Journal of Crystallization Process and Technology
Vol.3 No.2(2013), Article ID:30894,5 pages DOI:10.4236/jcpt.2013.32011
Synthesis, Crystal Structure and Antimicrobial Activity of (E)-ethyl-4-(2-oxoacenaphthylen-1(2H)-ylideneamino) benzoate
![]()
1School of Chemical Sciences, Universiti Sains Malaysia, Penang, Malaysia; 2School of Biological Sciences, Universiti Sains Malaysia, Penang, Malaysia.
Email: *abdussalam@usm.my
Copyright © 2013 Yi-Chen Chan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 3rd, 2013; revised April 5th, 2013; accepted April 15th, 2013
Keywords: Schiff Base; Acenaphthenequinone; Ethyl-4-aminobenzoate; Antimicrobial; Panama Disease
ABSTRACT
A Schiff base, (E)-ethyl-4-(2-oxoacenaphthylen-1(2H)-ylideneamino)benzoate, (E4AB) had been synthesized in good yield by the acid-catalyzed condensation reaction of acenaphthenequinone and ethyl-4-aminobenzoate in methanolic solution. The synthesized compound was elucidated by elemental analysis (CHN), FTIR, 1H-NMR, 13C-NMR and single crystal X-ray diffraction. E4AB crystallized in the monoclinic crystal system with space group P21/c, Z = 4, V = 1569.3(2) Å3 and unit cell parameters a = 9.1589(8) Å, b = 21.2003(17) Å, c =8.4502(7) Å, β= 106.972(2)˚. The crystal structure of the compound is stabilized by intermolecular C-H O hydrogen bonds and weak intermolecular π
O hydrogen bonds and weak intermolecular π π interactions. The title compound had been tested for the antimicrobial activity against Bacillus subtilis (B. subtilis), Enterobacter and Fusarium oxysporum f. sp. Cubense (Foc) by disc-diffusion method. E4AB is relatively active against Foc which is a pathogen that cause Wilt disease (also well known as Panama disease) in banana plantation.
π interactions. The title compound had been tested for the antimicrobial activity against Bacillus subtilis (B. subtilis), Enterobacter and Fusarium oxysporum f. sp. Cubense (Foc) by disc-diffusion method. E4AB is relatively active against Foc which is a pathogen that cause Wilt disease (also well known as Panama disease) in banana plantation.
1. Introduction
Schiff base was first prepared by Hugo Schiff in 1864 [1]. Schiff bases are nitrogen analogues of aldehydes and ketones, having a carbon-nitrogen double bond in place of the carbonyl group [2]. Their formations constitute part of the broad class of condensation reaction. Schiff bases have been playing a pivotal role in the development of coordination chemistry and bioorganic chemistry with the acenaphthenequinone-based Schiff bases are widely synthesized due to their potential applications in various scientific areas [3-7].
Azomethines of acenaphthenequinone were found to have antimicrobial activities where El-Ayaan et al. [8] had reported that bis[N-(2,6-diisopropylphenyl)imino] acenaphthene was active against S. aureus, E. coli and C. albicans. Many of these microorganisms are pathogenic to animals and plants. B. subtilis and Enterobacter had been suggested to be associated with the sick building syndrome such as eye irritation, asthma, and throat infection among the building occupants [9-10] while Panama disease caused by Fusarium oxysporum f. sp. cubense (Foc) is regarded as one of the most dreadful disease threatening the banana production worldwide [11]. Hence, in this paper we report the synthesis and characterization of (E)-ethyl-4-(2-oxoacenaphthylen-1(2H)-ylideneamino)benzoate, (E4AB) and the antimicrobial activity of the title compound against B. subtilis, Enterobacter and Foc.
2. Experimental
In the preparation of E4AB, all the reagents were used as received. Melting point was determined by Stuart Scientific (UK) apparatus. Elemental analysis (CHN) was carried out on a Perkin Elmer Series II, 2400 analyzer. IR spectrum was recorded as KBr pellets on a Perkin Elmer System 2000 FT-IR spectrophotometer in the wavenumber range of 4000 - 400 cm−1. NMR spectra were recorded on a Bruker Avance III 500 MHz spectrometer in DMSO-d6 using tetramethylsilane as an internal standard.
