Journal of Crystallization Process and Technology
Vol.1 No.3(2011), Article ID:7848,3 pages DOI:10.4236/jcpt.2011.13010
Tellurium Crystallisation in GeTeSb System
![]()
1Physics Department, Faculty of Science, Oran University, Oran, Algeria; 2Polymers Chemistry Laboratory, LCP Oran University, Oran, Algeria.
Email: *Nmaamar@yahoo.fr
Received July 23rd, 2011; revised August 27th, 2011; accepted September 6th, 2011.
Keywords: Chalcogenide, DSC, Switching, Kissinger Model, Crystallization Kinetics
ABSTRACT
Devitrification study of Ge15Te82Sb3 is compared to Ge14Te84.5Sb1.5 amorphous alloys using differential scanning calorimetry (DSC) and X-ray diffraction; we show tellurium effect in isothermal crystallization phenomena. The DSC traces of Ge15Te82Sb3 fixed at 413˚C show the phase separation in the sample with increasing transition temperatures Tg followed by two crystallization temperatures Tc1 and Tc2. This sample is compared to Ge14Te84.5Sb1.5 which crystallized by two transition temperatures and two crystallization temperatures Tc1 and Tc2. By X-Rays diffraction, we show that this behaviour is due to the presence of two phases in the amorphous sample with composition vitreous change during heating sample, the most important first one corresponds to the tellurium crystallization in the hexagonal form with 1.8 eV activation energy. The second correspond essentially to the crystallization of Te+ GeTe phase with 2.1 eV activation energy.
1. Introduction
Phase-change recording chalcogenide alloys are designed to have at least two structural forms, amorphous and crystalline, which can coexist at room temperature. Several factors [1], such as heat treatment are found to cause stability or crystallization of the alloys.
In the previous paper [2,3] we have determined the crystallization study of two systems Ge15–xTe82Sbx ternary eutectic and Ge20–xTe80Sbx. Among these compounds exhibit one glass transition temperatures followed by one crystallization temperatures. This paper aims to study the effect of annealing on the thermal properties and structure of Ge15.5–xTe84.5Sbx(0.5 ≤ x ≤ 2.5) system and the ageing effect of Ge15Te82Sb3 system. The first system exhibit two glass transition and two crystallization temperatures peaks. The studied compositions correspond to the transitory ternary peritectics displayed by Legendre and et al. [4]. The second system follows the same way only with aging effect. A process of nucleation and growth can describe a large majority of (a-c) phase transformations. Crystallization process of the Ge15.5–x Te84.5Sbx system is compared with the Ge15–xTe82 Sbx ternary eutectic system. Amorphous-crystalline transformations resulting from heat treatment were examined using an X-ray technique which can detect crystals in a glassy matrix, especially if the crystals are of dimensions greater than typically 100 nm [5].
2. Experimental
The preparation of the samples takes place in two steps. First, the three elements (99.999% purity) in suitable quantity are introduced into a quartz ampoule and sealed in a vacuum of 10–5 Pa. Then the ampoules were placed in an horizontally rotating oven and annealed at 1000˚C for 3 hours.
Finally, they were air quenched. The composition of the bulk alloys was checked by atomic absorption spectrometer (Perkin Elmer 2380). The ingots were ground down to a fine powder and placed in a capillary tube sealed under vacuum. The samples were annealed up to 1000˚C quenched in cold water. The amorphicity of the sample is checked by X-ray diffraction, the thermograms were recorded using a Setaram at sufficiently high heating rates (10 K/min).
3. Results
Figure 1 shows the typical thermograms obtained for the studied compositions Characteristic phenomena appear in the studied temperature range.
The values of these temperatures denote Tg and Tc, are reported in Table 1 for the different compositions. Except for the second glass transition temperature (Tg2), the other characteristic temperatures decrease as the amount of antimony increases. They correspond to the crystallization of two phases.
