Paper Menu >>
Journal Menu >>
 Open Journal of Physical Chemistry, 2011, 1, 1-5 doi:10.4236/ojpc.2011.11001 Published Online May 2011 (http://www.SciRP.org/journal/ojpc) Copyright © 2011 SciRes. OJPC Thermochemistry of Heteroatomic Compounds: Calculation of the heat of Combustion and the heat of Formation of some Bioorganic Molecules with Different Hydrophenanthrene Rows Vitaly V. Ovchinnikov Tupolev Kazan State Technical University, St- K. Marks 10, 420111 Kazan, Russian Federation E-mail: chem_vvo@mail.ru Received March 23rd, 2011; revised April 10th, 2011; accepted May 10th, 2011. Abstract On the basis of the known experimental heats of combustions of the seventeen alkanes in condensed state, the general equation has been deduced, in which i and f are correlation coefficients, N and g are a numbers of valence electrons and lone electron pairs of heteroatoms in molecule. The pre- sented dependence has been used for the calculation of the heats of combustion of thirteen organic molecules with biochemical properties: holestan, cholesterol, methyl-cholesterol, ergosterol, vitamin-D comb HifNg 2, estradiol, an- drostenone, testosterone, androstanedione, morphine, morphinanone, codeine and pentasozine. It is noted that good convergence was obtained within the limits of errors of thermochemical experiments known in the literature and calculations of the heats of combustion for some of them were conducted. With the application of Hess law and the heats of vaporization vap H 1 , which has been calculated with the use of a topological solvation index of the first order s x , the heats of formation fH for condensed and gaseous phases were calculated for the listed bioorganic molecules. Keywords: Alkanes, Biochemical Molecules, the Heat of Combustion, Heat of Formation, Heat of Vaporiza- tion, Topological Solvation Index 1. Introduction In living organisms the organic molecules named as ster- oids are synthesized many. Extremely low reactivity of steroids complicates their metabolism, which can be compared to full oxidation (burning) in a live organism. In such cases, researchers use the theoretical calculation of the heat (enthalpy) of combustion for the biochemical substances. The electronic conception of the interdependence of valence and the heat of combustion of organic com- pounds has been formulated in review of Kharasch and Sher [2] (Equation 1) * 109.0 4* combi i H abchc d (1) The number -109.0 has been deduced from the