New Journal of Glass and Ceramics
Vol.2 No.2(2012), Article ID:18607,6 pages DOI:10.4236/njgc.2012.22014
Effect of Neodymium on the Crystallization, Microstructure and Colorization of Li2O-Al2O3-SiO2 Glass Ceramics
![]()
Department of Materials Science and Engineering, Zhejiang university, Hangzhou, China.
Email: msewj01@zju.edu.cn
Received September 15th, 2011; revised October 21st, 2011; accepted December 21st, 2011
Keywords: Glass Ceramic; Crystallization; Colorization; Neodymium
ABSTRACT
The effect of single coloring agent Nd(NO3)3 on the crystallization, microstructure and colorization of Li2O-Al2O3-SiO2 (LAS) glass ceramics were investigated by differential thermal analysis (DTA), X-ray diffractometry (XRD) and scanning electron microscopy (SEM). The introduction of little neodymium has no effect on the crystallization manner and the formation of main crystallization phase, but more neodymium will weaken the crystallization of LAS glass. Little neodymium can increase the glossiness of LAS glass ceramic, while more neodymium can color LAS glass with light purple or red purple. The colorability of neodymium for LAS glass ceramic decreases with the increase of crystallization temperature.
1. Introduction
Lithium aluminosilicate (Li2O-Al2O3-SiO2, LAS) is wellknown as one of the most valuable glass ceramic systems, and has been extensively used in many fields because of its high heat resistance, low expansion, excellent mechanical properties and outstanding chemical durability [1-9] . Many studies on the LAS glass ceramics have mainly focused on crystallization mechanism, microstructure and properties of glass ceramics. At present the colorization of LAS glass ceramics has been becoming a research hotspot in this field. Macalik [10] studied the effects of Fe, Co and Mn on crystallization and structure of lithium aluminosilicate glass ceramic. ZHANG Zhi-yong [11] investigated the effects of Cr2O3, CoO, NiO, MnO2 and heating process on colorization of LAS glass ceramics. Li Yao-hui [12] analyzed the effects of V2O5 and Nd2O3 on the crystallization and coloration of transparent LAS glass ceramics. We have also studied the effects of Fe, Co and Ni on colorization and microstructure of LAS glass ceramics [13,14] .
The coloration mechanism of ions in the glass ceramic is greatly different from glass, resulting from lots of crystals existing in glass ceramics, and the colors of two materials are quite distinct from each other. The coloring agent can affect not only the color of glass ceramics, but also the melting, crystallization and microstructure of glass ceramic. There are not many studies to investigate the effects of single neodymium on crystallization, structure and performances of LAS glass ceramics. Based on our previous studies on the nucleation and crystallization mechanism of LAS glass [15-19] , this paper aims to investigate the effect of single neodymium on the crystallization, structure and colorization of LAS glass ceramics.
2. Experimental
2.1. Glass Preparation
The raw materials of glass batch include acid washed quartz sands and chemical reagents of high purity Li2CO3, Al2O3, MgO, ZnO, TiO2 and other compositions, and their compositions were listed in Table 1. The different
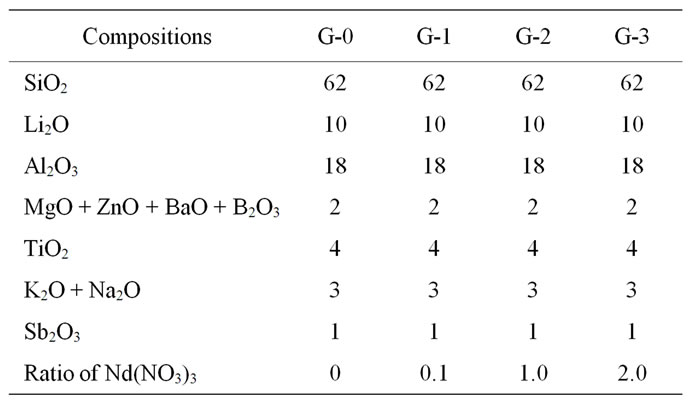
Table 1. Main compositions of the batches (wt%).
ratios of Nd(NO3)3 were added into the glass batch and employed as a coloring agents. The raw materials were mixed for 6 h at ball milling, melted for 4 - 6 h at 1673 - 1723 K and moulded to obtain glass in a pre-heated die. The glass samples were then annealed at 773 K for 1 h to eliminate internal stress.
