Open Journal of Internal Medicine
Vol.1 No.2(2011), Article ID:7696,4 pages DOI:10.4236/ojim.2011.12008
Right ventricular tear mimicking myocardial infarction following pericardiocentesis
![]()
Department of Cardiology, Hospital Group Twente, Hengelo, The Netherlands.
Email: *samsaid@home.nl
Received 1 June 2011; revised 13 July 2011; accepted 21 July 2011.
Keywords: Pericardial Effusion; pericardiocentesis; Right Ventricular Rupture; Myocardial Infarction; Pulmonary Adenocarcinoma
ABSTRACT
A 72-year-old female was admitted to the CCU with a recent onset of progressive breathlessness for bedside pericardial drainage for chronic pericardial effusion. After an uncomplicated drainage procedure, initially a serous straw coloured fluid was aspired with subsequent hemorrhagic aspiration with haemoglobin value similar to the peripheral blood. The patient showed initially transient improvement followed by rapid deterioration into severe shock and death. Signs of infero-posterior myocardial infarction (MI) were seen on the ECG. Before death, further interventions were refused by her and her family but a permission was given for autopsy. At autopsy, right ventricular rupture was seen with a 0.6 cm tear with a large amount of 800 cc bloody fluid with clots. The result of histopathologic study of the tear was resembling three-days old MI. The drain was found to be properly localized in the pericardial space, was not blocked and caused no harm to the myocardium. Furthermore, histopathologic examination revealed pulmonary adenocarcinoma of the left upper lobe, pleuritis and lymphangitis carcinomatosa and enlarged mediastinal lymph nodes. A case of fatal complication is reported following bedside pericardial drainage. Post-mortal, right ventricular tear mimicked myocardial infarction.
1. INTRODUCTION
Pericardial effusion may accumulate due to various causes including infection, malignancy, trauma or idiopathic. Major complications of pericardiocentesis accounts for 2%, including cardiac chamber laceration or pneumothoraces [1], acute pulmonary edema [2] and hemorrhagic peritonitis [3]. Echocardiography guided pericardiocentesis has a success rate of over 95% carrying a 4.9% complication rate [4] which is much lower than the blind approach [5]
We present a case of 72-years old female with a right ventricular tear following pericardiocentesis. At autopsy, the histopathologic findings resembled myocardial infarction.
2. CASE REPORT
A 72-years old female patient was admitted to the CCU due to progressive dyspnoea (New York Heart Association Functional Class III), orthopnoea and fatigue. Previous medical history included hypercholesterolemia, chronic obstructive pulmonary disease and recovery from cerebral vascular accident. She was treated with clopidogrel and atorvastatine.
On physical examination there was tachypnoea but normotensive with regular pulse rate, and normal jugular venous pressure. On auscultation an apical grade 1-2/6 systolic ejection murmur was heard without pericardial friction rub.
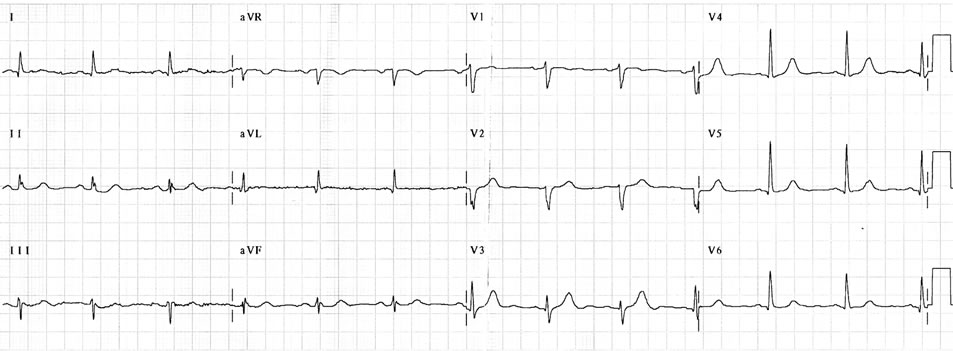
The ECG prior to pericardial drainage (Figure 1(a)) revealed sinus rhythm without microvoltage and postdrainage (Figure 1(b)) depicted signs of inferoposterior MI. The chest X-ray demonstrated cardiomegaly without pulmonary venous congestion or infectious lesions.
