International Journal of Organic Chemistry
Vol. 2 No. 1 (2012) , Article ID: 17847 , 4 pages DOI:10.4236/ijoc.2012.21005
Stereo-Selective Synthesis of 5-Norbornene-2-exo-carboxylic Acid—Rapid Isomerization and Kinetically Selective Hydrolysis
Graduate School of Bio-Applications and Systems Engineering, Tokyo University of Agriculture and Technology, Tokyo, Japan
Email: kogino@cc.tuat.ac.jp
Received January 11, 2012; revised February 16, 2012; accepted February 25, 2012
Keywords: Stereo-Selective Synthesis; 2-Substituted Norbornene; Isomerization; Kinetically Selective Hydrolysis
ABSTRACT
Simple and efficient stereo-selective synthesis of exo-5-norbornene-2-carboxylic acid (NBCA) is reported. Preliminary studies on base promoted isomerization of methyl 5-norbornene-2-carboxylate (MNBC) revealed that rapid isomerization was accomplished with sodium tert-butoxide (tBuONa), and the exo-content at the equilibrium was ca. 60%. The hydrolyses of endo-rich MNBC (endo/exo = 80/20) under various conditions were carried out. The exo selectivity for resulting NBCA was improved when the hydrolysis was conducted with equimolar water at room temperature in the presence of the stronger base (tBuONa) (endo/exo: 18/82). Whereas the use of excess amount of water led to rapid and non-selective hydrolysis affording high endo content of the product. The plausible reaction mechanism involving rapid equilibrium of thermodynamic isomerization and kinetically preferred hydrolysis of exo ester is proposed.
1. Introduction
5-Norbornene-2-carboxylic acid and its derivatives are important as intermediates of pharmaceutically and biologically active compounds and monomers for advanced polymeric materials. 2-Substituted norbornene compounds can be conventionally obtained by Diels-Alder cycloaddition between cyclopentadiene and acrylic compounds. It is well-known that Diels-Alder cycloaddiotion is endoselective due to secondary orbital overlap, and the selectivity is enhanced by Lewis acid [1,2]. Polynorbornenes, which can be synthesized by vinyl polymerization or ring-opening metathesis polymerization (ROMP) of norbornene derivatives, show high thermal stability and transparency, and are useful for optical applications such as optical fibers, disk, lens and displays [3,4]. Norbornene derivatives are also useful as monomers or intermediates for photoresist materials [5,6]. Exo-isomer of norbornene carboxylic ester shows higher reactivity in living ROMP than endo-isomer [7]. In the case of photoresist monomer synthesis, endo-isomer provides undesired lactone by intramolecular cycloaddition to result in a low yield of desired photoresist monomer [8]. As can be seen in the two examples, exo-selective synthesis of norbornene carboxylic acid derivatives is of importance from the practical viewpoint.
Some of exo-selective syntheses of norbornene derivatives have been reported. Gouverneur et al. reported asymmetric Diels-Alder reaction by using antibody catalyst [9]. Fraile reported asymmetric Diels-Alder reaction of furan with chiral acrylate [10]. Avenoza and Kawamura reported asymmetric Diels-Alder reaction of cyclopentadiene and chiral dienophile [11,12]. Because of high cost of these reactions, practically pure exo-isomer is obtained by removing of endo-isomer through lactonization [13, 14]. Previously we reported exo-selective synthesis of 5-norbornene-2-carboxylic acid from endo-rich carboxylate in order to investigate the effect of endo/exo ratio on performance of photoresist prepared from tert-butyl 5- norbornene-2-carboxylate [15]. High exo-content and yield of the product cannot be explained only by exoselective hydrolysis as reported by of Niwayama and Hiraga [16]. As the plausible mechanism, we proposed that hydrolysis should be carried out accompanying with endo/exo isomerization.
In this paper, various experimental parameters includeing reaction solvent, temperature, type of base, and the amount of water were optimized in order to afford simple and efficient synthetic method for exo-norbornene carboxylic acid, and confirm our hypothesis about exo-selective hydrolysis.