com- bustion enthalpy of n-octane. It has dimension kJ·mol–1 electron–1 and characterizes the shift energy of the one electron. Such power is nearly equal to the sum of the heats of C-H and C-C breaking bonds in molecule at the combustion. The parameters a and b are a numbers of all carbon and hydrogen atoms and c is a number of the moving electrons from carbon to the more electronega- tive atoms in molecule. The sum of the heat corrections i hc has been introduced by authors of the above men- tioned work for the identical types of bonds, spa- tial structures or groups in researching compounds. i d 2. Results and Discusion In the complex organic and biochemical compounds with various heteroatoms, it is difficult to define a degree the group electronegativity and correspondingly a number of moving to them or inside of them, accordingly to Kharasch and Sher conception, electrons. In our opinion the majority of missing structural and energetic correc-  2 V. V. OVCHINNIKOV tions can be in a complex considered within the limits of one-factorial regression analysis [3]. This consists in construction of a various correlations between the ex- perimentally known values of the heats of combustion comb H of organic or heteroatomic compounds and a general number of the bond-forming electrons N in its Equation (2), in which comb H ifNg (2) i and f are the correlation coefficients, describing struc- turally-energetic contributions in the enthalpy of com- bustion and sensitivity of the last to a general number of electrons N, from which subtracted a number (g) of lone electron pairs of heteroatoms in the different functional groups. So, for IV group of Periodic Table (C, Si and below) g is equal 0, for V group (N, P and below) g is equal 1, for VI group (O, S and below) g is equal 2. Proceeding from the listed above and also scheme of the process combustion of saturated, unsaturated and aromatic hydrocarbons (Equation 3) n2n2 22comb 2 CHO COHOpqrgas sliqH (3) in which p, q, r, s are stoichiometric coefficients (values of ∆fHо for CO2 = –393.5 and H2O = –285.8 kJ·mol–1 are taken from monography [4]), we have calculated de- pendence (4), which included itself the heat of combus- tion of saturated of alkanes (Table 1, compounds 1-17) of a various normal-and cyclic structure and a number valence electrons in its (g = 0) 1 comb /kJ mol 25.6327.37108.540.49 * H N (4) r 0.999, so 32.9, n 17. From the deduced Equation (4) it is possible to