Biochemically, normal cardiac markers and PRO-BNP but elevated D-dimers (>25000, normal range 0 - 500 ug/l). Pulmonary embolism was excluded by normal pulmonary perfusion-ventilation scintigraphy.
Trans-thoracic echocardiography showed trivial tricuspid regurgitation, dilated right atrium with end-diastolic collapse, pericardial effusion with fibrin mass and thrombus formation and left ventricular pseudohypertrophy with normal systolic function. The inferior vena cava was dilated and showed no collapse. An echogenic structure was visible attached to the outer surface of the right ventricle (Figure 2). Transthoracic echocardiography before pericardiocentesis was suggestive of pericardial effusion containing blood and clot (Figure 2).
Bedside pericardiocentesis was performed; initially a serous fluid (30 ml) was drained and subsequently bloody fluid. Cytology of the pericardial effusion was negative for malignant cells and on culture the fluid was sterile. The haemoglobin concentration of the drained fluid was similar to that of the peripheral sample. She developed shock; refractory to packed cells transfusion, intravenous fluid expansion and inotropics. The patient refused further surgical intervention and she died after several hours of refractory shock. The family gave permission for autopsy.
 (a)
(a)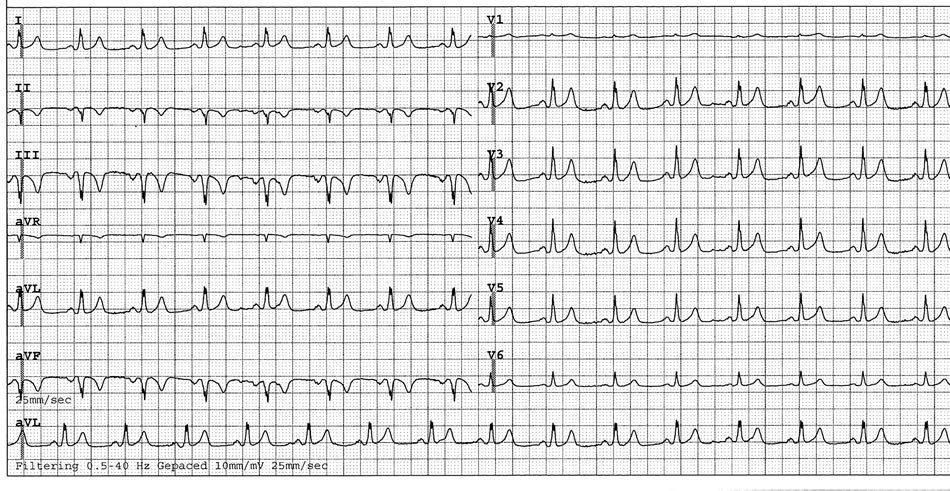 (b)
(b)
Figure 1. (a) An admission ECG Prior to pericardiocentesis and (b) following bedside pericardial drainage with signs mimicking myocardial infarction.