2. Experiment
2.1. Materials
5-Norbornene-2-carboxylate (MNBC) and tert-butyl 5- norbornene-2-carbocylate (tBNBC) were synthesized via conventional Diels-Alder cycloaddition reaction according to the previous work [15]. The ratio of endo/exo was determined as 80/20 by 1H-NMR and HPLC. Sulfuric acid was obtained from Aldrich. Tetrahydrofuran (THF) was purchased from Wako Pure Chemical Industries. Ltd., and dehydrated by distillation under nitrogen with sodium/benzophenone. All other materials were purchased from Wako Pure Chemical Industries. Ltd. and used without any further purification unless otherwise mentioned.
2.2. General Procedure for Synthesis of exo-Rich 5-Norbornene-2-carboxylic acid (NBCA)
THF solution of base (1 mol/L) was charged into a twonecked flask equipped with a dropping funnel under nitrogen atmosphere. MNBC (exo; 20%, 0.4 mol/L) was added into the flask and stirred at room temperature for 3 h. One or ten equivalents of deionized water diluted with THF was added into the reaction mixture dropwise, and the reaction was continued for 24 h at predetermined temperature. In order to complete the hydrolysis, excess amount of deionized water was added. The reaction mixture was stirred for 1 h at room temperature and was neutralized with acetic acid until pH was adjusted to 7.5. The excess solvent was removed under reduced pressure. After the addition of 35% HCl until pH was adjusted to 2.0, the reaction mixture was extracted with toluene three times. Toluene was removed under reduced pressure, and the product was dried in vacuo over night to give NBCA.
2.3. Synthesis of exo-Rich Methyl 5-Norbornene-2-carboxylate (MNBC)
5-Norbornene-2-carboxylic acid (9.40 g, 68 mmol, exo; 72), methanol (8.30 mL, 0.20 mol), dichloromethane (20 mL) and 98%-sulfuric acid (0.28 mL, 5.0 mmol) were added into 100 mL-three-necked flask. The mixture was heated and refluxed for 17 h, and cooled to room temperature. After addition of deionized water, the reaction mixture was extracted with dichloromethane and the extract was washed with sat. NaHCO3aq. The solvent was removed under reduced pressure to give methyl 5-norbornene 2-carboxylate (9.63 g, 93%, exo; 75%).
2.4. Characterization
1H-NMR spectra were obtained on a JEOL ECX-500 instrument at 500 MHz. The ratio of endoand exo-isomers of MNBC, NBCA, and tBNBC were determined from peak intensities of each isomer by 1H-NMR in chloroform-d at 25˚C. The ratio of MNBC and NBCA were also determined by HPLC; JASCO C-Net II/ADC system, UV-detector; JASCO UV-2075 (224 nm), column; SC PEGASIL ODS-2352 (4.6 mm i.d., 18 cm), eluent; distilled water/methanol = 4/6 (1 mL/min), pH was adjusted to 3 by phosphate buffer, retention time; exo-isomer 7.4 min, endo-isomer 8.6 min. The ratio determined by HPLC was calibrated by NMR.
3. Results and Discussion
Figure 1 shows 1H-NMR spectrum of MNBC synthesized via a conventional Diels-Alder cycloaddition reaction as a starting material. The mole ratio of endo/exo was determined as 80/20. In order to obtain 5-norbornene-2-exo-carboxylic acid (exo-NBCA), isomerization from endo-isomer to exo-isomer is required. As MNBC has an active proton at α-position of carbonyl group, the corresponding carbanion can be generated by elimination of the proton in basic conditions. In general, carbanion has sp3-type trigonal pyramid structure, and low activation barrier for the inversion of this geometry. By utilizing the continuous inversion, the mixture comprising mainly endo-isomer of MNBC can be isomerized to corresponding endo/exo mixture of which ratio is ruled by thermodynamic equilibrium under basic conditions.
