6) how to evaluate new treatments [22,26]. It is the process in which the program is taught that makes it effective. Classes are highly participative, and mutual support builds the participants’ confidence in their ability to manage their health and maintain active and fulfilling lives [22,25]. Weekly sessions lasted approximately two hours and were and administered by trained leaders affiliated with the South Carolina Department of Health and Environmental Control (SCDHEC). Sessions were administered in a group setting with all of the patients randomly assigned to the intervention arm of the study attending the same sessions together. Sessions were scheduled at a location that was familiar to participants at times that were convenient for the entire group and refreshments were provided.
2.2.2. Control Group
Control group patients received their usual care with the addition of a mailed book that provided tips for living a healthy life with a chronic condition [27]. Follow up phone calls were made to participants in both study arms in the period between intervention sessions to assess adherence in the intervention group and gauge general study satisfaction in both groups.
2.3. Measures
Data collected weredemographic (age, sex, etc.), behavioral (healthcare utilization, coping strategies, etc.), and biological (saliva samples for neuroendocrine responses to stress). Pre-intervention measures were obtained. Immediately following the six-session intervention phase and then at four months post-intervention, measures were repeated. Previous investigations have shown marked changes in neuroendocrine responses to stress and other biological markers of disease when postintervention measures are collected immediately following study activities [7,8]. Other studies have observed effects of the neuroendocrine stress response on the immune response between three months and two years post-intervention [4-6]. Those assigned to the control group completed post-intervention follow-up evaluations on the same schedule as those assigned to the experimental group.
Psychosocial stress was assessed by five validated measures. The State-Trait Anxiety Inventory (STAI) was initially conceptualized as a research instrument for the study of anxiety in adults [28]. It is a self-report assessment device which includes separate measures of state and trait anxiety. STAI is a 20 item 4 point likert scale, where responses range from “not at all” to “very much so” (where “0” represents no likelihood of experiencing a form of anxiety and “4” represents high likelihood of experiencing anxiety). The Arthritis Self-Efficacy Scale pain and other symptoms sub-scale[29] consists of 11 items designed to measure confidence in one’s ability to manage the pain, fatigue, frustration, and other aspects of disease; it was reworded in previous investigations to reflect lupus rather than arthritis[19]. The scale consists of ranges from 0 - 100, with three break points of “very uncertain” (0), “moderately uncertain” (50), and “very certain” (100). The Perceptions of Racism Scale is a 20- item self-report inventory concerning medical and lifetime experiences of discrimination [30]. The Perceptions of Racism Scale is on a 7 point likert scale whereas “several times a day” (0) represents the likelihood that an individual experiences racism more frequently and “never” (0) the opposite measurement effect. Health distress was assessed using a modified version of the Medical Outcomes Study (MOS) health distress scale, adapted by the Stanford Patient Education Research Center [31]. This scale consists of 3 questions in a likert format, whereas “none of the time” (0) indicates a patient does not experience health distress and “all of the time” (5) representing the opposite. The Beck Depression Inventory is a 21-question multiple-choice self-report inventory for measuring the severity of depression [32], in a 4 point likert scale format (lower end of the scale representing normality in emotional coping and the upper end representing extreme depression). It is composed of items relating to symptoms of depression such as hopelessness and irritability, cognitions such as guilt or feelings of being punished, as well as physical symptoms such as fatigue, weight loss, and lack of interest in sex. Quality of life was assessed using two instruments that describe a spectrum of quality of life outcomes. The LUP-QOL incorporates the Medical Outcomes Study (MOS) Short Form 36 Health Survey (SF-36) and the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), which are reliable and valid instruments that are frequently used in quality of life studies of persons with lupus [33,34]. The questionnaire includes questions pertaining to physical function, role function, social function, mental health, health perception and pain. Behavior Change was assessed using Stanford Patient Education Research Center Questionnaires assessing medical outcomes such as hospital visits, illness intrusiveness, and use of stress management techniques [26, 35]. These are behavior change scales, modified from the Medical Outcomes Study, to determine if participants are practicing cognitive stress reduction (pain reduction) and non-cognitive (mental stress management/relaxation) techniques. These scales also assess whether key behaviors concerning communicating with health care providers and health care utilization have changed.
To assess the effectiveness of implementing a validated stress management program in African American lupus patients, valid measures of both psychosocial and neuroendocrine responses to stress were included to determine whether intervening at the psychosocial level translated into relevant effects at the biological level. Cortisol (hydrocortisone) is a steroid hormone that is released in response to stress, acting to restore homeostasis [36]. Because the amount of cortisol present in the blood undergoes diurnal variation (the level peaks in the early morning and reaches its lowest level at about midnight-4 am, or three to five hours after the onset of sleep), efforts were made to collect samples at a common time of day across participants. Dehydroepiandrosterone (DHEA) is the major steroid hormone secreted by the adrenal cortex, suggesting its role in the immune and stress response. DHEA has been implicated in a broad range of biological effects that include anti-depressant effects and protection from cortisol over-concentration [36-38]. To ensure the integrity of biological samples for future study, strict protocols were adhered to for specimen collection and storage. Saliva was collected by passive drool into polypropylene vials on ice, processed in refrigeration, and frozen at −20˚C within 4 hours of collection and then −80˚C within 24 hours. If this method is used and samples batched at −80˚C, samples are stable for up to 1.5 years (per manufacturer stability studies). All samples were analyzed within this timeframe.