2.2. Crystallization and Characterization
The annealed glass samples were heat-treated at 873 K, 893 K, 993 K, 1093 K and 1193 K for 1 h with a heating rate of 20 K·min–1. Subsequently, the heat-treated glass samples were fast-cooled, ground and sieved through a 200-mesh screen.
Differential thermal analysis (DTA) of the annealed and heat-treated glass samples was measured on a differential thermal analyzer (NETZSCH STA 409 PC Luxx, Germany) with alumina as the reference and the samples were heated at 5 - 20 K·min–1 from 293 K to 1573 K. The crystalline phases of the glass ceramic samples were analyzed by the X-ray diffraction (XRD) method on a XJ 10 - 60 X-ray diffractometer using nickel filtered Cu Kα radiation in the range of 2θ = 10˚ - 80˚ with a scanning speed of 2˚/min. The morphology of the glass ceramic were observed on the SEM (scanning electron microscopy, FEI SIRION), and the surface of observed samples was polished and eroded by HF (2 wt%) for 30 - 40 s. The template is used to format your paper and style the text. All margins, column widths, line spaces, and text fonts are prescribed; please do not alter them. You may note peculiarities. For example, the head margin in this template measures proportionately more than is custommary. This measurement and others are deliberate, using specifications that anticipate your paper as one part of the entire proceedings, and not as an independent document. Please do not revise any of the current designations.
3. Results and Discussion
3.1. Crystallization Kinetics
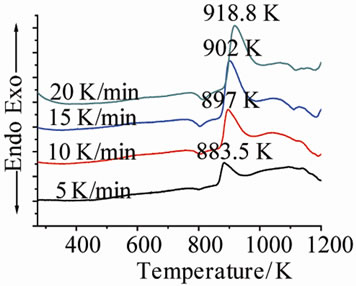
Figure 1 shows the DTA curves of LAS glass containing different neodymium at different heating rates. Table 2 shows the crystallizing peak maximum temperatures (Tp) from DTA curves at different heating rates. It is noted that crystallizing peak maximum temperatures (Tp) of all
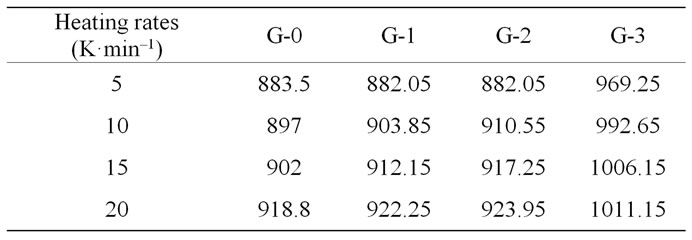
Table 2. Tp (K) values from DTA curve of LAS glass samples at different heating rates.
 (a)
(a)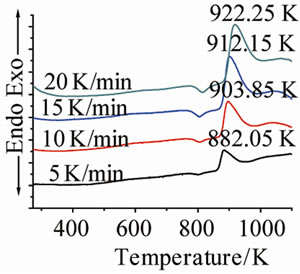 (b)
(b)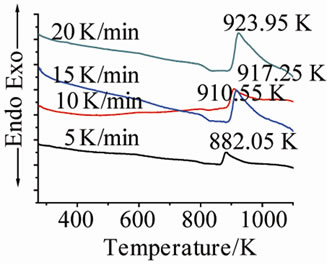 (c)
(c)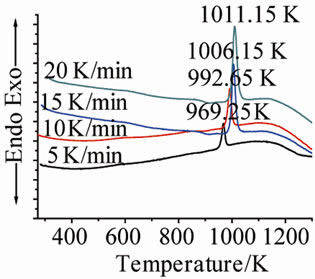 (d)
(d)
Figure 1. DTA traces obtained from: (a) G-0; (b) G-1; (c) G-2; and (d) G-3 LAS glass powders.
glass samples increase with the increase of heating rates, and also increase with the ratios of Nd(NO3)3 at the same heating rate.