see, pletely corresponds to the suggested earlier by Kharasch and Sher, that testifies to qualitative and their quantita- tive conformity. Besides, using last dependence, we calculated the combustion enthalpy of cis-decalin (18) and compared it with known in the literature. It is equal –6320.4 ± 31.6 and within the error, estimated by us in ± 0.5 %, coincides with experimental –6287.7 ± 0.9 kJ·mol–1 [4]. The specified circumstances gives the pos- sibility to apply the last equation for calculations of the heat of combustions for the variously substituted al- kanes: cholestane, (19), cholesterol (20), O-methyl- cho- lesterol (21), ergosterol (22), vitamin D2, received by the isomerization of ergosterol [5], (23), estradiole (24), androstenone (25), testosterone (26), androstanedione (27), having the important biochemical properties. It is necessary to note also a good correspondence (within the limits of ± 2%) of the thermochemical parameters calculated by us with the known in the literature (Table 2, Equation (5)) 1 comb exp 1 comb / kJmol476.4308.2 96.6216.7*/ kJmol calc H H (5) r 0.999, so 158.7, n 6 (20-22, 25-27). Moreover, necessary to note, that the experimental re- sults for cholesterol (20), androstenone (25), testosterone (26) and androstanedione (27) (Table 2 , column 5, 8) are very different each to other in the essential values, but they are rather near to calculated by us values (Table 2, column 4). In structure of other well-known bioorganic molecules [5], such as morphine (28), morphinanone (29), codeine (30) and pentasozine (31) the aromatic rings are included. Nevertheless, accordingly to Kharasch-Sher-conception there is a mention about the application of the Equation (1) to the saturated and aromatic compounds. For this reason expediently to calculate the combustion enthalpy of these substances with the use dependence (4) of for saturated hydrocarbons. Table 1. The number of valence electrons (N) and the heats of combustion (kJ·mol–1 of the different structural n-and cyclo- lkanes [4]. P 101kPa; T 298.15; all compounds are in condensed state. No Compound, formula N -ΔcombHNoCompound, formula N -ΔcombH 1 Methylcyclopentane (С6Н12 ) 36 3938.6 10 2-Methylheptane, (С8Н18 ) 50 5456.5 2 Cyclohexane, (С6Н12 ) 36 3920.0 11 2,5- Dimethylhexane, (С8Н18 ) 50 5460.2 3 n-Hexane, (С6Н14 ) 38 4163.3 12 3,4- Dimethylhexane, (С8Н18 ) 50 5468.7 4 Cycloheptane, (С7Н14 ) 42 4597.0 13 Ethylhexane, (С8Н18 ) 50 5470.2 5 