Figure 2(a) shows temperature dependence of isomerization of endo-rich MNBC in the presence of sodium methoxide (CH3ONa). At room temperature, the rate of isomerization was slow, and the reaction did not reach its equilibrium (the exo-content increased to about 35% from 20%). When the solvent was changed to THF from methanol, the kinetics of isomerization and thermodynamic equilibrium were not changed. The kinetics of isomerization and exo-ratio was not dependent on the solvent. At elevated temperatures (40˚C and 60˚C), the exo content approached to 50%, and the equilibrium was almost established within 2 h. Maximum exo content remained at 50% even heated at 67˚C (not shown). As shown in Figure 2(b), a strong base (tBuONa) effectively promoted the isomerization to reach thermodynamic equilibrium (exo content ca. 60%) rapidly even at room temperature in 10min. When exo-rich MNBC (exoratio; 75%) was used as a starting materials, the ratio of exo-isomer in the equilibrium was also the same as that obtained with endo-rich MNBC. In the case of norbornene dicarboxilic acid anhydride, exo-isomer is known to be thermodynamically more stable than endo isomer [16]. These results suggest that a slight difference of thermodynamic stability between endoand exo-isomers of norbornene monocarboxylate carbanion exist, but it is negligible above 40˚C.
The kinetics difference of hydrolysis of endoand exo-
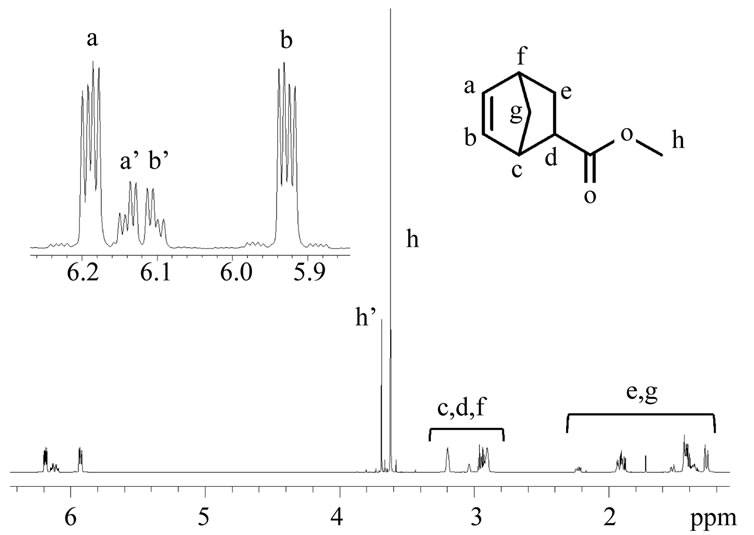
Figure 1. 1H-NMR spectrum of endo-rich mixture of methyl 5-norbornene-2-carboxylate measured at 500 MHz in CDCl3 (endo/exo = 82/18).
 (a)
(a) (b)
(b)
Figure 2. Endo-exo isomerization of MNBC (a) Temperature dependence using CH3ONa in methanol (solid line) or THF (broken line) (MNBC/CH3ONa = 1/2.2, [MNBC] = 0.45 M); (b) Base dependence in THF at 25˚C (MNBC/base = 1/2.2, [MNBC] = 0.45 M).
MNBC can be utilized to obtain exo-rich mixture of NBCA. As suggested by Niwayama [16], the rate of hydrolysis of exo-isomer is expected to be faster than that of endo-isomer due to the steric hindrance of norbornene ring.
In order to obtain exo-rich mixture of NBCA in a high yield, it is considered that kinetically exo-selective hydrolysis should occur accompanied by the rapid isomerization from endoto exo-isomer. As described in the previous section, part of esters is considered to exist as the corresponding carbanions under basic conditions allowing mutual isomerization. However, once the hydrolysis proceeds, resulting carboxylate anion does not generate carbanion because of high energy level of resulting dianion, which means that isomerization carboxylate anion is suppressed, while ester is isomerized through continuous inversion via the carbanion. Therefore, kinetically exo-selective hydrolysis accompanied by a rapid isomerization of ester enables to generate exo-carboxylic acid in a high yield as illustrated in Scheme 1.