The crystallization kinetic characteristics of LAS glass can be deduced as follows by Arrhenius [1] , Kissinger [20] and Augis-Bennett [21] , which are respectively expressed as
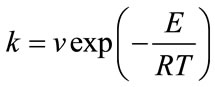 (1)
(1)
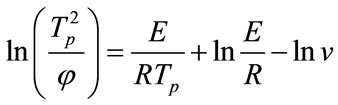 (2)
(2)
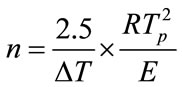 (3)
(3)
where E is the activation energy (kJ·mol–1), R is the gas constant, ν is the frequency factor (min–1), φ is the DTA heating rate (K·min–1), k is the reaction rate constant, n is the crystallization index and ΔT is the half-height temperature wideness of the maximum exothermical peak of DTA. Low E value and high ν indicate high crystallization rate and crystallinity. Crystallization index n is related to crystallization manner, n ≈ 1, surface crystallization, n ≈ 2 two-dimension growth crystallization, n ≈ 3, volumetric crystallization, n ≈ 4, homogeneous nucleation crystallization.
According to Table 2, the relationship between 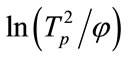 and
and 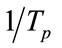 is constructed (Figure 2) to calculate the effective activation energy, frequency factor and crystallization index. Table 3 is the E, v, and n crystallization values of the LAS glass samples. It is shown
is constructed (Figure 2) to calculate the effective activation energy, frequency factor and crystallization index. Table 3 is the E, v, and n crystallization values of the LAS glass samples. It is shown

Figure 2. Relationship between 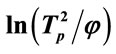 and
and ![]() of G-0, G-1, G-2 and G-3 LAS glass samples.
of G-0, G-1, G-2 and G-3 LAS glass samples.
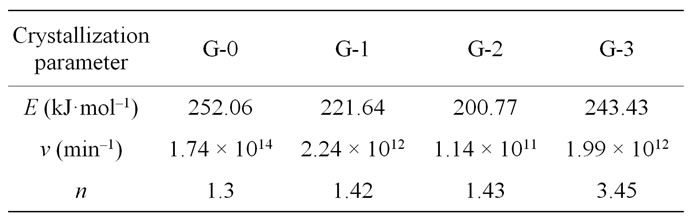
Table 3. E, v, and n crystallization values of the LAS glass samples.
that the G-0 specimen has higher E and v than other specimens. As the ratio of Nd(NO3)3 increases, the E and v of the samples become lower while both E and v increase again when the ratio reaches to 2 wt% (G-3). It is also suggested that the additions of more neodymium will weaken the ability of crystallizations of LAS glass. The fact n values of G-0, G-1 and G-2 samples are near 1 indicates that crystallization manner of LAS glass is surface crystallization, while the n value of G-3 sample is more than 3, suggesting volumetric crystallization. It indicates the addition of little Nd has no obvious effect on crystallization manner.
3.2. Crystalline Phases and Microstructure
Figure 3 shows the X-diffraction patterns of G-0 and G-3 samples, which were heat treated for 1 h at several temperatures above 873 K. Both of G-0 and G-3 glass samples are amorphous phase below 893 K. There are only hexagonal LiAl(SiO3)2 crystal phase (JCPDS-PDF 31-0706) at 993 K, which is similar to low-temperature stable phase α-spodumene. The high-temperature stable phase β-spodumene (JCPDS-PDF 35-0797) was main crystallization phase in both samples at 1193 K. In addition, the specimen became opaque due to the crystallinity. It is also confirmed that the phase transformation of α-spodumene into β-spodumene finished at 1193 K, and the additions of Nd have no effect on the formation of main crystallization phase.
The volume fraction, grain size and completion of crystallization phase, which determine the performances of glass ceramics, are influenced by glass composition
 (a)
(a)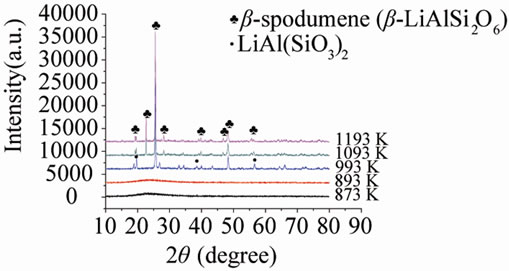 (b)
(b)
Figure 3. XRD patterns of: (a) G-0 and (b) G-3 LAS glass samples heat treated at different crystallization temperatures.