2.1. Synthesis of E4AB
Acenaphthenequinone (0.182 g, 1 mmol), ethyl-4-aminobenzoate (0.165 g, 1 mmol) and 30.0 mL of methanol were placed in 100 mL round bottom flask. The mixture was allowed to heat under reflux for overnight in the presence of formic acid. The solvent was removed to give the crude compound. Single crystal suitable for Xray crystallography was obtained after recrystallization from ethanol. Yield: 73%. m.p.: 211˚C - 212˚C. Anal. Calcd for C21H15NO3: C 76.60, H 4.56, N 4.26%; found: C 76.94, H 4.33, N 4.38%. Main IR bands (KBr, cm−1): 2983 (w), 1729 (m), 1702 (s), 1666 (m), 1600 (s), 1275 (s), 1237 (m), 1107 (m), 1014 (m), 781 (m). 1H-NMR 500 MHz, (DMSO-d6, ppm): δ 1.37 (3H, t, CH3), 4.36 (2H, q, CH2), 6.79 (1H, d, H8), 7.22 (2H, d, H14), 7.59 (1H, t, H7), 7.93 (1H, t, H3), 8.11 (2H, d, H15), 8.16 (1H, d, H2), 8.22 (1H, d, H6), 8.39 (1H, d, H4). 13C-NMR (ppm): δ 14.21, 60.67, 117.77, 121.94, 123.19, 126.19, 126.39, 128.45, 128.62, 129.83, 130.15, 130.66, 130.90, 132.41, 142.68, 154. 98, 158.96 (C=N), 165.39 (C=O), 188.32 (C=O).
2.2. Crystal Structure Determination
Crystal data was collected using Bruker APEXII DUO area-detector diffractometer with graphite monochromated MoKα radiation at a detector distance of 50 mm and collected under 100 K using Oxford Cryosystem Cobra low temperature attachement [12]. The cell refinement and data reduction were performed using SAINT program [13] and the empirical absorption correction was performed using the SADABS program [13]. The structure was solved by direct methods and refined by leastsquares using SHLEXTL software package [14]. All non-hydrogen atoms were refined anisotropically. All hydrogen atoms were placed in idealized position, and refined using a riding model. The details of the crystal data and structure refinements are given in Table 1.
2.3. Antimicrobial Activity Determination
E4AB was tested for the antibacterial activity against B. subtilis and Enterobacter by slight modification of the disc-diffusion method described by Murray et al. [15]. 0.1 mL of 108 CFU/mL of bacteria suspension was streaked evenly onto the agar containing Petri plate. The plate was then allowed to air dry in laminar flow. E4AB was dissolved in DMSO to get 1000, 500 and 250 µg/mL concentrations of the compound. Four sterilized filter paper discs (diameter 7 mm) were placed at four equidistant places on the inoculated plate. 20 µL of each concentration of the compound was spiked on the disc accordingly by using micropipette. Filter paper disc treated with DMSO was served as the control in the test. Lastly, the inoculated plate was allowed to air dry and incubated under room temperature (25˚C ± 2˚C) for 48 hours before the inhibition zone was recorded in diameter (mm). Each test was conducted in triplicate. In the
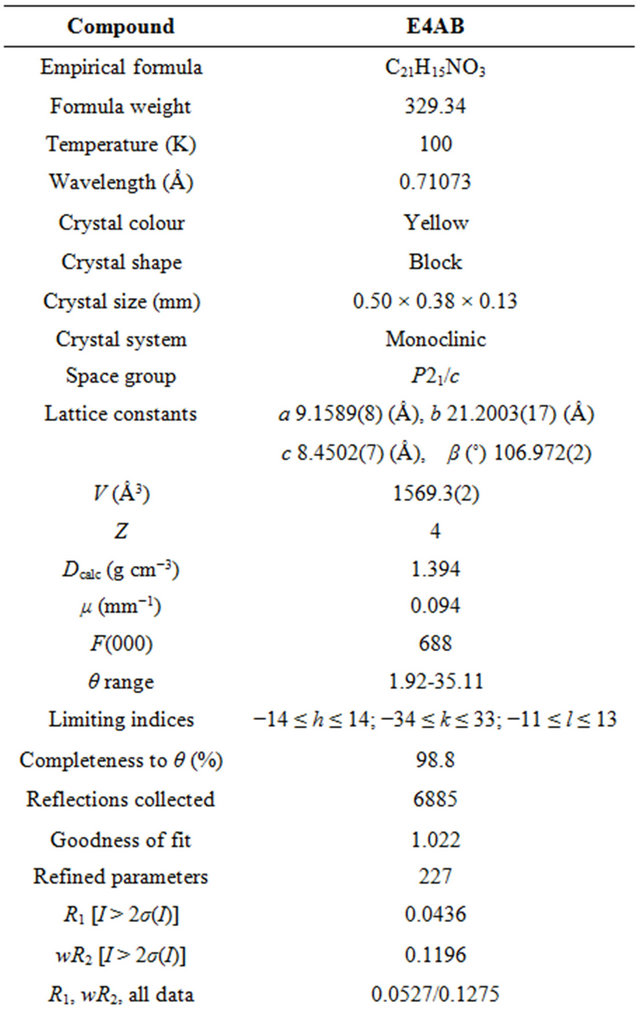
Table 1. Crystal data, data collection and refinement parameters of E4AB.