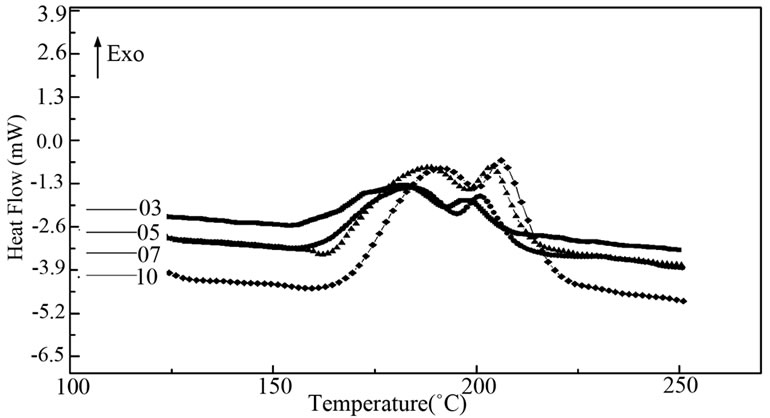
Figure 1. DSC traces Ge14Te84.5Sb1.5 at different hating rate.
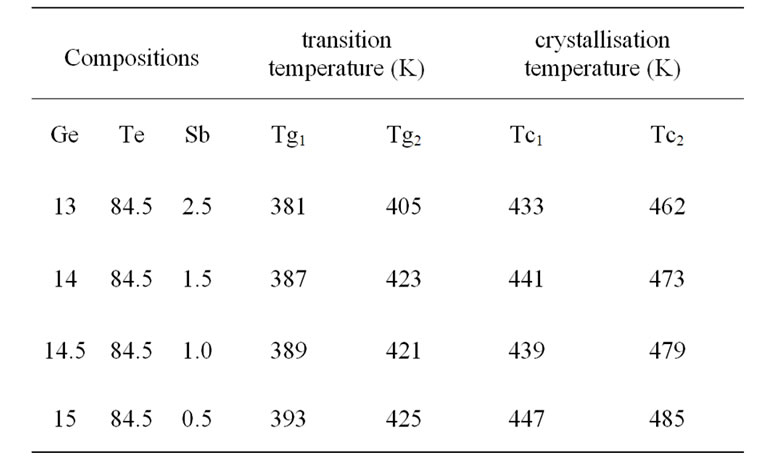
Table 1. Thermal parameters of GeTeSb.
Figure 2 correspond to the thermal effect of the sample. As a result of one hour thermal annealing of the bulk Ge15Te82Sb3 specimen at T = 433 K. some peaks with different intensities appeared; see Figure 3 Comparing their positions (2θ) with the PDF (powder diffraction (file) cards, the identified crystalline phases are Te phase with hexagonal structure of unit-cell constants a = 4.456 Å; c = 5.921 Å and GeTe rhombohedra structure of lattice constants a = 8.342 Å; c = 10.668 Å.
Kissinger model was used to determine activation energy for each phase for the study sample, we noted 1.8 eV for tellurium activation energy and 2.1 eV for the second phase see Figure 4.