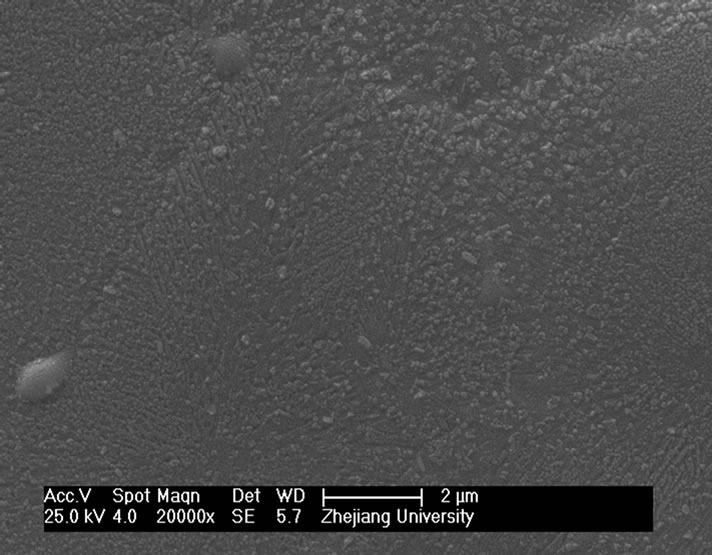
and heat-treatment. Figure 4 shows the microstructures of G-0 and G-3 samples heat treated for 1h at different crystallization temperatures. It can be seen that the crystal size of both samples increases with increase of the crystallization temperatures. The crystal shape of G-0 sample becomes needle while that of the G-3 sample is of block or particle, which coordinates with the results of crystallization manner.
3.3. Colorization of LAS Glass Ceramic
Figure 5 shows the color of different glass samples at different crystallization temperatures. It is seen that the color of glass before crystallization is greatly distinct from that after crystallization, and the phenomenon become more apparent at higher ratio of Nd(NO3)3. The color of glass ceramic samples crystallized below 993 K varies from light purple to red purple with the ratios of Nd(NO3)3, while those colors of glass ceramic samples crystallized above 993 K fades, and in the meantime the main crystal phases have formed above 993 K (according to Figures 3 and 4). It is also observed that the color of glass ceramic samples with lower ratio of Nd(NO3)3 become brighter than that of glass ceramic sample without Nd(NO3)3, in that the neodymium can decrease the faint yellow of LAS glass ceramics, which results from the addition of TiO2. It improves that addition of single neodymium has a weak colorability to the coloration of LAS glass ceramic with higher crystal size, but little neodymium can improve the glossiness of glass ceramic.
4. Discussions
Neodymium exists as the state of Nd3+ ion in glass, and lies in low-symmetry lattice site outside the network [12]. Nd3+ ion is known to absorb visible light wavelengths and used to colorize glass and has unusual two-color effect. The coloring of Nd3+ ion is stable because its valence electrons are in the 4f track which is shielded by 5s25p6 track, and it leads to f-f energy level transition.