Methylcyclohexane, (С7Н14 ) 42 4565.3 14 n-Decane, (С10 Н22) 62 6778.6 6 1,1-Diethylcyclohexane, (С8Н16 ) 48 5216.0 15 n-Dodecane, (С12 Н26 ) 74 8090.6 7 trans-1,3- Dimethyl-cyclohexane, (С8Н16 ) 48 5219.0 16 trans-syn-trans-Pentahydroanthracene,(С14 Н24 ) 80 8608.5 8 trans-1,4- Dimethyl-cyclohexane, (С8Н16 ) 48 5212.3 17 n-Hexadecane, (С16Н34 ) 98 10700.1 9 n-Octane, (С8Н18 ) 50 5471.8 Copyright © 2011 SciRes. OJPC 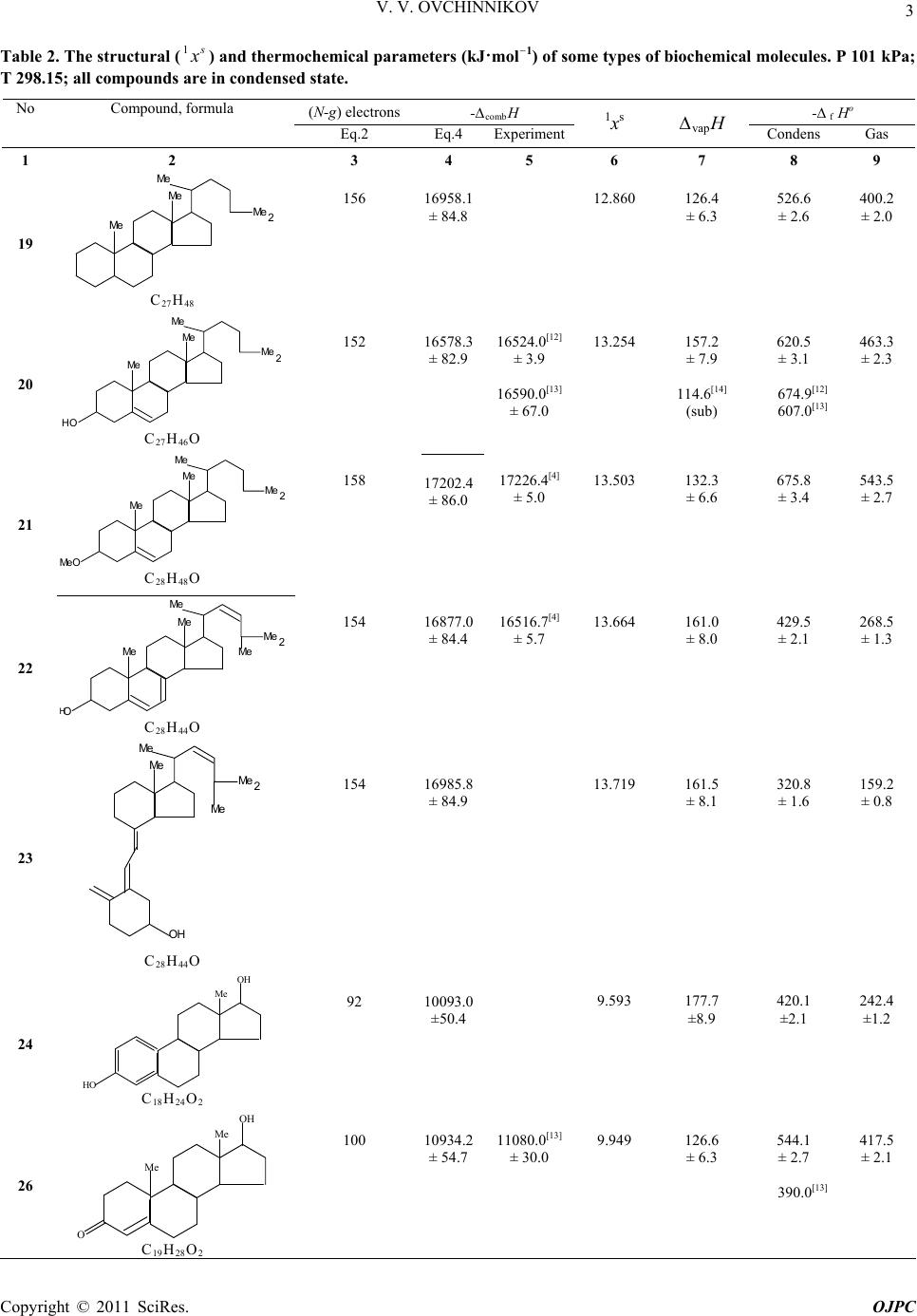 V. V. OVCHINNIKOV 3 Table 2. The structural (1 s x ) and thermochemical parameters (kJ·mol–1) of some types of biochemical molecules. P 101 kPa; T 298.15; all compounds are in condensed state. (N-g) electrons-combH - f Hо No Compound, formula Eq.2 Eq.4 Experiment 1xs vapH Condens Gas 1 2 3 4 5 6 7 8 9 19 Me Me Me Me 2 C27 H48 156 16958.1 ± 84.8 12.860 126.4 ± 6.3 526.6 ± 2.6 400.2 ± 2.0 20 Me Me Me Me 2 C27 H46O 152 16578.3 ± 82.9 16524.0[12] ± 3.9 16590.0[13] ± 67.0 13.254 157.2 ± 7.9 114.6[14] (sub) 620.5 ± 3.1 674.9[12] 607.0[13] 463.3 ± 2.3 21 Me Me Me Me Me 2 C28 H48O 158 17202.4 ± 86.0 17226.4[4] ± 5.0 13.503 132.3 ± 6.6 675.8 ± 3.4 543.5 ± 2.7 22 Me Me Me Me Me 2 H C28 H44O 154 16877.0 ± 84.4 16516.7[4] ± 5.7 13.664 161.0 ± 8.0 429.5 ± 2.1 268.5 ± 1.3 23 Me Me Me Me 2 C28 H44O 154 16985.8 ± 84.9 13.719 161.5 ± 8.1 320.8 ± 1.6 159.2 ± 0.8 24 HO Me OH C18 H24O2 92 10093.0 ±50.4 9.593 177.7 ±8.9 420.1 ±2.1 242.4 ±1.2 26 O Me OH Me C19 H28O2 100 10934.2 ± 54.7 11080.0[13] ± 30.0 9.949 126.6 ± 6.3 544.1 ± 2.7 390.0[13] 417.5 ± 2.1 Copyright © 2011 SciRes. OJPC 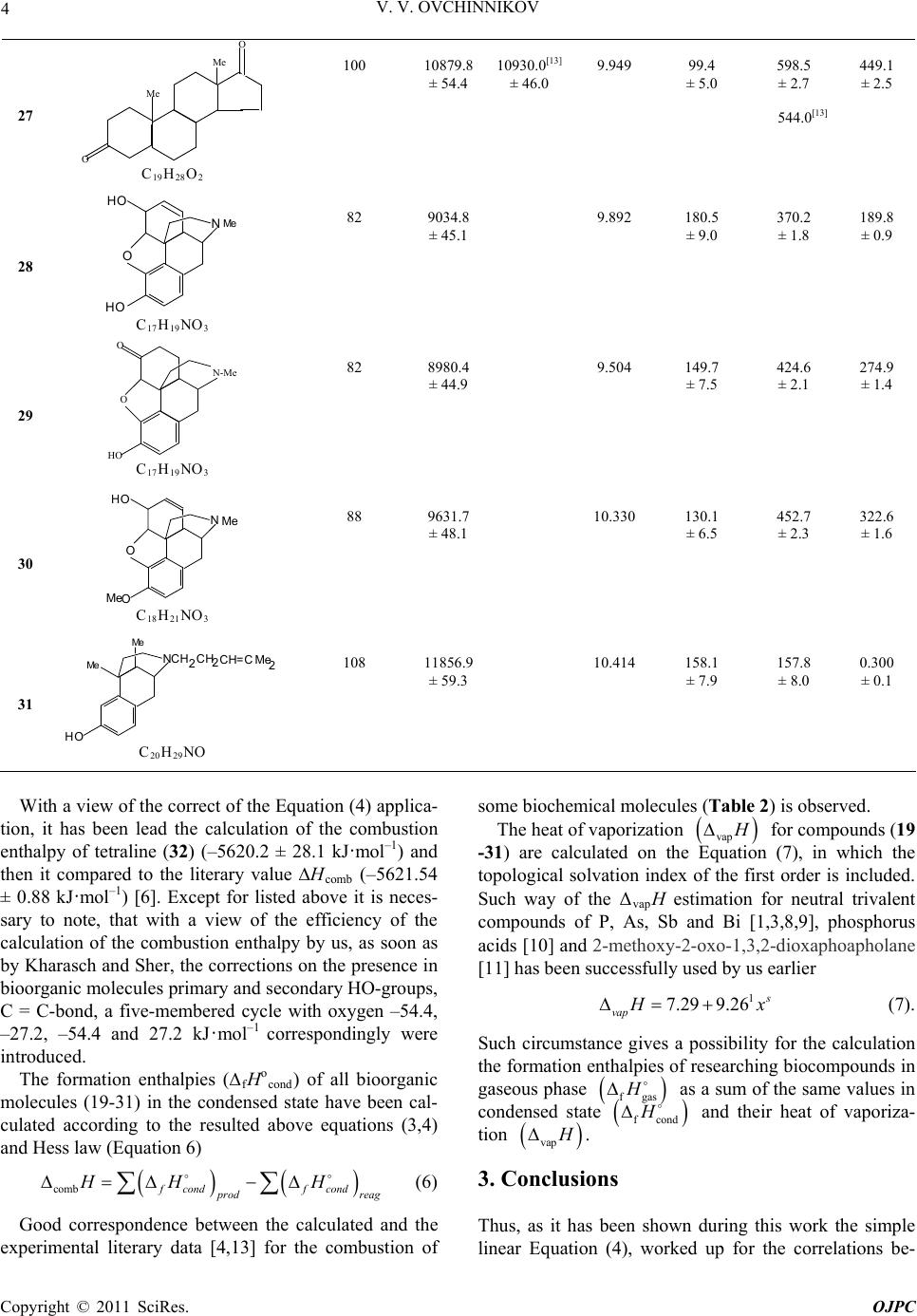 V. V. OVCHINNIKOV Copyright © 2011 SciRes. OJPC 4 27 Me Me O O C19 H28O2 100 10879.8 ± 54.4 10930.0[13] ± 46.0 9.949 99.4 ± 5.0 598.5 ± 2.7 544.0[13] 449.1 ± 2.5 28 Me C17 H19NO3 82 9034.8 ± 45.1 9.892 180.5 ± 9.0 370.2 ± 1.8 189.8 ± 0.9 29 O N-Me O HO C17 H19NO3 82 8980.4 ± 44.9 9.504 149.7 ± 7.5 424.6 ± 2.1 274.9 ± 1.4 30 Me Me C18 H21NO3 88 9631.7 ± 48.1 10.330 130.1 ± 6.5 452.7 ± 2.3 322.6 ± 1.6 31 Me Me Me C20 H29NO 108 11856.9 ± 59.3 10.414 158.1 ± 7.9 157.8 ± 8.0 0.300 ± 0.1 With a view of the correct of the Equation (4) applica- tion, it has been lead the calculation of the combustion enthalpy of tetraline (32) (–5620.2 ± 28.1 kJ·mol–1) and then it compared to the literary value Hcomb (–5621.54 ± 0.88 kJ·mol–1) [6]. Except for listed above it is neces- sary to note, that with a view of the efficiency of the calculation of the combustion enthalpy by us, as soon as by Kharasch