2.4. Statistical Analyses
Although the study was compromised of 30 participants, two of the participants assigned to the intervention group did not attend any intervention sessions and were eliminated from all post-intervention analyses. In addition, several participants did not complete post-intervention questionnaires and were also excluded from analyses. Therefore, data were analyzed on 30 participants at baseline, 25 (N = 13 for control group and N = 12 for intervention group) at post-intervention, and 22 (N = 12 for control group and N = 10 for intervention group) at four months post-intervention. “Per-protocol” (or the elimination of any participants that did not complete treatment) rather than “intent-to-treat” (inclusion of all participants regardless of whether they completed treatment) analyses were undertaken due to missing survey data at specified data collection points from most of the excluded participants. Intent-to-treat analyses would have been suitable if excluded participants had completed the study (i.e., provided responses at specified data collection points), even if they did not receive treatments they should have (i.e., completed intervention sessions). Given participant dropout and the investigative nature of this study, statistical tests were deemed inappropriate to assess changes from baseline to post-intervention and at four months post-intervention due to violation of assumptions and low power. Therefore, descriptive statistics of measures assessing perceived stress, quality of life and biological markers of stress at each collection point are reported along with correlation to estimate the effect of the intervention. In addition, Cohen’s (1988) effect sizes (d) were computed as a measure of power using the software program g-power. Typically, Cohen’s d is only reported as a unidirectional statistic ranging from 0 to infinity (typically not above 2 or 3). The magnitude of Cohen’s d is similar to that of Pearson’s r, wherein a value of 0.2 indicates small effect, 0.5 indicates medium effect, and 0.8 indicates large effect. Reporting Cohen’s d was chosen for this study because it is independent of sample size, unlike statistical methods such as t-tests. In this case, effect sizes show the actual magnitude of the difference—not just how likely the results are to have occurred by chance.
3. RESULTS
Thirty participants were randomly assigned to intervention and control groups. All participants were African American, 28 were female, and more than half either attended trade school or college (Table 1).
Group means were significantly different on several measures of perceived stressimmediately following the intervention and four months post-intervention. Specifically, participants in the intervention group reported improved self-efficacy pertaining to coping with having lupus over controls (M = 19.17). Although differences in cognitive symptom management were non-significantly different, given the small sample size, it is worth noting that the average change score of intervention participants (M = 0.92) was higher than those in the control group (M = 0.33). In addition, participants in the intervention group reported sizable decreases in symptoms of depresssion on the BDI-II (M = −7.21). Conversely, those in the control group had decreases in reported self-efficacy (M = −3.21), only minimal changes in cognitive symptom management (M = 0.33) and increases in reported depression (M = 2.89). No significant differences were found on the State-Trait Anxiety Inventory. Similar patterns were evidenced at four months post-intervention compared to baseline. Although mean scores on the Lupus Self-Efficacy survey and BDI-II were improved compared to baseline, both were reduced compared to post-intervention. Lupus Self-Efficacy scores decreased an average of 9.50 points between post-intervention and four months post-intervention and depression scores decreased 2.71. Those in the control group had similar mean difference scores as compared to post-intervention (Table 2).
Experimental group participants displayed improve-
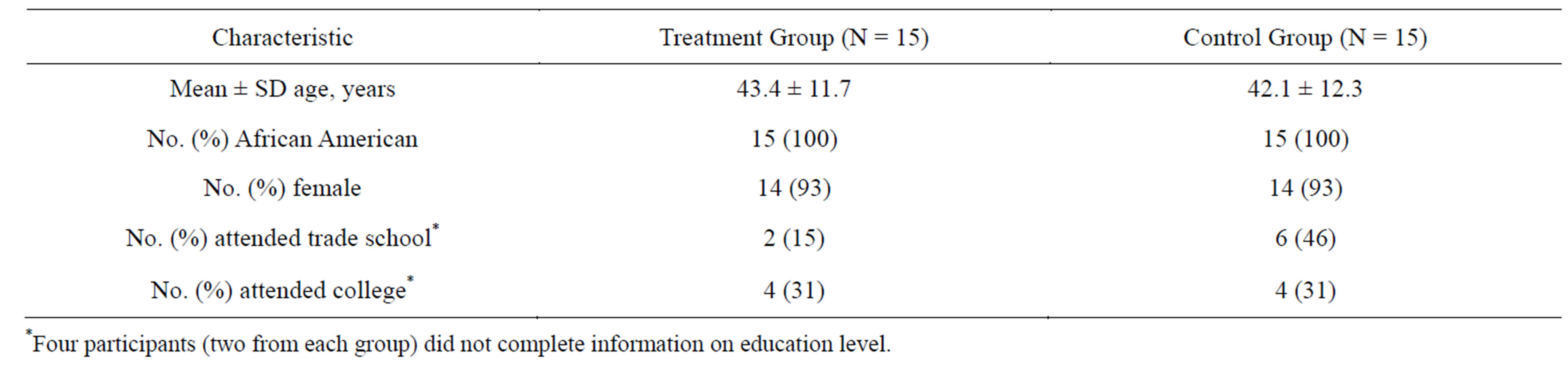
Table 1. Characteristics of study participants.