In this study typical hydrolysis experiments were carried out by adding water into the solution of MNBC and CH3ONa or tBuONa, or potassium tert-butoxide (tBuOK). Table 1 lists experimental conditions and results. In all experiments, exo-rich NBCA was obtained compared with starting ester (exo-ratio; 20%) in high conversions, which supports the mechanism described in Scheme 1. Run 1 was carried out under almost the same conditions as a previous report [8,15]. By changing the solvent from methanol to anhydrous THF, exo selectivity increased slightly and the conversion was improved. In Run 3, excess amount of water was rapidly added into the reaction mixture. As a result, low exo-ratio was observed since nonselective hydrolysis occurred due to high concentration of water. Lowering temperature is expected to increase exo-ratio by the improvement of the selectivity of hydrolysis, but resulted in lower exo-ratio at room temperature (Run 4) probably due to slow isomerization as shown in Figure 2(a). The rate of isomerization was accelerated by stronger base, tBuONa as shown in Figure 2(b). By using tBuONa as a base, exo-ratio increased significantly (Run 6, Figure 3 shows 1H-NMR spectrum). When the reaction was carried out at higher temperature (Run 5), exo-ratio slightly decreased because the selectivity decreased because of the high hydrolysis rate. Decreasing temperature to 5˚C provided slightly lower exocontent than at room temperature (Run 7). It may be due to slow isomerization similar to the case of Run 3. In the case using tBuOK as a base (Run 8), exo-ratio was almost the same as that with tBuONa (Run 6). Acidic hydrolysis led to low exo-content with relatively low conversion, suggesting that endo/exo isomerization occurs under only basic conditions (Run 9). Hydrolyses of another ester, tert-butyl 5-norbornene-2-carboxylate (tBNBC)
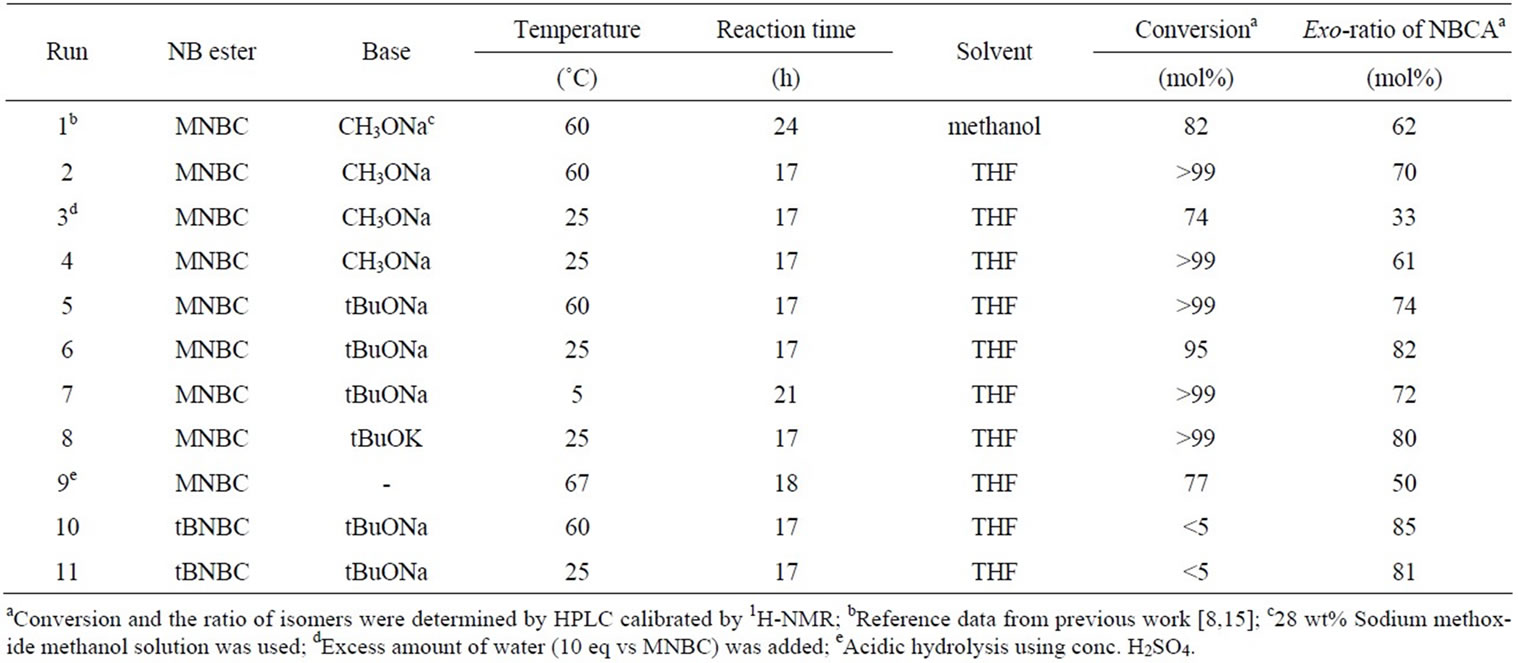
Table 1. Conditions and results of hydrolysis.