and Sher, the corrections on the presence in bioorganic molecules primary and secondary НО-groups, С = С-bond, a five-membered cycle with oxygen –54.4, –27.2, –54.4 and 27.2 kJ·mol–1 correspondingly were introduced. The formation enthalpies (fHо cond) of all bioorganic molecules (19-31) in the condensed state have been cal- culated according to the resulted above equations (3,4) and Hess law (Equation 6) comb f condf cond p rod reag HH H (6) Good correspondence between the calculated and the experimental literary data [4,13] for the combustion of some biochemical molecules (Table 2) is observed. The heat of vaporization vap H for compounds (19 -31) are calculated on the Equation (7), in which the topological solvation index of the first order is included. Such way of the ΔvapН estimation for neutral trivalent compounds of P, As, Sb and Bi [1,3,8,9], phosphorus acids [10] and 2-methoxy-2-oxo-1,3,2-dioxaphoapholane [11] has been successfully used by us earlier 1 7.29 9.26 s vap H x (7). Such circumstance gives a possibility for the calculation the formation enthalpies of researching biocompounds in gaseous phase fgas H as a sum of the same values in condensed state f cond H and their heat of vaporiza- tion vap H . 3. Conclusions Thus, as it has been shown during this work the simple linear Equation (4), worked up for the correlations be-  V. V. OVCHINNIKOV 5 tween the heats of combustion of saturated alkanes of the different spatial structure and the general number of the valence electrons, excluding of the lone electron pare of heteroatoms in its, could be useful applicable to the cal- culation of the same thermochemical parameters of the bioorganic molecules of hydrophenanthrene rows. The calculated the heats of formation in a gaseous phase are necessary for an estimation of the bond ener- gies in biochemical substances. 4. References [1] V. V. Ovchinnikov and N. R. Muzafarov, “Thermochem- istry of Heteroatomic Compounds 26*. Calculation of the combustion and formation enthalpy of carbohydrate bi- cyclophosphites,” Russian Chemical Bulletin, Interna- tional Edition, Vol. 58, No. 4, April 2009, pp. 851-853. doi:10.1007/s11172-009-0105-4 [2] M. S. Kharasch and B. Sher, “The Electronic Conception of Valence and Heats of Combustion of Organic Com- pounds,” Journal Physical Chemistry, Vol. 29, No. 6, January 1925, pp. 625-658. doi:10.1021/j150252a001 [3] V. V. Ovchinnikov, “Thermochemistry of Heteroatomic Compounds: Enthalpies