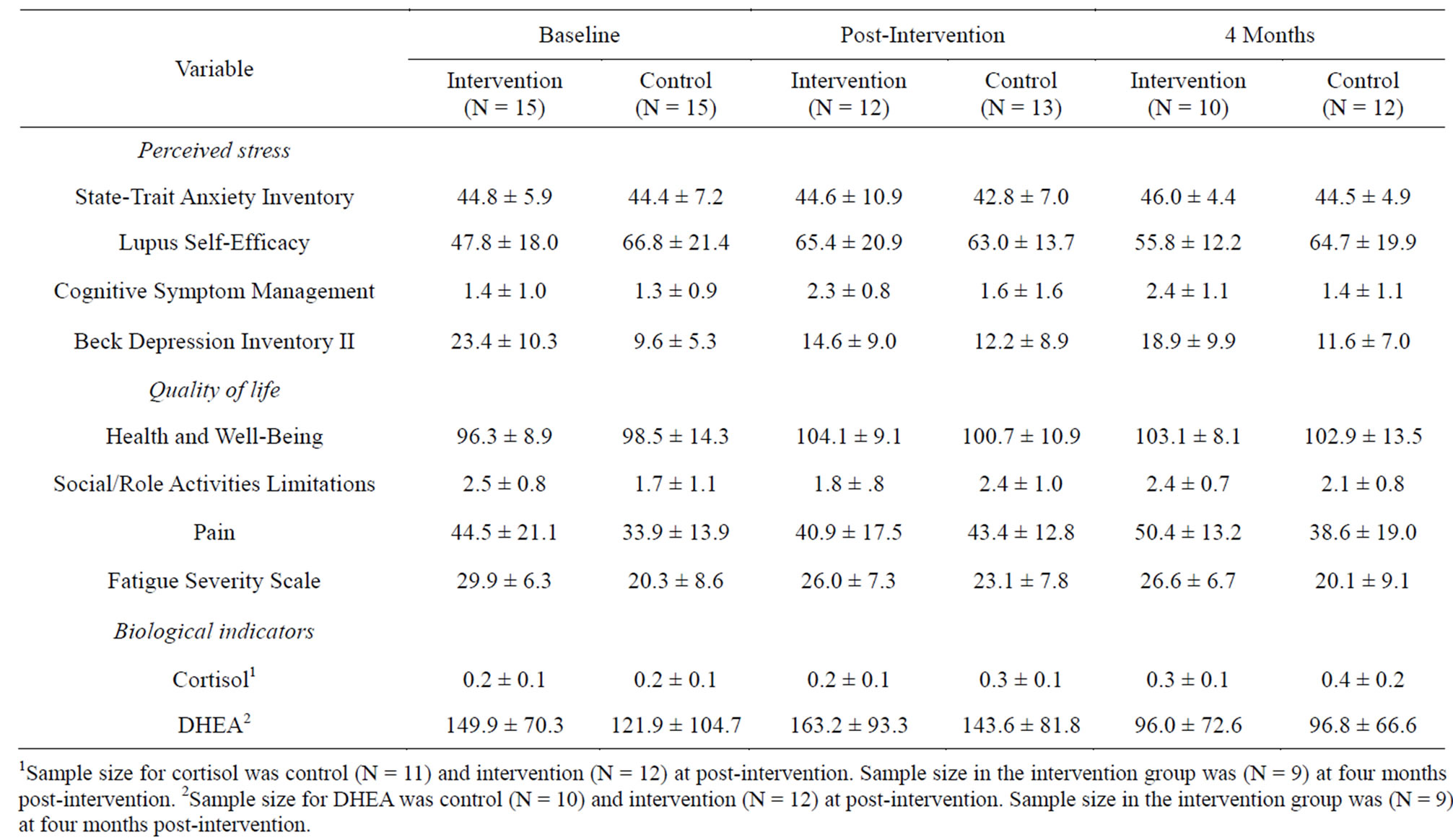
Table 2. Means and Standard Deviations of Variables by Intervention and Control Groups.
ments on each measure of quality of life from baseline to post-intervention (see Table 2). Overall, participants in the intervention group had higher difference scores on the Health and Well-Being survey after the intervention (M = 7.17). They reported less limitations in social/role activities (M = −0.30), pain (M = −4.67), and fatigue (M = −2.67) compared to baseline. While control group participants also had a modest improvement in reported health and well-being (M = 1.80), they reported more limitations in social/role activities (M = 0.69), increased pain (M = 9.46), and more fatigue (M = 4.23) (Table 3). At four months post-intervention, participants in the intervention group continued to have higher scores on the Health and Well-Being survey (M = 6.40) compared to baseline as well reductions in social/role limitations (M = −0.25) and fatigue (M = −1.60). However, they reported more pain (M = 8.70). Participants in the control group had small increases in reported health and well-being (M = 0.71), but reported slightly more social/role limitations (M = 0.38) and fatigue (M = 0.83) and more pain (M = 6.83) (Table 3).
