 Natural Science, 2009, 1, 10-16 NS http://dx.doi.org/10.4236/ns.2009.11003 Copyright © 2009 SciRes. OPEN ACCESS Studies on the effects of pretreatment on production hydrogen from municipal sludge anaerobic fermentation Feng Wu1,2, Shao-Qi Zhou1,*, Yang-Lan Lai1, Wen-Jiao Zhong2 1College of Environmental Science and Engineering, South China University of Technology, Guangzhou, 510006, China; 2Guangdong Provincial People’s Armed School, Guangzhou, 510006, China. Corresponding author: *fesqzhou@scut.edu.cn Received 31 March 2009; revised 15 April 2009; accepted 21 April 2009. ABSTRACT Municipal sludge was rich in organic matter, period of natural degradation was long and low efficiency, leachate would pollute underground water. In this paper, a comparative study of the ways of pretreatment with acid alkali treatment, heat digestion and ultrasonic treatment were done. The results showed that the dehydro- genase activity was increased, the SCOD (solu- able chemical oxygen demand, SCOD) in- creased more than 2.47~2.83, 1.70~1.76, 2.6~ 2.77 times respectively. The hydrogen yield in- creased more than 11.5~12.2, 24.1~24.7, 34.2~ 34.9 mL.g-1 (VS) respectively. The period of pro- hydrogen was shorten to 7.5, 8.0, 6.5 d respec- tively. The degradation rate was up to 72.04%, 81.4%, 80% respectively, the methane concen- tration in the gas was close to “zero” and ul- trasonic treatment was better than others. Gompertz model curve fitting on hydrogen production was carried out. All the values of correlation factor R2 were more than 0.97. Keywords: Municipal Sludge; Pretreatment; An- aerobic Digestion; Biological Pro-Hydrogen 1. INTRODUCTION The wastesolid treatment mainly includes filleading, compost and incineration. Filleading and heap in brief results in resource waste and pollute the body of water, even endanger the human health. Which don’t attach to coincidence method of “circulation economy”. The wastesolid anaerobic fermentation producted hydrogen develope a new road of wastesolid resource. And many research workers are interested in it [1,2,3,4,5,6,7]. Go- mez [8] has study on the two stages of bio-hydrogen production, hydrogen production and methanogenic, using organic solid waste and slaughterhouse waste as substrate, high temperature activated sludge as inoculum. Levin’s study [9] showed the wooden fiber of delignifi- cation is a good hydrogen production substrate. Liliana [6] use anaerobic sludge to degraded organic solid waste and synthetic wastewater in UASB whose capacity is 3.85 L, and produce hydrogen successfully. The volume content and hydrogen production rate of H2 is 47%, 99 N mL.g-1(VS); 51%, 127 N mL.g-1(VS) respectively. Zuo Yi [10] used river sediments as seed sludge, at the opti- mal condition of the pH of 5.0-5.2, temperature of 35, ℃ and HRT of 6-8 h, a steady anaerobic bio-hydrogen production was obtained in a lab scale reactor success- fully with gluose as substrate. The highest hydrogen production was 6.7 L.d-1. Tai Mulin [11] showed that the optimal initial pH for bio-hydrogen production from sewage sludge was around 11.0, Under this optimal con- dition, the bio-hydrogen yield of raw sludge was 8.1 mL.g-1, and it would reach to 16.9 mL.g-1 when the sludge was pretreated by alkali. Steven W. Van Ginkel [12] used food wastewater as substrate indicated Biogas produced from all four food processing wastewaters consistently contained 60% hydrogen, with the balance as