4. Discussion
The Te-based amorphous alloys exhibit either one Tg and two Tc as in (GeSe)60(GeTe)20(Sb2Te3)20[6], two Tg and two Tc as in Al23Te87 [7] and in SixTe1–x [8]. However, in this last case the second glass transition appears after an annealing at a temperature near the first crystallization one. This may be explained by the fact that the first crystallization modifies the amorphous phase generally Te precipitates so that the remaining amorphous phase contains less Te atoms and the environment of the other atoms is modified. A further annealing above Tc1 enables the determination of the glass transition temperature of this second amorphous and the temperature Tc2 corresponds to the crystallization of this second phase
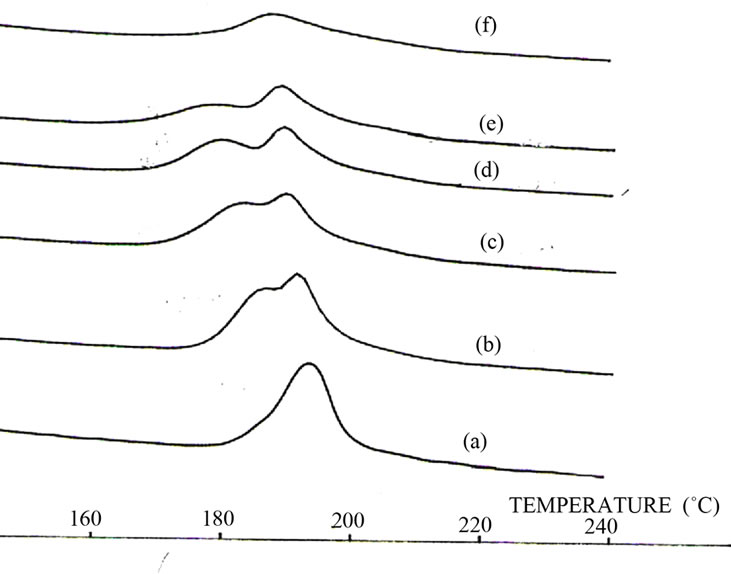
Figure 2. Thermal effect on Ge15Te82Sb3 DSC traces.
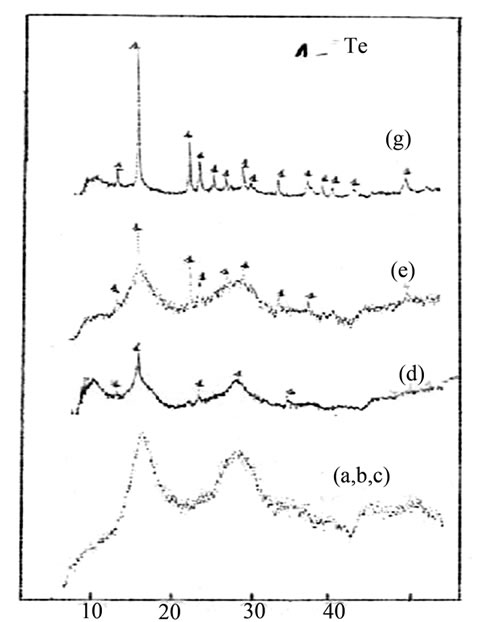
Figure 3. Thermal effect on Ge15Te82Sb3 X-ray traces.
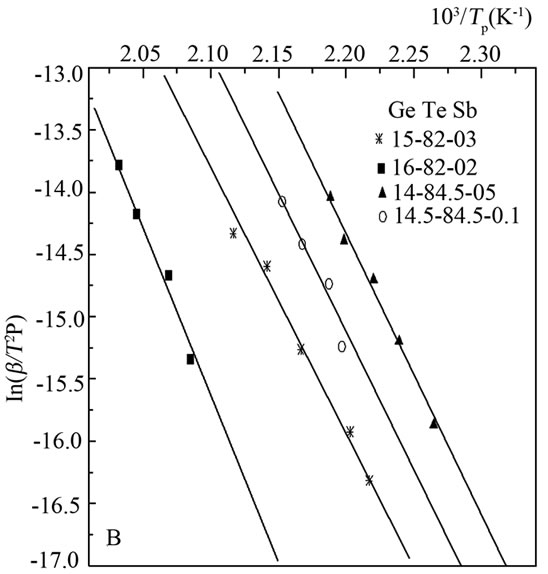
Figure 4. Determination of activation energy.
in the case Se60Ge20Sb20 both glass transition temperatures Tg1 and Tg2 appear before any crystallization. After the first crystallization at Tc1 and cooling at room temperature a further annealing lets Tg2 unchanged and enables the determination of the second crystallization temperature. These results and X-ray analysis lead to the assumption that the as-prepared material contains two amorphous phases: Sb2Se3 and GeSe2.