There were no significant differences found between the experimental and control groups in terms of biological indicators of stress (Table 4). Only negligible differ-
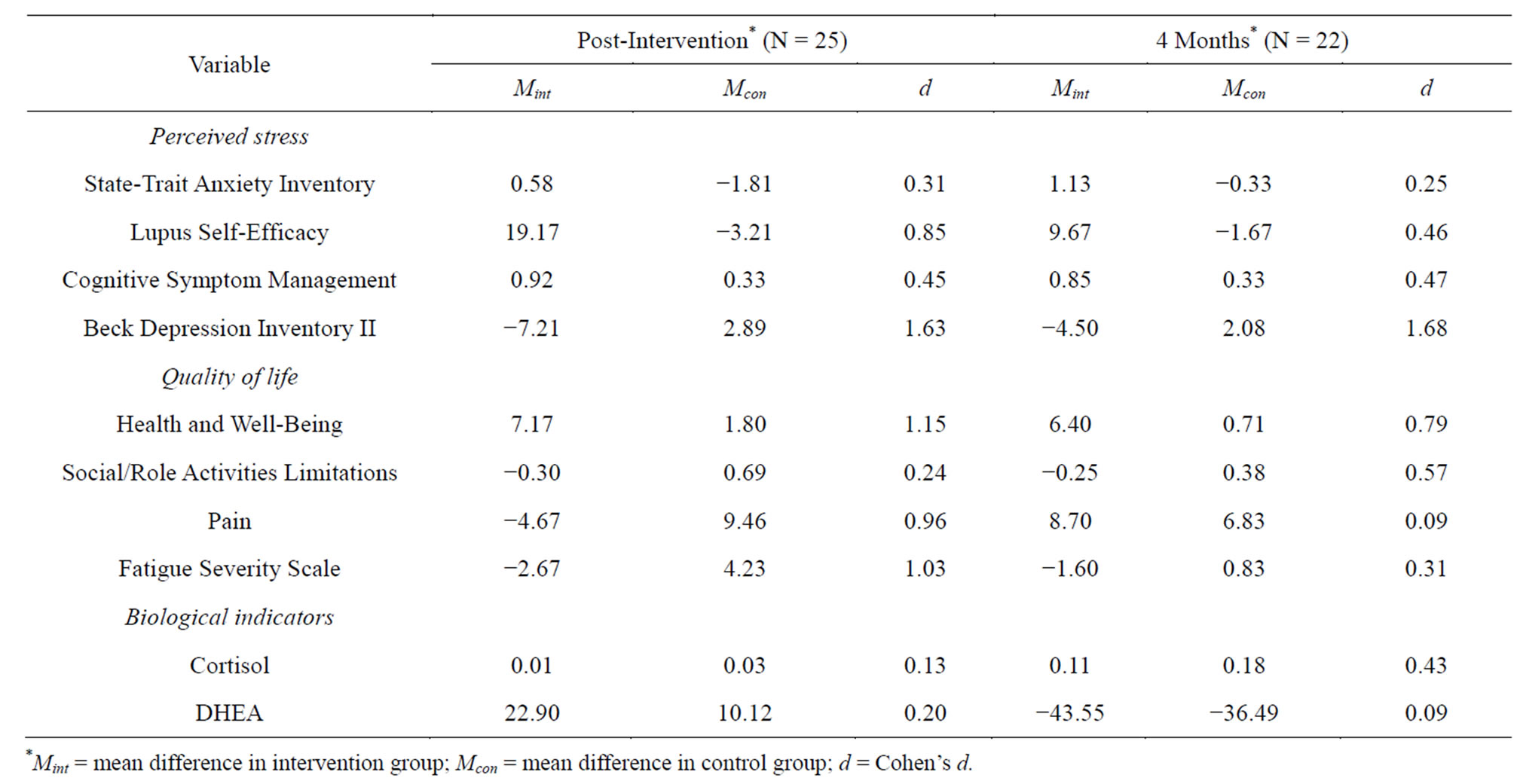
Table 3. Mean difference scores between baseline and post-intervention by group.