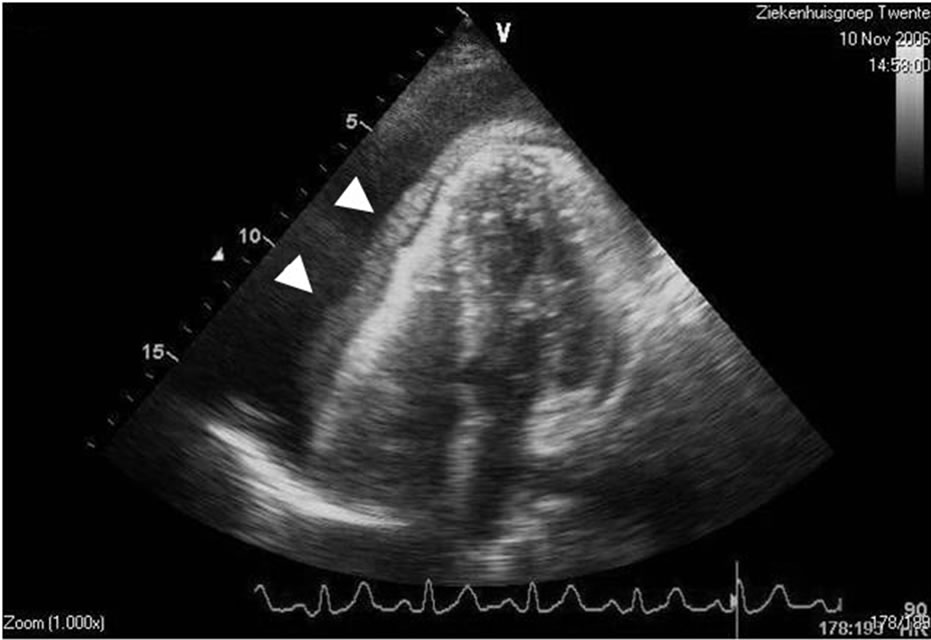
Figure 2. Echocardiogram before pericardiocentesis demonstrating A pericardial effusion (arrowheads) and hematoma surrounding the outer surface of the right ventricle (RV).
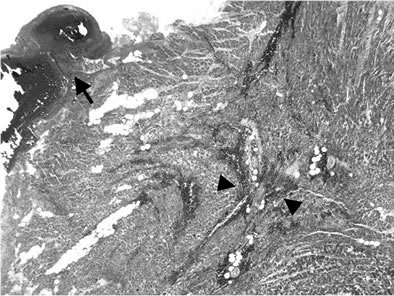
An autopsy limited to heart and lungs was performed. RV rupture was recognized with a 0.6 cm tear (Figures 3(a)-(d)) accompanied with a large pericardial effusion of 800 ml bloody fluid with thrombi. The gross pathology of the RV tear appeared frayed. Histo-pathologically, the RV tear was found in a recently developed damage in the region of RV wall and septum resembling a 3 days old MI. The drain was found to be properly positioned in the pericardial space coursing laterally to the left ventricle next to the left atrium and was not blocked. The tip was obstructed with a post-mortal clot. The coronary arteries were not severely obstructed. Furthermore, there was an adenocarcinoma (Figure 3(e)) of the left upper
 (a)
(a)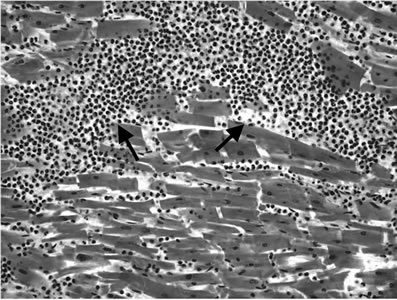 (b)
(b)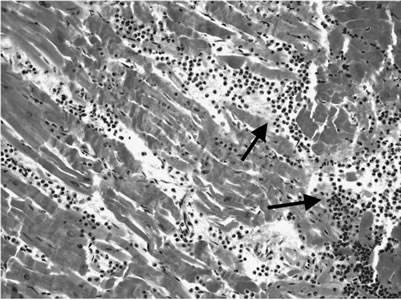 (c)
(c)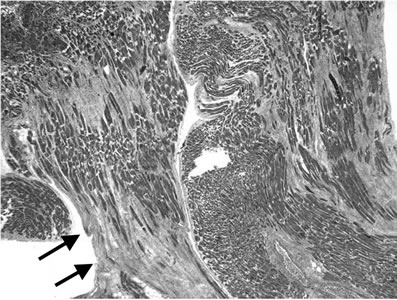 (d)
(d) (e)
(e)
Figure 3. Histopathological findings: Photomicrographs of a section from the heart and lung. Low and high power views with Hematoxylin and Eosin (H-E) stain are demonstrated: 3(a): Section of the area of the right ventricular rupture: blood clot at the rupture surface (arrow); intramural haemorrhage (arrowheads). H-E stain × 25. 3(b): RV wall: Microscopic findings of recent RV tear. The RV wall was infiltrated with neutrophilic granulocytes (arrows). H-E stain × 200; 3(c): Septum: The myocardial septal wall was infiltrated with neutrophilic granulocytes (arrows). H-E stain × 200; 3(d): Right ventricular wall with areas of scarring fibrosis (arrows) representing old damage. H-E stain × 25. 3(e): Bronchogenic adenocarcinoma of the left lower lobe (arrows). H-E stain × 100.
lobe of the lung with pulmonary tumour expansion, pleuritis accompanied with lymphangitis carcinomatosa and lymphogenic dissemination to the mediastinal lymph nodes. The pericardium was not involved.