antifungal activity against Foc the same method as described for antibacterial activity was applied except for the amount of fungal suspension and incubation period used which were 1 mL of 1 × 104 spore/mL suspension and seven days, respectively.
3. Results and Discussion
3.1. Description of the Crystal Structure
In E4AB (Figure 1), the acenaphthylenone moiety is essentially planar, with atom C10 deviates from the mean plane formed by a maximum deviation of −0.057(1) Å. Nevertheless, the whole molecule is not planar, as indicated by the dihedral angle between the mean planes through the acenaphthylenone moiety and phenyl ring being 74.44(3)˚. The ethyl formate group is slightly inclined at a dihedral angle of 12.01(5)˚ with respect to the attached phenyl ring. In the crystal packing (Figure 2), adjacent molecules are interconnected into one-dimensional hydrogen-bonded chains propagating along the a axis via intermolecular C-H O hydrogen bonds
O hydrogen bonds
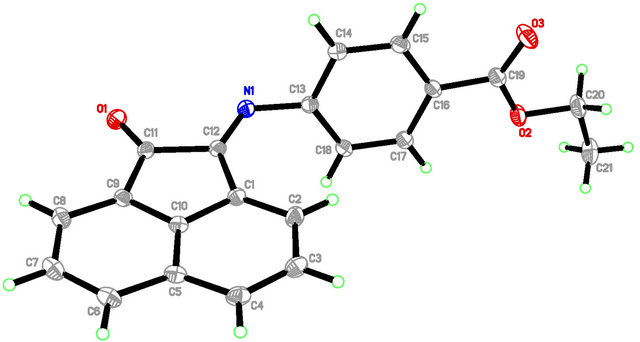
Figure 1. Molecular structure of E4AB. The non-hydrogen atoms are drawn as thermal ellipsoids with 50% probability displacement level.
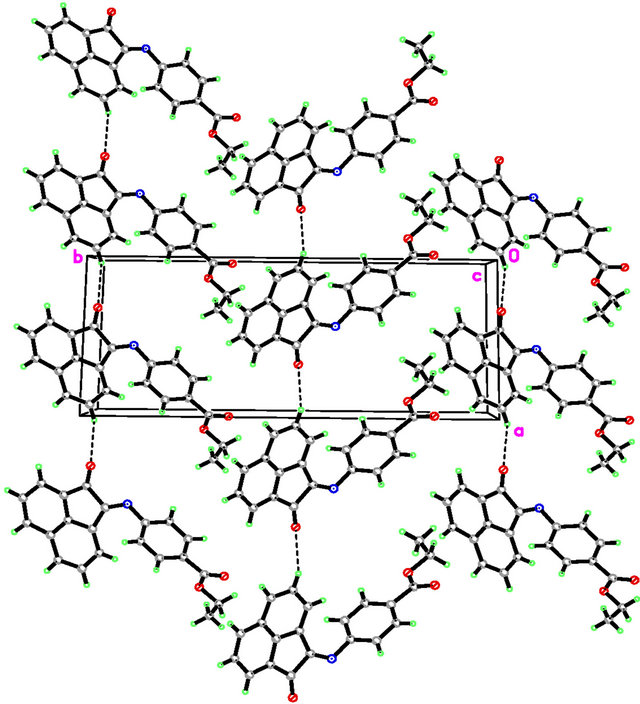
Figure 2. Crystal packing of E4AB, showing molecules being interconnected into one-dimensional chains along the a axis. Intermolecular hydrogen bonds are shown as dashed lines.