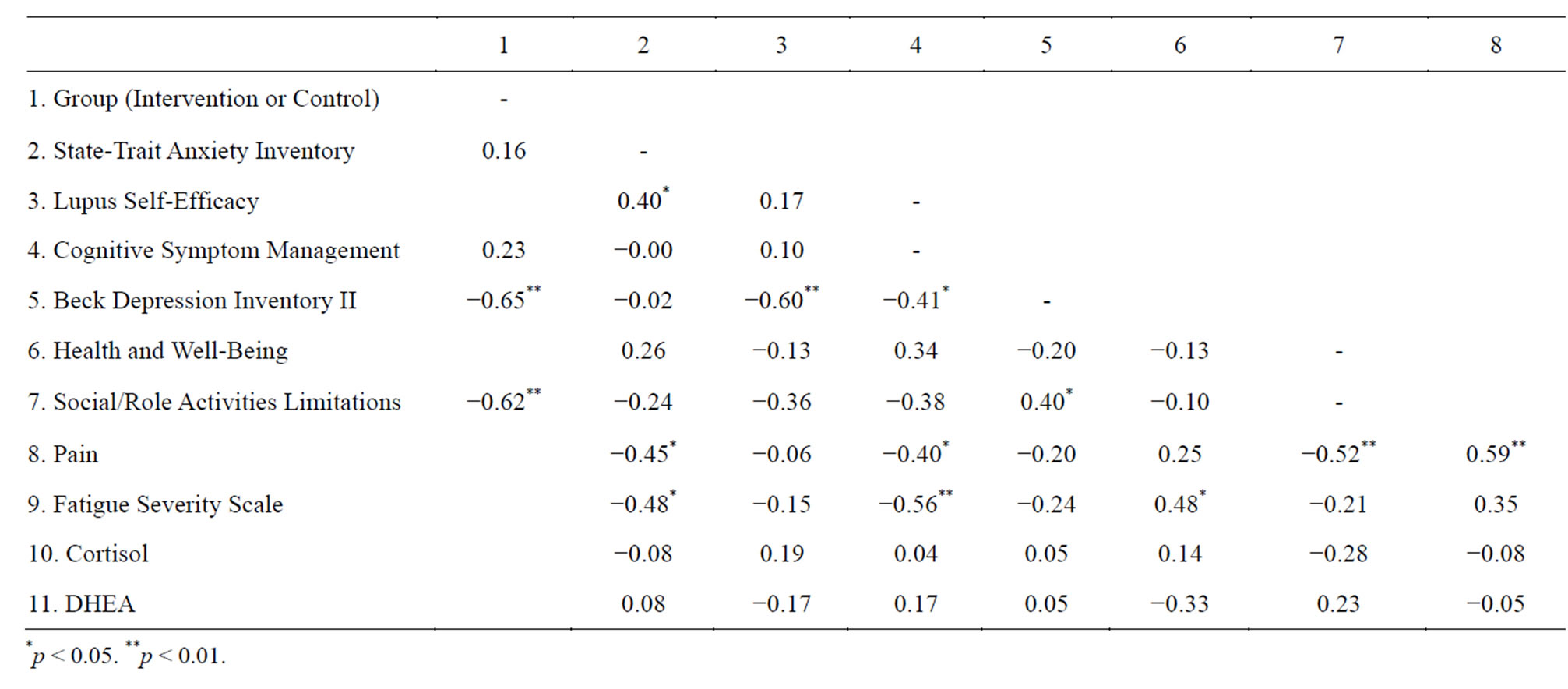
Table 4. Correlation matrix of difference scores between baseline and post-intervention.
ences were found in cortisol levels between baseline and post-intervention in both the intervention and control groups. Neither the differences in cortisol or DHEA levels preand post-intervention were found to be significantly different between intervention participants and controls. At four months post-intervention, there were modest increases in cortisol levels in both groups. DHEA levels at post-intervention were higher in both the intervention (M = 22.90) and control group (M = 10.12). However, at four months post-intervention compared to baseline, both groups had decreases in DHEA (Table 3).
Results indicate that participation in the workshops had large effects upon depression (d = 1.63), social/role activities limitations (d = 1.15), health distress (d = 1.13), fatigue (d = 1.03), pain (d = 0.96), and lupus self-efficacy (d = 0.85). In all of the effects, the intervention workshops acted to reduce perceived stress and improve quality of life as assessed by the aforementioned measures. At four months post-intervention, large effects were again found on depression (d = 1.68) and health and well-being (d = 0.78).
4. DISCUSSION
This pilot demonstrated that a validated chronic disease self-management program to support and assist participants in finding practical ways to deal with pain, fatigue, and stress, as well as introduce better nutrition and exercise choices, new treatment options, and better ways to talk with doctors and family about health matters could reduce perceived stress and improve quality of life in African American lupus patients. The intervention resulted in significant improvement in depression, social/role activities limitations, health distress, fatigue, pain, and lupus self-efficacy immediately following intervention activities and four months post-intervention. Overall, the control group began with better scores than the experimental group, but did not improve or even worsened post-intervention.
Self-efficacy has been correlated with SLE-related health outcomes in a number of studies [39,40]. In this pilot, we demonstrated that self-efficacy could be enhanced by our intervention. These results are consistent with other trials of counseling for SLE patients in a group setting that demonstrated significant improvements in psychological status [39,41]. These trials were conducted in predominantly white study samples but was similar to our pilot in the patient behaviors targeted; namely, self-care activities in managing fatigue, patient’s communication skills, removing barriers to medical care, medication self-management, symptom monitoring, and stress control methods. Our findings imply that comparable, if not more significant gains in relevant health indicators are possible in African American patients when provided the opportunity to participate in such an intervention.
Depression was improved by an approximate average of 9 points (post-intervention) and 5 points (four months post-intervention) on the BDI-II in intervention participants, which is greater than the minimum clinically significant change suggested for interpreting change scores in lupus trials [42-44]. Overall, depression findings were consistent with other studies that have observed higher depression scores in SLE cases compared with controls [43,45]. Our findings demonstrate that intervention participation resulted in the mean post-intervention depression score dropping below the normally accepted cut-off of 15 points for the BDI-II [43].