3. DISCUSSION
The most frequent symptoms of pericardial effusion were found to be dyspnoea, chest pain, orthopnoea, edema, and cough [6]. Our patient presented with progresssive dyspnoea. Major complications of pericardiocentesis accounts for 2%, such as cardiac chamber laceration or pneumothoraces were reported earlier [ 1 ].
Recently, acute pulmonary edema with preserved myocardial function [2] and hemorrhagic peritonitis [3] has been reported. Various hypotheses can be formulated to explain the pathophysiological mechanisms involved in the development of dyspnoea and death in the absence of pulmonary embolism in this case. Transthoracic echocardiography before pericardiocentesis was suggestive of pericardial effusion containing blood and clots. After pericardiocentesis, pericardial decompression causes a sudden decrease in right atrial pressure inducing a dramatic increase in venous return, especially in spontaneously breathing patients [7]. In our current case, failure to recovery was because the compression by blood and clot in the pericardial space which could not be completely relieved by pericardiocentesis. Alternatives for pericardiocentesis are open drainage by subxiphoid pericardiotomy [6], sternotomy [8] or left anterolateral thoracotomy [9]. Both modalities were refused by patient and her family.
In our case, the 0.6 cm frayed tear in the RV wall is thought to be iatrogenic during pericardiocentesis though histopathologic examination revealed intramural hemorrhage, infiltration of neutrophilic granulocytes and areas of fibrosis and scarring resembling a 3-days old MI. Manipulation during pericardial drainage is incriminated for this complication.
Metastatic dissemination in the myocardium could have been implicated but this is an extremely rare occurrence. In the current case, such findings could not be confirmed at the post-mortal examination.
To avoid major complications associated with blind bedside pericardiocentesis manoeuvre, such as iatrogenic RV tear or laceration, Echocardiography guided pericardiocentesis (EGP) should be considered.
EGP has a success rate of over 95% but it does carry a 4.9% complication rate [ 4 ] which is much lower than with the blind approach [5]. The major complications include laceration of the cardiac chambers, damage of intercostals vessels, pneumothorax, arrhythmias, infection, and death. Minor complications are transient entry in the cardiac chambers, vasovagal reactions, and pleuropericardial fistula.
Cheng suggested the utilization of dehydrocholate or magnesium sulphate, through the aspirating needle, in case of aspiration of hemorrhagic pericardial effusion. Typical circulation time determination response indicates that the needle is in the cardiac cavity and should be withdrawn immediately [10]. It has been suggested to use perflutren lipid microspheres for echocardiographic contrast during EGP of bloody fluid to assess the needle’s location [ 11 ].
Cardiac and pericardial metastases are discovered at autopsy in 10% - 12% of all patients with malignancy [12,13]. The most common underlying malignancy is carcinoma of the lung, in part because of the proximity to the heart and its common prevalence [14]. Our patient had no signs of cardiac or pericardial metastasis of the post-mortem discovered pulmonary neoplasm.
Autopsy series show that lung cancer is the most common primary tumour and adenocarcinoma the most frequent cell type of cardiac metastases [13]. The most common clinical presentation of neoplastic pericardial disease is shortness of breath but cardiac tamponade is rarely the first manifestation of a metastatic neoplasm [15].
4. CONCLUSIONS
Bedside pericardiocentesis for diagnostic and therapeutic purposes may be performed but carry certain hazards. EGP is the method of choice to perform such procedure.