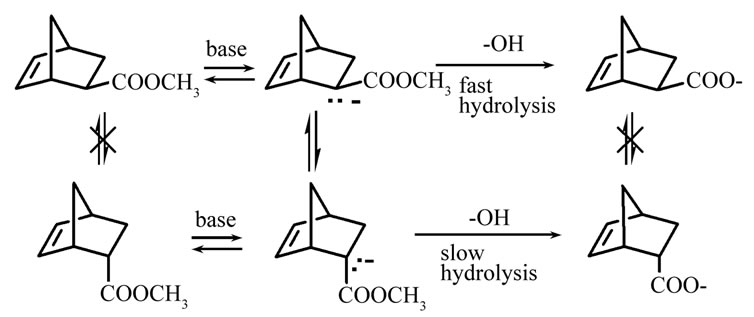
Scheme 1. Reaction mechanism of stereo-selective synthesis.

Figure 3. 1H-NMR spectrum of exo-rich mixture of 5-norbornene-2-carboxylic acid measured at 500 MHz in CDCl3 (endo/exo = 18/82, run 6 in Table 1).
were also investigated under similar conditions. As expected, high exo-content of products were obtained with low conversions due to the steric hindrance (Runs 10, 11). It is considered that high selectivity in hydrolysis is still valid.
Presented results indicate that hydrolysis with equimolar water in the presence of a strong base at moderate temperature provides high stereo-selectivity in the conversion of MNBC to NBCA. In order to obtain high exoratio of NBCA, both rapid isomerization and slow hydrolysis are required.
4. Conclusion
We investigated the relationship between the exo-selectivity and conversion in hydrolysis reaction of MNBC, and the experimental conditions including basicity, solvent, and temperature. High selectivity with high conversion was accomplished by the slow hydrolysis with equimolar water in the presence of a strong base (tBuONa) at room temperature. Based on the results, a plausible mechanism for high selectivity is proposed which involves the rapid isomerization of a reactant during slow and selective hydrolysis, and relatively slow isomerization of resulting carboxylate anions. In conclusion, exo-5-norbornene carboxylic acid was synthesized by simple and efficient procedure, and the proposed method provides isomerically rich compound without chiral materials, catalysts, strict reaction conditions.
REFERENCES
- R. Hoffmann and R. B. Woodward, “Orbital Symmetries and endo-exo Relationships in Concerted Cycloaddition Reactions,” Journal of American Chemical Society, Vol. 87, No. 19, 1965, pp. 4388-4389. doi:10.1021/ja00947a033
- A. C. Kinsman and M. A. Kerr, “Highly Selective Diels-Alder Reactions of Dienophiles with 1,3-Cyclohexadiene Mediated by Yb(OTf)3·2H2O and Ultrahigh Pressures,” Organic Letters, Vol. 2, No. 22, 2000, pp. 3517- 3520. doi:10.1021/ol0065773
- C. Janiak and P. G. Lassahn, “Metal Catalysts for the Vinyl Polymerization of Norbornene,” Journal of Molecular Catalysis A, Vol. 166, No. 2, 2001, pp. 193-209. doi:10.1016/S1381-1169(00)00475-1
- C. W. Bielawski and R. H. Grubbs, “Living Ring-Opening Metathesis Polymerization,” Progress in Polymer Science, Vol. 32, No. 1, 2007, pp. 1-29. doi:10.1016/j.progpolymsci.2006.08.006
- M. D. Rahman, D. S. McKenzie, J. B. Bae, T. Kudo, W. K. Kim, M. Padmanaban and R. R. Dammel, “Novel Hybrid Copolymers of Cycloolefin/Maleic Anhydride (COMA)/Methacrylate for 193-nm Resist Compositions,” Proceedings of the 18th International Society for Optical Engineering, Advances in Resist Technology and Processing, Santa Clara, Vol. 4345, 25 February-2 March 2001, pp. 159-167.