Fatigue improved by an approximate average of 4 points (post-intervention) and 3 points (four months postintervention) in intervention participants, which is greater than the minimum clinically significant change suggested for interpreting change scores in lupus trials [46,47]. It is notable that while the mean fatigue score for intervention participants decreased post-intervention, the mean fatigue score for control participants actually increased.
Pain decreased by an approximate average of 4 points (post-intervention), but then increased 6 points (four months post-intervention) in intervention participants, which suggests that intervention activities were effective for short-term pain relief, but gains were not sustained. Literature has shown that pain has been frequently reported as an unmet need by African American lupus patients [48], so future intervention efforts should target strategies for sustaining positive health outcomes that have been observed in other areas [26].
Overall, our results confirm findings from research done by other investigators, and support the importance of psychosocial factors in SLE. It seems that pain, fatigue, self efficacy, and depression go together and suggest that treating the disease alone does not adequately treat the underlying psychosocial elements that affect patients’ ability to cope with and manage disease. The clinical observation is that if the patient is depressed and unable to cope, they don’t tend to improve.
Various examinations of biological indicators of stress before and after induced stressful circumstances have demonstrated increases in levels of such markers as salivary cortisol and testosterone and urinary cortisol and neopterin, commensurate with stress induction [1-3]. Such examinations have also demonstrated attenuated decreases in stress markers after cessation of the stressinducing activity [1-3], providing objective evidence of neuroendocrine responses to stress in the absence of disease. Our findings suggest that the effects of this intervention had more subtle effects on biologic indicators of stress and more profound effects on coping with and managing disease. We do not attribute this trend to within-sample variability affecting our ability to observe significant differences. Other studies that have observed significant DHEA and cortisol levels among women exposed to stress over a specified time period have reported greater variability than was observed in the current study [49,50]. Additionally, our findings were consistent with another examination of diurnal cortisol and DHEA levels of women with rheumatoid arthritis in that we also observed no significant differences between the control and the experimental groups [50].
Given that this study was an exploratory pilot, the small sample size will reduce generalizability of the results. However, since Cohen’s d is a measure of the magnitude of the effect irrespective of sample size, we would expect a larger sample to improve the odds of significance, but would also expect the magnitude of the relationship to remain the same. Therefore, our results are still impressive in the perceived stress and quality of life domains. In spite of a limited sample size, our findings fill a critical gap left by similar investigations that have not been racially representative. This study clearly demonstrates that comparable interventions may be effective or even more effective in more racially diverse populations.
Additionally, our intervention was multi-faceted and combined elements of efficacy enhancement and problem solving. With our design, we cannot definitively separate the effects of each element. Further research with larger samples that are racially representative and can be randomized into varying levelsand elements of intervention is recommended to determine which kinds of programs work best, for which kinds of patients, and in which situations.
5. CONCLUSION
In summary, our pilot stress intervention combined elements of efficacy enhancement and problem solving in an understudied group. African American patients with SLE experienced significant improvements in depression, social/role activities limitations, health distress, fatigue, pain, and lupus self-efficacy. This widely available intervention has the potential to reduce health problems and costs in a debilitating, management-intensive chronic disease in the population subset at highest risk for the disease and should be more widely implemented and studied to more rigorously assess benefits.
REFERENCES
- Chida, Y. and Hamer, M. (2008) Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin, 134, 829-885. http://dx.doi.org/10.1037/a0013342
- Dunbar, P., Hill, J. and Neale, T. (1993) Urinary neopterin quantification indicates altered cell-mediated immunity in healthy subjects under psychological stress. Australian & New Zealand Journal of Psychiatry, 27, 495-501. http://dx.doi.org/10.3109/00048679309075808
- Hoglund, C., Axen, J. and Kemi, C. (2006) Changes in immune regulation in response to examination stress in atopic and healthy individuals. Clinical & Experimental Allergy, 36, 982-992. http://dx.doi.org/10.1111/j.1365-2222.2006.02529.x
- Bricou, O., Taïeb1, O., Baubet, T., Gal, B., Guillevin, L. and Moro1, M.-R. (2004) Stress and coping strategies in systemic lupus erythematosus. La Presse Medicale, 33, 1284-1292. http://dx.doi.org/10.1016/S0755-4982(04)98908-7
- Peralta-Ramirez, M., Coin-Mejias, M. and Jimenez-Alonso, J. (2006) Stress as a predictor of cognitive functioning in lupus. Lupus, 15, 858-864. http://dx.doi.org/10.1177/0961203306071404
- Pawlak, C., Witte, T. and Heiken, H. (2003) Flares in patients with systemic lupus erythematosus are associated with daily psychological stress. Psychotherapy and Psychosomatics, 72, 159-165. http://dx.doi.org/10.1159/000069735
- Birmingham, D., Nagaraja, H. and Rovin, B. (2006) Fluctuation in self-perceived stress and increased risk of flare in patients with lupus nephritis carrying the serotonin receptor 1A-1019G allele. Arthritis & Rheumatism, 54, 3291-3299. http://dx.doi.org/10.1002/art.22135
- Peralta-Ramirez, M., Jiménez-Alonso, J., Godoy-García, J. F., Pérez-García, M. and on behalf of the group Lupus Virgen de las Nieves. (2004) The effects of daily stress and stressful life events on the clinical symptomatology of patients with lupus erythematosus. Psychosomatic Medicine, 66, 788-794. http://dx.doi.org/10.1097/01.psy.0000133327.41044.94
- Oates, J., Levesque, M. and Hobbs, M. (2003) Nitric oxide synthase 2 promoter polymorphisms and systemic lupus erythematosus in african-americans. Journal of Rheumatology, 30, 60-67.