5. ACKNOWLEDGEMENTS
The authors appreciate the technical support of Mrs. M. Roeloffzen and Mr. B. van Rennes for the echocardiographic images and the librarians of the medical library of Hospital Group Twente, Mrs. A. Geerdink and Mr. D. Maas for their secretarial assistance during the preparation of the manuscript.
REFERENCES
- Tsang, T.S., Barnes, M.E., Hayes, S.N., Freeman, W.K., Dearani, J.A., Osborn Butler, S.L. and Seward, J.B. (1999) Clinical and echocardiographic characteristics of significant pericardial effusions following cardiothoracic surgery and outcomes of echo-guided pericardiocentesis for management: Mayo Clinic experience, 1979-1998. Chest, 116, 322-331. doi:10.1378/chest.116.2.322
- Bernal, J.M., Pradhan, J., Li, T., Tchokonte, R. and Afonso, L. (2007) Acute pulmonary edema following pericardiocentesis for cardiac tamponade. Canadian Journal of Cardiology, 23, 1155-1156. doi:10.1016/S0828-282X(07)70887-5
- Luckraz, H., Kitchlu, S. and Youhana, A. (2004) Haemorrhagic peritonitis as a late complication of echocardiography guided pericardiocentesis. Heart, 90, 16. doi:10.1136/hrt.2003.024075
- Tsang, T.S., Enriquez-Sarano, M., Freeman, W.K., Barnes, M.E., Sinak, L.J., Gersh, B.J., Bailey, K.R. and Seward J.B. (2002) Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: Clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clinic Proceedings, 77, 429-436. doi:10.4065/77.5.429
- Krikorian, J.G. and Hancock, E.W. (1978) Pericardiocentesis. American Journal of Medicine, 65, 808-814. doi:10.1016/0002-9343(78)90800-8
- van Trigt, P., Douglas, J., Smith, P.K., Campell, P.T., Wall, T.C., Kenney, R.T., O’Connor, C.M., Sheikh, K.H. and Corey G.R. (1993) A prospective trial of subxiphoid pericardiotomy in the diagnosis and treatment of large pericardial effusion. A follow-up report. Annals of Surgery, 218, 777-782. doi:10.1097/00000658-199312000-00012
- Magder, S. (1998) More respect for the CVP. Intensive Care Medicine, 24, 651-653. doi:10.1007/s001340050640
- Geffroy, A., Beloeil, H., Bouvier, E., Chaumeil, A., Albaladejo, P. and Marty, J. (2004) Prolonged right ventricular failure after relief of cardiac tamponade. Canadian Journal of Anesthesia, 51, 482-485. doi:10.1007/BF03018312
- Sunday, R., Robinson, L.A. and Bosek V. (1999) Low cardiac output complicating pericardiectomy for pericardial tamponade. Annals of Thoracic Surgery, 67, 228-231. doi:10.1016/S0003-4975(98)01143-6
- Cheng, T.O. (2000) What to do when bloody fluid is obtained on pericardiocentesis? Chest, 117, 1525-1526. doi:10.1378/chest.117.5.1525-a
- Escabi-Mendoza, J.E., Martinez-Diaz, J.D. and Aviles-Rivera, E.D. (2006) Perflutren microspheres for contrast echocardiography in a bloody pericardiocentesis. Texas Heart Institute Journal, 33, 214-217.
- Abrahamm, K.P., Reddy, V. and Gattuso, P. (1990) Neoplasms metastatic to the heart: Review of 3314 consecutive autopsies. American Journal of Cardiovascular Pathology, 3, 195-198.
- Klatt, E.C. and Heitz, D.R. (1990) Cardiac metastases. Cancer, 65, 1456-1459. doi:10.1002/1097-0142(19900315)65:6<1456::AID-CNCR2820650634>3.0.CO;2-5
- Thurber, D.L., Edwards, J.E. and Achor, R.W.P. (1962) Secondary malignant tumors of the pericardium. Circulation, 26, 228-241.
- Haskell, R.J. and French, W.J. (1985) Cardiac tamponade as the initial presentation of malignancy. Chest, 88, 70-73.