of Combustion and Formation of Organic Derivatives of P, As, Sb and Bi,” Doklady Physical Chemistry, Vol. 411, No. 2, February 2006, pp. 328-330. doi:10.1134/S0012501606120025 [4] J. D. Cox and G. Pilcher, “Thermochemistry of Organic and Organometallic Compounds,” Academic Press, New York, 1970. [5] M. Goodman and F. Morehouse, “Organic Molecules in Action,” Gordon and Breach Science Publishers, New York-London-Paris, 1973. [6] J. B. Pedley, R. D. Naylor and S. P. Kirby, “Thermo- Chemical Data of Organic Compounds,” Chapman and Hall, New York, 1986. [7] I. S. Antipin and A. I. Konovalov, “Prognostication of the Enthalpy of Vaporization and Solvation of Organic Com- pounds on the Basis of Topological Index 1 s,” Russian Journal of General Chemistry, Vol. 66, No.3, March 1996, pp. 389-401. [8] V. V. Ovchinnikov, L. I. Lapteva and M. G. Kireev, “Thermochemistry of Heteroatomiccompounds 19. En- thalpies of Combustion and Formation for Alkyl- phosphines,” Russian Chemical Bulletin, International Edition, Vol. 53, No. 8, August 2004, pp. 1693-1694. [9] V. V. Ovchinnikov, N. R. Muzafarov and L. I. Lapteva, “Thermochemistry of Heteroatomic Compounds 22. En- thalpies of combustion and formation of alkyl (aryl) ar- sines and arsenites in the condensed and gaseous phases,” Russian Chemical Bulletin, International Edition, Vol. 56, No. 5, May 2007, pp. 1042-1043. [10] V. V. Ovchinnikov and N. R. Muzafarov, “Calculations of the Enthalpies of Combustion and Formation of 2- Hydroxy-2oxo-1, 3, 2-dioxaphosphocyclanes,” Russian Journal of Physical Chemistry, Vol. 82, No. 11, Novem- ber 2008, p. 1979. [11] V. V. Ovchinnikov and N. R. Muzafarov, “Thermochem- istry of Heteroatomic Compounds: XXIV. Calculation of the Formation Enthalpy of 2-methoxy-2- oxo-1, 3, 2-Di- oxaphospholane,” Journal Physical Chemistry, Vol. 79, No. 6, June 2009, p. 1220. [12] W. H. Johnson, “The Enthalpies of Combustion and For- mation of Choletsterol [cholest-5-en-3-ol (3β)],” Journal of Research National Bureau Standards. Section A, Vol. 79, January 1975, pp. 493 -496. [13] D. Paoli, J.-C. Garrigues and H. Patin, “Combustion Mi- crocalorimetry: Application to Steroids,” C. R. Acad. Sci. Paris, Vol. 268, February 1969, pp. 780-783. [14] K. C. D. Hickman, J. C. Hecker and N. D. Embree, “Di- rect Determination of Low Vapor Pressures,” Industrial & Engineering Chemistry, Vol. 9, No. 6, January 1937, pp. 264-267. doi:10.1021/ac50110a005 Copyright © 2011 SciRes. OJPC |