- Alarcon, G., Beasley, T. and Roseman, J. (2005) Ethnic disparities in health and disease: The need to account for ancestral admixture when estimating the genetic contribution to both (LUMINA XXVI). Lupus, 14, 867-868. http://dx.doi.org/10.1191/0961203305lu2184xx
- Rus, V. and Hochberg, M. (2002) Chapter 4 the epidemiology of systemic lupus erythematosus. In: Wallace, D. and Hahn, B. Eds., Dubois’ Lupus Erythematosus, Lippincott Williams & Wilkins, Philadelphia, 65-83.
- Wyatt, S., et al. (2003) Racism and cardiovascular disease in African Americans. American Journal of Medical Sciences, 325, 315-331. http://dx.doi.org/10.1097/00000441-200306000-00003
- Williams, D. (2003) The health of men: Structured inequalities and opportunities. American Journal of Public Health, 93, 724-731. http://dx.doi.org/10.2105/AJPH.93.5.724
- Hertzman, C. and Wiens, M. (1996) Child development and long-term outcomes: A population health perspective and summary of successful interventions. Social Science & Medicine, 43, 1083-1095. http://dx.doi.org/10.1016/0277-9536(96)00028-7
- Cattell, V. (2001) Poor people, poor places, and poor health: The mediating role of social networks and social capital. Social Science & Medicine, 52, 1501-1516. http://dx.doi.org/10.1016/S0277-9536(00)00259-8
- Williams, D. and Collins, C. (2001) Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Reports, 116, 404-416.
- O’Donnell, M. (2004) Health-promotion behaviors that promote self-healing. The Journal of Alternative and Complementary Medicine, 10, S49-S60. http://dx.doi.org/10.1089/1075553042245809
- Gaab, J., Sonderegger, L., Scherrer, S. and Ehlert., U. (2006) Psychoneuroendocrine effects of cognitive-behavioral stress management in a naturalistic setting-a randomized controlled trial. Psychoneuroendocrinology, 31, 428-438. http://dx.doi.org/10.1016/j.psyneuen.2005.10.005
- Greco, C., Rudy, T. and Manzi, S. (2004) Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: A randomized controlled trial. Arthritis & Rheumatism, 51, 625-634. http://dx.doi.org/10.1002/art.20533
- Bijlani, R., Vempati, R. and Yadav, R. (2005) A brief but comprehensive lifestyle education program based on yoga reduces risk factors for cardiovascular disease and diabetes mellitus. The Journal of Alternative and Complementary Medicine, 11, 267-274. http://dx.doi.org/10.1002/art.20533
- Dobkin, P., Da Costa, D. and Joseph, L. (2002) Counterbalancing patient demands with evidence: Results from a Pan-Canadian randomized clinical trial of brief supportive-expressive group psychotherapy for women with systemic lupus erythematosus. Annals of Behavioral Medicine, 24, 88-99. http://dx.doi.org/10.1207/S15324796ABM2402_05
- Lorig, K., Ritter, P. and Plant, K. (2005) A disease-specific self-help program compared with a generalized chronic disease self-help program for arthritis patients. Arthritis & Rheumatism, 53, 950-957. http://dx.doi.org/10.1002/art.21604
- Hochberg, M. (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis & Rheumatism, 40, 1725. http://dx.doi.org/10.1002/art.1780400928
- Lorig, K. and Holman, H. (1993) Arthritis self-management studies: A twelve-year review. Health Education Quarterly, 20, 17-28. http://dx.doi.org/10.1177/109019819302000104
- Lorig, K., Sobel, D. and Stewart, A. (1999) Evidence that a chronic disease self-management program can improve health status while reducing utilization and costs: A randomized trial. Medical Care, 37, 5-14. http://dx.doi.org/10.1097/00005650-199901000-00003
- Lorig, K., Mazonson, P. and Holman, H. (1993) Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis & Rheumatism, 36, 437-446. http://dx.doi.org/10.1002/art.1780360403
- Lorig, K., et al. (2008) Living a healthy life with chronic conditions: Self management of heart disease, arthritis, diabetes, asthma, bronchitis, emphysema and others. Bull Publishing Company, Boulder.
- Spielberger, C. and Gorsuch, R. (1983) Manual for the state-trait anxiety inventory (form y) (“self evaluation questionnaire”). Consulting Psychologists Press, Palo Alto.
- Lorig, K., Chastain, R.L. Ung, E., Shoor, S. and Holman, H.R. (1989) Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis & Rheumatism, 32, 37-44. http://dx.doi.org/10.1002/anr.1780320107
- Green, N. (1995) Development of the perceptions of racism scale. Image: the Journal of Nursing Scholarship, 27, 141-146. http://dx.doi.org/10.1111/j.1547-5069.1995.tb00838.x
- Stewart, A., Hays, R. and Ware, J. (1992) Health perceptions, energy/fatigue, and health distress measures. In: Stewart, A. and Ware, J. Eds, Measuring Functioning and Well-Being: The Medical Outcomes Study Approach, Duke University Press, Durham, 143-172.