 (a)
(a)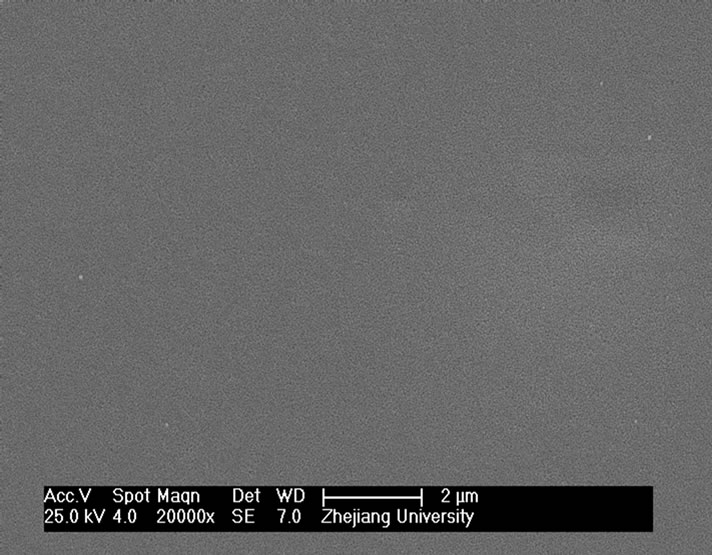 (b)
(b)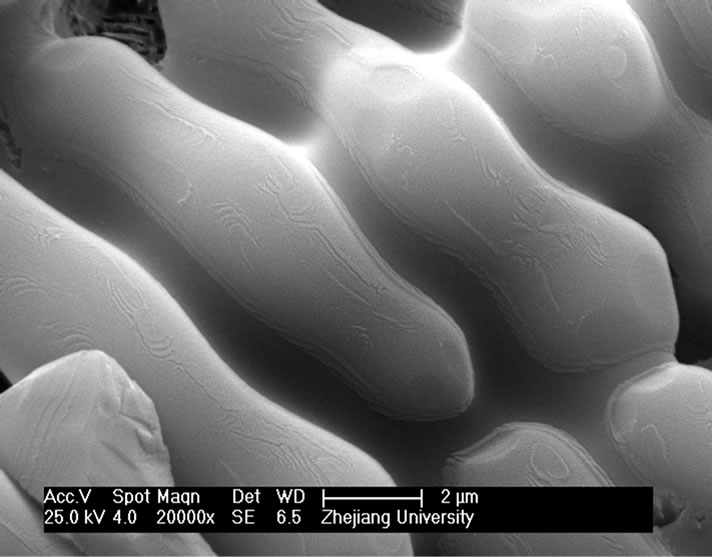 (c)
(c)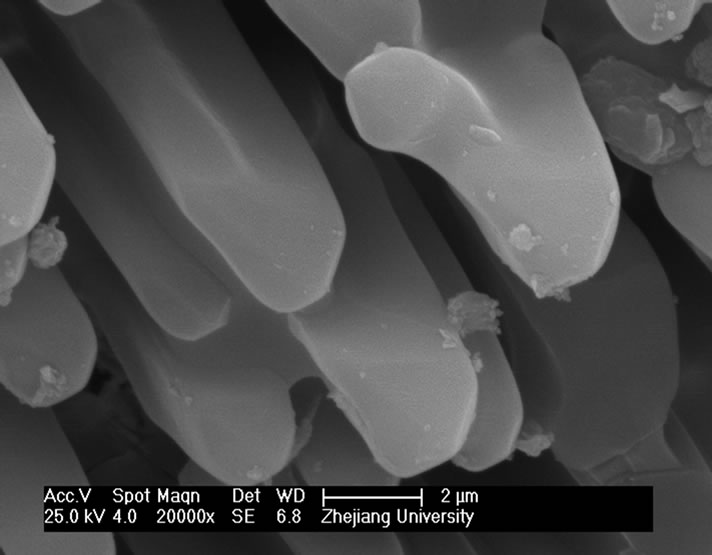 (d)
(d) (e)
(e) (f)
(f)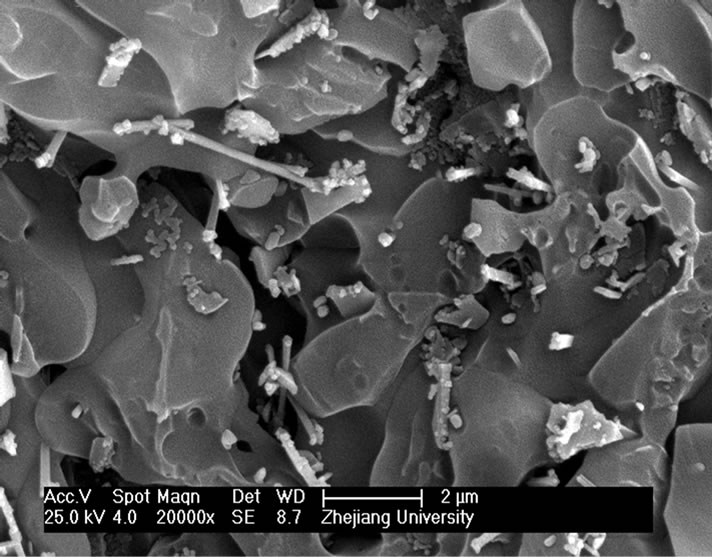 (g)
(g) (h)
(h)
Figure 4. SEM images of G-0 and G-3 LAS glass samples heat treated for 1h at different crystallization temperatures. (a) G-0 873 K; (b) G-0 993 K; (c) G-0 1093 K; (d) G-0 1193 K; (e) G-3 873 K; (f) G-3 993 K; (g) G-3 1093 K; (h) G-3 1193 K.