[C O distance of 3.4010(11) Å]. Further stabilization of the crystal packing is provided by weak intermolecular π
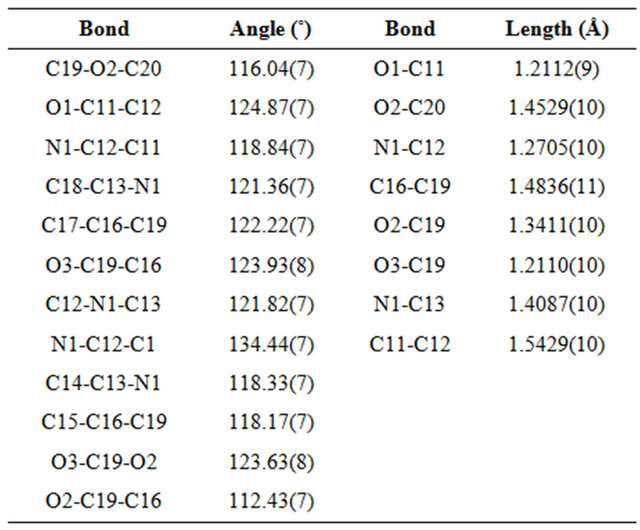
O distance of 3.4010(11) Å]. Further stabilization of the crystal packing is provided by weak intermolecular π π aromatic stacking interactions [centroid-centroid distance of 3.7102(6) Å] involving the C5-C10 phenyl ring. The selected bond lengths and bond angles are given in Table 2.
π aromatic stacking interactions [centroid-centroid distance of 3.7102(6) Å] involving the C5-C10 phenyl ring. The selected bond lengths and bond angles are given in Table 2.
3.2. FT-IR Spectrum
E4AB displays two stretching bands at 1729 and 1702 cm−1 which are corresponded to the C=O stretching vi
Table 2. Selected bond angles (˚) and bond lengths (Å) of E4AB.
brations of the ester and the ketone, respectively [16]. Meanwhile the ν (C=N) band is assigned to an absorption at 1666 cm−1 [17]. The characteristic aromatic ring C=C stretching frequency is observed at 1600 cm−1 [2]. In the IR spectrum of E4AB, the C-H stretch of sp3 hybrid carbon atom appears at 2983 cm−1.
3.3. 1H-NMR Spectroscopy
1H-NMR spectrum of E4AB is shown in Figure 3. A triplet at δ 1.37 ppm and a quartet at δ 4.36 ppm are assigned to H19 and H18, respectively. H8 which is ortho magnetically coupled to H7 appears as a doublet at δ 6.79 ppm with a coupling constant of 7.5 Hz. Meanwhile the doublet at δ 7.22 ppm is attributed to H14 which is coupled to H15 (δ 8.11 ppm). H7 and H3 are observed as triplets at δ 7.59 and 7.93 ppm, respectively. H7 is coupled to H8 and H6 while H3 is coupled to H2 and H4
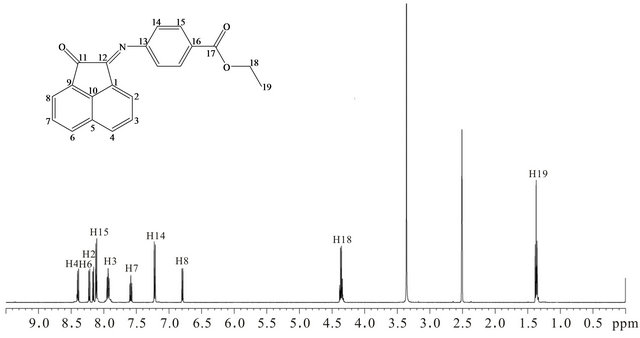
Figure 3. 1H-NMR spectrum of E4AB.
where H2, H4 and H6 are assigned to doublets at δ 8.16, 8.39 and 8.22 ppm, respectively. The assignment of naphthalene protons to the corresponding signals are in agreement with the previously reported observations by Wang et al. [18] and Yang et al. [19].