- Beck, A. (2006) Depression: Causes and treatment. Philadelphia, University of Pennsylvania Press, Philadelphia.
- Webster, K., Cella, D. and Yost, K. (2003) The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, application, and interpretation. Health and Quality of Life Outcomes, 1, 79. http://dx.doi.org/10.1186/1477-7525-1-79
- Yu, E., et al. (2006) Validation of LUP-QOL: A lupus-specific measure of health-related quality of life. Eular.
- Lorig, K., Ritter, P., Stewart, A.L., Sobel, D.S., William Brown, Jr. B., Bandura, A., Gonzalez, V.M., Laurent, D.D. and Holman, H.R. (2001) Chronic disease self-management program: 2 year health status and health Care utilization outcomes. Medical Care, 39, 1217-1223. http://dx.doi.org/10.1097/00005650-200111000-00008
- Gallagher, P. and Young, A. (2002) Cortisol/DHEA ratios in depression. Neuropsychopharmacology, 26, 410.
- Oderbeck, R., Benschop, R.J., Jacobs, R., Hosch, W., Jetschmann, J.U., Schürmeyer, T.H., Schmidt, R.E. and Schedlowski, M. (1998) Endocrine mechanisms of stressinduced DHEA secretion. Journal of Endocrinological Investigation, 21, 148-153.
- Hechter, O., Grossman, A. and Chatterton, R.J. (1887) Relationship of dehydroepiandrosterone and cortisol in disease. Medical Hypotheses, 49, 85-91. http://dx.doi.org/10.1016/S0306-9877(97)90258-9
- Karlson, E., Liang, M.H., Eaton, H., Huang, J., Fitzgerald, L., Rogers, M.P. and Daltroy, L.H. (2004) A randomized clinical trial of psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis & Rheumatism, 50, 1832-1841. http://dx.doi.org/10.1002/art.20279
- Kosinski, M., Zhao, S.Z., Dedhiya, S., Osterhaus, J.T. and Ware Jr., J.E. (2000) Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis & Rheumatism, 43, 1478-1487. http://dx.doi.org/10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M
- Austin, J., Maisiak, R.S., Macrina, D.M. and Heck, L.W. (1996) Health outcome improvements in patients with systemic lupus erythematosus using two telephone counseling interventions. Arthritis & Rheumatism, 9, 391-399. http://dx.doi.org/10.1002/1529-0131(199610)9:5<391::AID-ANR1790090508>3.0.CO;2-V
- Petri, M., Naqibuddin, M., Carson, K.A., Wallace, D.J., Weisman, M.H., Holliday, S.L., Sampedro, M., Padilla P.A. and Brey, R.L. (2010) Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. Journal of Rheumatology, 37, 2032-2038. http://dx.doi.org/10.3899/jrheum.091366
- Kozora, E., Ellison, M.C., Waxmonsky, J.A., Wamboldt, F.S. and Patterson, T.L. (2005) Major life stress, coping styles, and social support in relation to psychological distress in patients with systemic lupus erthematosus. Lupus, 14, 363-372. http://dx.doi.org/10.1191/0961203305lu2094oa
- Iverson, G.L., Sawyer, D.C., McCracken, L.M. and Kozora, E. (2001) Assessing depression in systemic lupus erythematosus: Determining reliable change. Lupus, 10, 266-271. http://dx.doi.org/10.1191/096120301680416959
- Ward, M., Marx, A. and Barry, N. (2002) Psychological distress and changes in the activity of systemic lupus erythematosus. Rheumatology, 41, 184-188.
- Cleanthous, S., Tyagi, M., Isenberg, D.A. and Newman, S.P. (2012) what do we know about self-reported fatigue in systemic lupus erythematosus? Lupus, 21, 465-476.
- Tench, C., McCurdie, I., White, P.D. and D’Cruz, D.P. (2000) The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology, 39, 1249-1254. http://dx.doi.org/10.1093/rheumatology/39.11.1249
- Danoff-Burg, S. and Friedberg, F. (2009) Unmet needs of patients with systemic lupus erythematosus. Behavioral Medicine, 35, 5-13. http://dx.doi.org/10.3200/BMED.35.1.5-13
- Izawa, S., Saito, K., Shirotsuki, K., Sugaya, N. and Nomura, S. (2012) Effects of prolonged stress on cortisol and dehydroapiandrosterone: A study of a two-week teaching practice. Psychoneuroendocrinology, 37, 852-858. http://dx.doi.org/10.1016/j.psyneuen.2011.10.001
- Blackman, M., Muniyappa, R., Wilson, M., Moquin, B.E., Baldwin, H.L., Wong, K.A., Snyder, C., Magalnick, M., Alli, S., Reynolds, J., Steinberg, S.M. and GoldbachMansky, R. (2007) Diurnal secretion of growth hormone, cortisol and dihydroepiandorsterone in preand postmenopausal women with active rheumatoid arthritis: A pilot case-control study. Arthritis Research & Therapy, 9, 73- 82. http://dx.doi.org/10.1186/ar2271