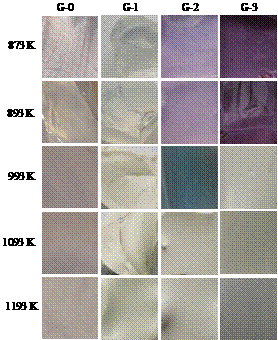
Figure 5. Color of different samples at different crystallization temperatures.
Nd3+ ions are surrounded by O2– ions to form different coordination ligands and states, which play a vital part in the glass structure and spectrum characteristics. Nd3+ ions is strong in terms of electric field intensity with the ion radii of 0.1281 nm, and it can form ligands and join the silicon oxygen tetrahedron together, which is the reason why more Nd(NO3)3 can reduces the crystallization ability of LAS glass.
Nd3+ ions joins into the network of glass and participates the restructuring, correspondingly, the crystallization and crystal phases have an effect on the absorption spectrum of Nd3+ ions. The crystallinity and crystal size of LAS glass ceramic increase with the increase of crystallization temperature, which affects the absorption of Nd3+ ions in visible light wavelengths.
5. Conclusion
The crystallization mechanism, microstructure of Li2OAl2O3-SiO2 system glass ceramics containing neodymium were investigated. The introduction of little neodymium has no effect on the crystallization manner and the formation of main crystallization phase, but more neodymium will reduces the crystallization ability of LAS glass and change the shape of crystals. Little neodymium can increase the glossiness of LAS glass ceramic, more neodymium can color LAS glass with light purple or red purple, but the colorability decreases for LAS glass ceramic with higher crystallization temperature.
REFERENCES
- P. W. McMillan, “Glass-ceramics,” Academic Press, New York, 1979.
- P. F. James, “Glass Ceramics: New Compositions and Uses,” Journal of Non-Crystalline Solids, Vol. 181, No. 1, 1995, pp. 1-15.
- P. A. Tick, N. F. Borrelli and I. M. Reaney, “The relationship between structure and transparency in glassceramic materials,” Optical Materials, Vol. 15, No. 1, 2000, pp. 81-91. doi:10.1016/S0925-3467(00)00017-3
- X. Z. Guo, H. Yang and M. Cao, “Nucleation and Crystallization Behavior of Li2O-Al2O3-SiO2 System GlassCeramic Containing Little Fluorine and No-Fluo rine,” Journal of Non-Crystalline Solids, Vol. 351, No. 24-26, 2005, pp. 2133-2137. doi:10.1016/j.jnoncrysol.2005.04.071
- P. Riello, S. Bucella, R. Krsmanovic, S. Meneghetti, S. Pietrantoni and R. Francini, “Synthesis, X-ray Diffraction Characterization, and Radiative Properties of Er2O3- ZrO2 Nanocrystals Embedded in LAS Glass Ceramic,” Journal of Physical Chemistry B, Vol. 109, No. 28, 2005, pp. 13424-13430.
- O. Garcia-Moreno, A. Fernandez and R. Torrecillas, “Conventional sintering of LAS-SiC Nanocomposites with Very Low Thermal Expansion Coefficient,” Journal of the European Ceramic Society, Vol. 30, No. 15, 2010, pp. 3219-3225. doi:10.1016/j.jeurceramsoc.2010.07.003
- A. Arvind, R. Kumar, M. N. Deo, V. K. Shrikhande and G. P. Kothiyal, “Preparation, Structural AndthermoMechanical Properties of Lithium Aluminum Silicate Glass-Ceramics,” Ceramics International, Vol. 35, 2009, pp. 1661-1666.