carbon dioxide. Heguang Zhua [13] showed enhanced hydrogen production potential as compared with All combinations of the feedstocks (FW+PS, FW+WAS and FW+PS+WAS). A mixing ratio of 1:1 was found to be the best among the ratios tested and hydrogen yield of 112 mL.g-1 volatile solid (VS). M. Krupp and R. Wid- mann [14] studied Biohydrogen production by dark fer- mentation, the result showed The gas amount varied with the different OLRs, but could be stabilised on a high level as well as the hydrogen concentration in the gas with 44~52%. Ela Eroglua [15] introduced Biological hydrogen production from olive millwastewater with two-stage proc- esses. In some cases of dark-fermentation, activated sludge was initially acclimatized to the OMW to provide the ad- aptation of microorganisms to the extreme conditions of OMW. The highest hydrogen production potential obtained was 29 L H2/LOMW. Dongmin Li [16] used corn straw as substrate, Hydrogen was produced by simultaneous sac- charification and fermentation from steam-exploded corn straw (SECS) using Clostridium butyricum AS1.209.  F. Wu et al. / Natural Science 1 (2009) 10-16 11 Copyright © 2009 SciRes. OPEN ACCESS Maximum specific hydrogen production rate and maximal hydrogen yield were 126 mL.g-1 (VSS) d and 68 mL.g-1 SECS, respectively. The yield of soluble metabolites was 197.7 mg.g-1 SECS. Acetic acid accounted for 46% of the total was the most abundant product and this shows that hydrogen production from SECS was essentially ace- tate-type fermentation. Consequently, fermentative bio-hydrogen production technique is at the stage of laboratory research, many hydrogen production bottlenecks binding factors are urgently needed to be solved. This study focused on the factors of fermentative bio-hydrogen production of mu- nicipal sludge. In this paper, a comparative study on the effect of pretreatment-acid alkali treatment, heat digestion and ultrasonic treatment on hydrogen production were done, and the optimum pretreatment approach was ascer- tained, which break new a way for sludge treatment. 2. MATERIALS AND METHODS 2.1. Source and Characteristic of Sludge Concentrated sludge came from a sewage treatment plant in Guangzhou, China. Table 1 showed The char- acteristics of the municipal sludge. In the experiment, the proper complement of N, P and inorganic micro- nutrients should be added in the sludge. The nutrient solution contained: NH4HCO3 2.0 g·L-1, MgSO4·7H2O 50 mg·L-1, NaCl 10 mg·L-1, Na2MoO4·2H2O 10 mg·L-1, CaCl2·2H2O 10 mg·L-1, MnSO4·7H2O 15mg·L-1, FeCl2 70 mg·L-1, KH2PO4 10 mg·L-1. 2.2. Experimental Equipment Cylindrical anaerobic reactor (patent number: ZL 20052 0053384.X) with the dimensions: ødiameter = 22 cm, øexternal diameter = 24 cm, h = 30 cm, effective volume = 11 liters; JY99-IID ultrasonic cell disruptor (Ningbo Xinzhi); XLJ-IIB low-speed tabletop centrifuge (Shanghai an ting Scientific Instruments and Apparatus Co.); SC-15 ther- mostatic water-circulator bath box (Ningbo Xinzhi); JJ-4 digital display motor stirrer (Jintan City Zhengji Instru- ments Co. Ltd); BSD0.5 wet-gas flow meter (Shanghai Blue Jewelry); GC-7900 gas chromatograph, thermal conductivity detector, and FID detector (Shanghai Tian- mei); ZXZ-1 sliding vane rotary vacuum pump (Zheji- ang Huangyanqiujing, modified as shown in Fig. 1). 