3.4. Antimicrobial Activity
The antimicrobial activity of the title compound in DMSO solution was assayed against two bacteria and one fungus by disc-diffusion method employing 1000, 500 and 250 µg/mL concentrations of the compound. The effectiveness of an antimicrobial agent in sensitivity testing is based on the size of zone of inhibition. The diameter of the zone is measured to the nearest millimeter. The result (Table 3) shows that at higher concentration, the Gram-negative bacterium (Enterobacter) is more sensitive towards E4AB than the Gram-positive bacterium (B. subtilis). E4AB also exhibits relatively potent inhibitory activity against Foc.
4. Conclusion
(E)-ethyl-4-(2-oxoacenaphthylen-1(2H)-ylideneamino)benzoate, E4AB had been synthesized in good yield. Melting point determination was performed to check the purity of the compound. Results obtained from the elemen
Table 3. Antimicrobial activity of E4AB.
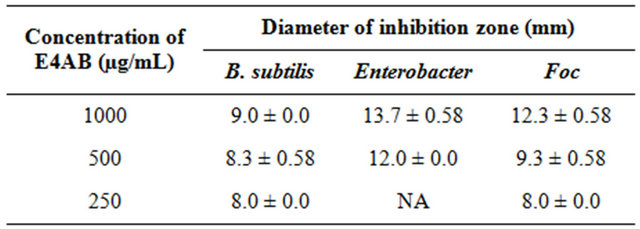
NA = not active tal, spectral (FTIR, NMR) and X-ray crystallography had confirmed the proposed structure of the title compound. E4AB was found to be able to inhibit B. subtilis, Enterobacter and Foc.
5. Acknowledgements
The authors would like to thank Universiti Sains Malaysia—Science Fund Grant No. 1001/PKIMIA/ 823003 and RU Grant No. 1001/PKIMIA/811196 for the financial support of this work.
REFERENCES
- R.-B. Xu, N. Zhang, H.-Y. Zhou, S. P. Yang, Y.-Y. Li, D.-H. Shi, W.-X. Ma and X.-Y. Xu, “Synthesis, Crystal Structure and Antibacterial Activity of N,N’-bis(4-metoxy-benzylidene)-1,4-bis(3-aminopropyl)piperazine,” Journal of Chemical Crystallography, Vol. 42, No. 9, 2012, pp. 928-932. doi:10.1007/s10870-012-0338-1
- L. G. Wade, “Organic Chemistry,” 6th Edition, Pearson Prentice Hall, Upper Saddle River, 2005.
- R. J. Maldanis, J. S. Wood, A. Chandrasekaran, M. D. Rausch and J. C. W. Chien, “The Formation and Polymerization Behaviour of Ni(II) α-Diimine Complexes Using Various Aluminium Activators,” Journal of Organometallic Chemistry, Vol. 645, No. 1-2, 2002, pp. 158-167. doi:10.1016/S0022-328X(01)01340-7
- I. Mhaidat, J. A. Mergos, S. Hamilakis, C. Kollia, Z. Loizos, A. Tsolomitis and C. T. Dervos, “One-Stage Synthesis of Poly[(p-phenylimino)-acenaphthene], a New Macromolecular Material with Interesting Electric Properties,” Materials Letters, Vol. 63, No. 29, 2009, pp. 2587- 2590. doi:10.1016/j.matlet.2009.09.016
- Y.-G. Li, L. Pan, Z.-J. Zheng and Y.-S. Li, “Polymerization of Ethylene to Branched Polyethylene with Silica and Merrifield Resin Supported Nickel(II) Catalysts with α-Diimine Ligands,” Journal of Molecular Catalysis A: Chemical, Vol. 287, No. 1-2, 2008, pp. 57-64. doi:10.1016/j.molcata.2008.03.003
- G. W. Son, K. B. Bijal, D. W. Park, C. S. Ha and I. L. Kim, “Novel Nickel(II)-Based Catalysts for the Polymerization of Ethylene,” Catalysis Today, Vol. 111, No. 3- 4, 2006, pp. 412-416. doi:10.1016/j.cattod.2005.10.054