- K. Nakano, S. Iwasa, K. Maeda and E. Hasegawa, “Thermally Stable Alkylsulfonium Salts for ArF Excimer Laser Resists,” Journal of Photopolymer Science and Technology, Vol. 14, No. 3, 2001, pp. 357-362. doi:10.2494/photopolymer.14.357
- J. M. Pollino, L. Stubbs and M. Weck, “Living ROMP of exo-Norbornene Esters Possessing PdII SCS Pincer Complexes or Diaminopyridines,” Macromolecules, Vol. 36, No. 7, 2003, pp. 2230-2234. doi:10.1021/ma025873n
- Mitsubishi Rayon Co. Ltd., “Production Method of 5-Norbornene-2-carboxylic Acid and Its Esters,” JP patent No. JP2006-160712AO, 2006.
- V. E. Gouverneur, K. N. Houk, B. de-Pascual-Teresa, B. Beno, K. D. Janda and R. A. Lerner, “Control of the exo and endo Pathways of the Diels-Alder Reaction by Antibody Catalysis,” Science, Vol. 262, No. 5131, 1993, pp. 204-208. doi:10.1126/science.8211138
- J. M. Fraile, J. I. Garcıa, D. Gracia, J. A. Mayoral and E. Pires, “First Asymmetric Diels-Alder Reactions of Furan and Chiral Acrylates Usefulness of Acid Heterogeneous Catalysts,” Journal of Organic Chemistry, Vol. 61, No. 26, 1996, pp. 9479-9482. doi:10.1021/jo961513k
- A. Avenoza, M. P. Bueno, C. Cativiela and J. A. Mayoral, “Asymmetric Synthesis of Exo-Norbornane-2-carboxylic Acids,” Tetrahedron: Asymmetry, Vol. 3, No. 3, 1992, pp. 343-346. doi:10.1016/S0957-4166(00)80270-0
- M. Kawamura and K. Kudo, “Exo-Selective Asymmetric Diels-Alder Reaction of Acrylate Ester,” Chirality, Vol. 14, No. 9, 2002, pp. 727-730. doi:10.1002/chir.10130
- J. D. Roberts, E. R. Trumbull Jr., W. Bennett and R. Armstrong, “The Reaction of Norbornylene with N-Bromosuccinimide Nortricyclene and Its Derivatives,” Journal of American Chemical Society, Vol. 72, No. 7, 1950, pp. 3116-3124. doi:10.1021/ja01163a086
- C. D. Ver Nooy and C. S. Rondestvedt Jr., “Formation of Nortricyclene Derivatives by Bromination of exo-2,5- Methylene-1,2,5,6-tetrahydrobenzoic Acids,” Journal of American Chemical Society, Vol. 77, No. 13, 1955, pp. 3583-3586. doi:10.1021/ja01618a048
- M. Kanao, A. Otake, K. Tsuchiya and K. Ogino, “Stereo-Selective Synthesis of exo-Norbornene Derivatives for Resist Materials,” Journal of Photopolymer Science and Technology, Vol. 22, No. 3, 2009, pp. 365-370. doi:10.2494/photopolymer.22.365
- S. Niwayama and Y. Hiraga, “New exo/endo Selectivity Observed in Monohydrolysis of Dialkyl Bicyclo[2.2.1]- hept-5-ene-2,3-dicarboxylates,” Tetrahedron Letters, Vol. 44, 2003, pp. 8567-8570. doi:10.1016/j.tetlet.2003.09.131