- A. Ananthanarayanan, G. P. Kothiyal, L. Montagne and B. Revel, “MAS-NMR investigations of the Crystallization Behaviour of Lithium Aluminum Silicate (Las) Glasses Containing P2o5 and Tio2 Nucleants,” Journal of Solid State Chemistry, Vol. 183, No. 6, 2010, pp. 1416-1422.
- O. D. Gutierrez, E. Osorio, C. G. Paucar, R. Cogollo and C. Z. Hadad, “Synthesis and Characterization of Thermoluminescent Glass-Ceramics Li2O-Al2O3-SiO2:CeO2,” Journal of Luminescence, Vol. 129, No. 8, 2009, pp. 836-839.
- B. Macalik and T. Morawska-Kowal, “Coloration Processes in Soda-Lime Silicate Glasses,” Nuclear Instruments and Methods in Physics Research Section B, Vol. 191, No. 1-4, 2002, pp. 379-381. doi:10.1016/S0168-583X(02)00543-8
- Z. Zhang and L. Zhang, “Study on the colouration of Li2O-Al2O3-SiO2 Transparent Glass Ceramics,” Glass and Porcelain, Vol. 29, No. 4, 2001, pp. 13-17.
- L. Yaohui, C. Jianwei, X. Bo, L. Jinshan and L. Kaiming, “Effect of Vanadium and Neodymium on Crystallization and Coloration of Transparent Li2O-Al2O3-SiO2 GlassCeramics,” Rare Metal Materials and Engineering, Vol. 38, 2009, pp. 1128-1132.
- H. Yang and X. Z. Guo, “Effect of Colouring Agent on Colourisation and Structure of Li2O-Al2O3-SiO2 Glass Ceramics,” International Journal of Materials and Product Technology, Vol. 37, No. 3-4, 2010, pp. 280-286.
- X. z. Guo, H. Yang, et al., “Crystallization Mechanism of Lithium Aluminosilcate Glass Containing Co, Ni and Fe,” Chinese Journal of Nonferrous Metals, Vol. 17, No. 6, 2007, pp. 903-908.
- X. Z. Guo and H. Yang, “Effects of fluorine on Crystallization, Structure and Performances of Lithium Aluminosilcate Glass Ceramic,” Materials Research Bulletin, Vol. 41, No. 2, 2006, pp. 396-405. doi:10.1016/j.materresbull.2005.08.002
- X. Z. Guo, H. Yang, C. Han and F. Song, “Nucleation of Lithium Aluminosilicate Glass Containing Complex Nucleation Agent,” Ceramics International, Vol. 33, No. 7, 2007, pp. 1375-1379. doi:10.1016/j.ceramint.2006.04.019
- X. Z. Guo, H. Yang, C. Han and F. Song, “Crystallization and microstructure of Li2O-Al2O3-SiO2 Glass Containing Complex Nucleating Agent,” Thermochimica Acta, Vol. 444, No. 2, 2006, pp. 201-205. doi:10.1016/j.tca.2006.02.016
- X. Z. Guo, H. Yang, M. Cao, C. Han and F. F. Song, “Crystallinity and Crystallization Mechanism of Lithium Aluminosilicate Glass by X-Ray Diffractometry,” Transactions of Nonferrous Metals Society, Vol. 16, No. 3, 2006, pp. 593-597.
- X. z. Guo, et al., “Effects of Li Replacement on the Nucleation, Crystallization and Microstructure of Li2O-Al2O3- SiO2 Glass,” Journal of Non-Crystalline Solids, Vol. 354, No. 34, 2008, pp. 4031-4036. doi:10.1016/j.jnoncrysol.2008.05.013
- H. E. Kissinger, “The Crystallization Kinetics with Heating Rate in Differential Thermal Analysis,” Journal of research of the National Bureau of Standards, Vol. 57, 1956, pp. 217-221.
- J. A. Augis and J. E. Bennett, “Calculation of the Avrami parameters for Heterogeneous Solid State Reactions Using a modification of the Kissinger method,” Journal of Thermal Analysis and Calorimetry, Vol. 13, No. 2, 1978, pp. 283-292. doi:10.1007/BF01912301