- M. C. Rodriguez-Argüelles, M. B. Ferrari, G. G. Fava, C. Pelizzi, G. Pelosi, R. Albertini, A. Bonati, P. P. Dall’ Aglio, P. Lunghi and S. Pinelli, “Acenaphthenequinone Thiosemicarbazone and Its Transition Metal Complexes: Synthesis, Structure, and Biological Activity,” Journal of Inorganic Biochemistry, Vol. 66, No. 1, 1997, pp. 7-17. doi:10.1016/S0162-0134(96)00146-8
- U. El-Ayaan, and A. A. M. Abdel-Aziz, “Synthesis, Antimicrobial Activity and Molecular Modelling of Cobalt and Nickel Complexes Containing the Bulky Ligand: Bis[N-(2,4-diisopropylphenyl)imino]acenaphthene,” European Journal of Medicinal Chemistry, Vol. 40, No. 12, 2005, pp. 1214-1221. doi:10.1016/j.ejmech.2005.06.009
- P. L. Ooi and K. T. Goh, “Sick Building Syndrome: An Emerging Stress-Related Disorder,” International Journal of Epidemiology, Vol. 26, No. 6, 1997, pp. 1243-1249. doi:10.1093/ije/26.6.1243
- M. J. Mendell, W. J. Fisk, K. Kreiss, H. Levin, D. Alexander, W. S. Cain, J. R. Girman, C. J. Hines, P. A. Jensen, D. K. Milton, L. P. Rexroat and K. M. Wallingford, “Improving the Health of Workers in Indoor Environments: Priority Research Needs for a National Occupational Research Agenda,” American Journal of Public Health, Vol. 92, No. 9, 2002, pp. 1430-1440. doi:10.2105/AJPH.92.9.1430
- Jumjunidang, Riska and A. Soemargono, “Identification and Distribution of Fusarium Oxysporum f. sp. Cubense Isolates through Analysis of Vegetative Compatibility Group in Lampung Province, Indonesia,” Journal of Engineering and Applied Sciences, Vol. 7, No. 4, 1990, pp. 279-284.
- J. Cosier and A. M. Glazer, “A Nitrogen-Gas-Stream Cryostat for General X-Ray Diffraction Studies,” Journal of Applied Crystallography, Vol. 19, No. 2, 1986, pp. 105- 107. doi:10.1107/S0021889886089835
- Bruker, “APEX2, SAINT and SADABS,” Bruker AXS Inc., Madison, 2009.
- G. M. Sheldrick, “A Short History of SHELX,” Acta Crystallographica Section A, Vol. 64, No. 1, 2008, pp. 112-122. doi:10.1107/S0108767307043930
- R. P. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken, “Manual of Clinical Microbiology,” 6th Edition, ASM Press, Washington DC, 1995.
- J. Kovach, M. Peralta, W. W. Brennessel and W. D. Jones, “Synthesis and X-Ray Crystallographic Characterization of Substituted Aryl Imines,” Journal of Molecular Structure, Vol. 992, No. 1-3, 2011, pp. 33-38. doi:10.1016/j.molstruc.2011.02.027
- K. M. El-Shaieb, “Condensation of 1-(Dicycanomethylene)acenaphthene-2-one with Aromatic Diamines,” Journal of the Chinese Chemical Society, Vol. 55, No. 5, 2008, pp. 1150-1155.
- X. Y. Wang, J. N. Cui, W. M. Ren, F. Li, C. L. Lu and X. H. Qian, “Baker’s Yeast Mediated Reduction of Substituted Acenaphthenequinones: Regioand Eneatioselective Preparation of Mono-Hydroxyacenaphthenones,” Chinese Chemical Letters, Vol. 18, No. 6, 2007, pp. 681-684. doi:10.1016/j.cclet.2007.04.022
- W. Yang, Z. Yin, Z. Li, J. He and J.-P. Cheng, “A New Acenaphthenequinone-Based Calyxpyrrole: Synthesis, Structure and Anion Binding Study,” Journal of Molecular Structure, Vol. 889, No. 1-3, 2008, pp. 279-285. doi:10.1016/j.molstruc.2008.02.014
NOTES
*Corresponding author.