In our study, both glass transition temperatures Tg1 and Tg2 are followed by two exothermic peaks Tc1and Tc2 in Ge15.5–xTe84.5Sbx. A first annealing at a temperature near Tc1 leads to the precipitation of tellurium, in its hexagonal form, as in the other Te-based amorphous alloys. A Ge15Te82Sb3 study show one transition temperature and one crystallization temperature ,thermal effect show the phase separation in this sample with one Tg and two Tc with tellurium precipitation and constant crystallization enthalpy (area peak) for sample heated under 30 min (curve (a), (b) and (c) ).
The X-ray diffraction diagrams show an additional Te precipitation accompanying by the GeTe precipitation. Following Moss [9], This is probably due to the fact that the residual glass prior to the GeTe crystallization contains more than 50 at % of the Te.
The X-ray diffraction diagrams do not exhibit any ray corresponding to know Sb-based compounds. It is possible, as suggested by Moss, that the Sb atoms are concentred into the GeTe crystals and, due to the low concentration of Sb, is not visible X-ray diffraction.
5. Conclusions
It is obvious that the as-prepared Ge15.5–xTe84.5Sbx alloys contain two amorphous phases characterised by the two transition temperatures. However, the thermal effect in eutectic composition show the same phenomena.
During the first crystallization we only observe Te precipitation which leads to the impoverishment of one or both amorphous phases. The second amorphous phases could have the approximative composition of Te+ GeTe which crystallize at Tc2.
REFERENCES
- S. R. Ovshinsky, “The Relationship between Crystal Structure and Performance as 58. Optical Recording,” Journal of Non-Crystalline Solids, Vol. 141, 1992, pp. 200-203.
- M. Belhadji, N. Ziani and M. Mostefa, “Phase Separation and Devitrification Study of GeTeSb,” Chinese Journal of physics, Vol. 43, No. 11, 200, p. 119.
- N. Ziani, M. Belhadji, L. Heireche, Z. Bouchaour and M. Belbachir, “Crystallization Kinetics of Ge20Te80 Chalcogenide Glasses Doped with Sb,” Physica B: Condensed Matter, Vol. 358, No. 1-4, 2005, pp. 132-137. doi:10.1016/j.physb.2004.12.068
- S. Bordas, M. T. Clavaguer-Mora, B. Legendre and C. Hancheng, “Phase Diagram of the Ternary System GeSbTe: II. The Subternary Ge-GeTe-Sb2Te3-Sb,” Thermochimica Acta, Vol. 107, 1986, pp. 239-265. doi:10.1016/0040-6031(86)85051-1
- M. A. Hassan and C. A. Hogarth, “A Comparison of the Optical Properties of Glass and of Evaporated Amorphous Thin Films of BaO-TeO2,” Journal of Materials Science, Vol. 24, No. 5, 1989, pp. 1607-1611. doi:10.1007/BF01105679
- S. Asokan, G. Partansarathy and E. S. R. Gopal, “Evidence for a New Metastable Crystalline Compound in GeTe,” Journal of Materials Science Letters, Vol. 4, 1985, p. 50.
- J. Colmenero and J. M Barandiaran, “Crystallization of Al23Te77 Glasses,” Journal of Non-Crystalline Solids, Vol. 30, No. 3, 1979, pp. 263-271. doi:10.1016/0022-3093(79)90165-0
- N. Afify, “Crystallization Kinetics of Overlapping Phases in Se0.6Ge0.2Sb0.2 Chalcogenide Glass,” Journal of NonCrystalline Solids, Vol. 126, No. 1-2, 1990, pp. 130-140. doi:10.1016/0022-3093(90)91030-U
- S. C. Moss and J. P de Neufville, “Research on the Properties of Amorphous Semiconductors,” Materials Research Bulletin, Vol. 7, No. 5, 1972, pp. 423-441. doi:10.1016/0025-5408(72)90145-6