2.3. Experimental Methods 1kg dried sludge was dissolved in 10 L water,stirred uniformly, divided into A, B, C group. This sample would carry out acid alkali treatment, heat digestion and ultrasonic treatment, using 2#, 3#, 4# to mark the sample performed cid alkali treatment, 5#, 6#, 7# to mark the sample performed heat digestion and 8#, 9#, 10# to mark the sample performed ultrasonic treatment. Put 200 mL the liquor in a cone type bottle as reference object which was marked 1#. They were respectively performed an- aerobic digestion in shaking table whose rate was 1,050 rpm at 36℃. 2.4. Analysis Methods Gas components were detected using a gas chromato- graph (model: GC-7,900). A flame ionization detector (FID) and a 2-m stainless steel column packed with 5A Table 1. Characteristics of condensed sludge from municipal wastewater treatment plan. pH SS (g/Kg) Water content (%) TN COD 6.7~7.913~2778.7~90 1750~2000 3900~5000 1. SC-15 water bath; 2. pH adjusting port; 3. organic reactor; 4. agitating blade; 5. reaction substrate outlet; 6. material inlet; 7. vent; 8. digital stirrer; 9. thermometer; 10. CO2 removal; 11. desiccant; 12. wet gas flowmeter; 13. GC-7900 gas chromatography; 14. nitrogen purging port. Figure 1. Equipment and sequence of steps in the anaerobic digestion of sludge. 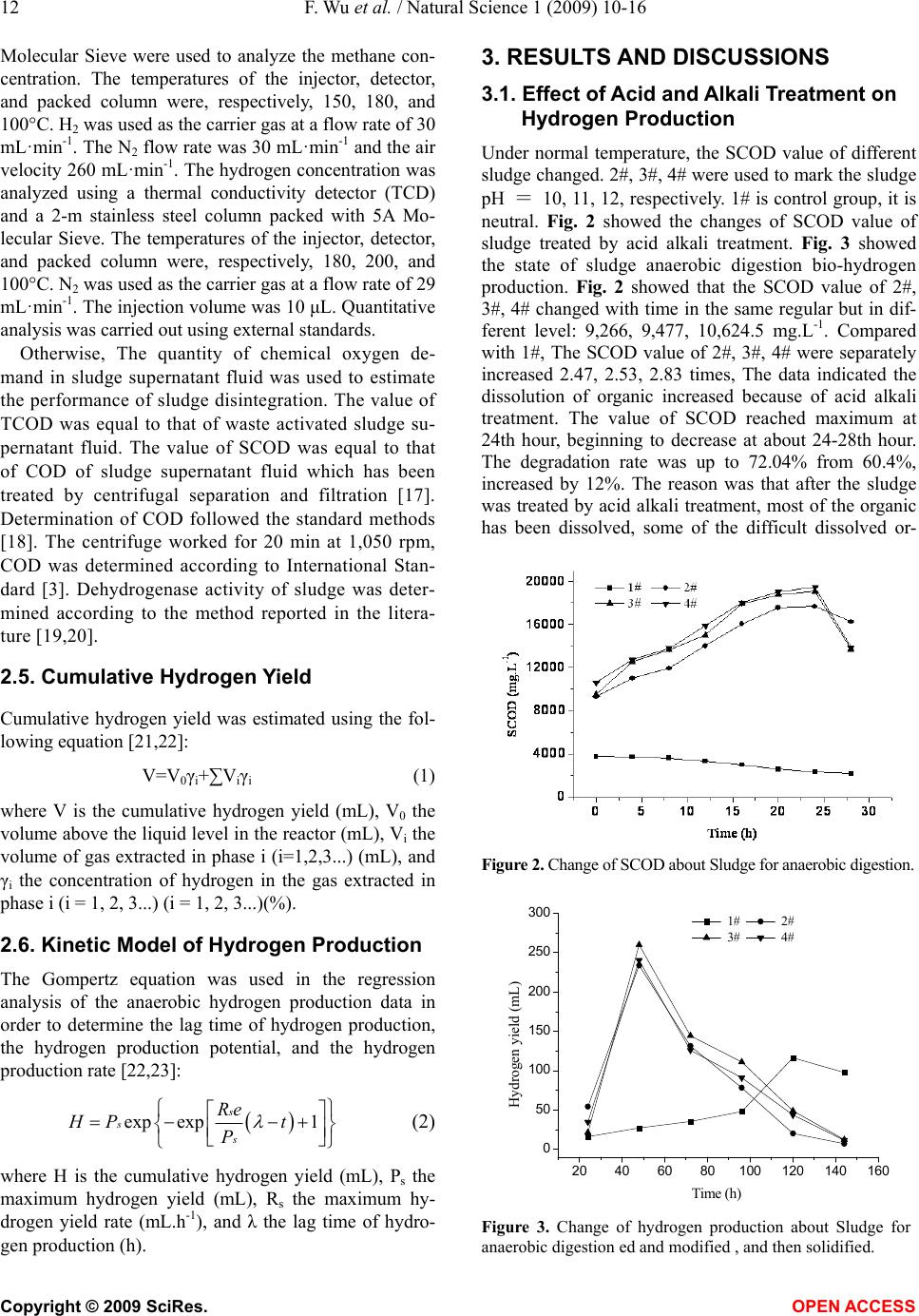 12 F. Wu et al. / Natural Science 1 (2009) 10-16 Copyright © 2009 SciRes. OPEN ACCESS Molecular Sieve were used to analyze the methane con- centration. The temperatures of the injector, detector, and packed column were, respectively, 150, 180, and 100°C. H2 was used as the carrier gas at a flow rate of 30 mL· mi n -1. The N2 flow rate was 30 mL·min-1 and the air velocity 260 mL·min-1. The hydrogen concentration was analyzed using a thermal conductivity detector (TCD) and a 2-m stainless steel column packed with 5A Mo- lecular Sieve. The temperatures of the injector, detector, and packed column were, respectively, 180, 200, and 100°C. N2 was used as the carrier gas at a flow rate of 29 mL· mi n -1. The injection volume was 10 μL. Quantitative analysis was carried out using external standards. Otherwise, The quantity of chemical oxygen de- mand in sludge supernatant fluid was used to estimate the performance of sludge disintegration. The value of TCOD was equal to that of waste activated sludge su- pernatant fluid. The value of SCOD was equal to that of COD of sludge supernatant fluid which has been treated by centrifugal separation and filtration [17]. Determination of COD followed the standard methods [18]. The centrifuge worked for 20 min at 1,050 rpm, COD was determined according to International Stan- dard [3]. Dehydrogenase activity of sludge was deter- mined according to the method reported in the litera- ture [19,20]. 2.5. Cumulative Hydrogen Yield Cumulative hydrogen yield was estimated using the fol- lowing equation [21,22]: V=V0γi+∑Viγi (1) where V is the cumulative hydrogen yield (mL), V0 the volume above the liquid level in the reactor (mL), Vi the volume of gas extracted in phase i (i=1,2,3...) (mL), and γi the concentration of hydrogen in the gas extracted in phase i (i = 1, 2, 3...) (i = 1, 2, 3...)(%). 2.6. Kinetic Model of Hydrogen Production The Gompertz equation was used in the regression analysis of the anaerobic hydrogen production data in order to determine the lag time of hydrogen production, the hydrogen production potential, and the hydrogen production rate [22,23]: exp exp1 s s s Re HP t P (2) where H is the cumulative hydrogen yield (mL), Ps the maximum hydrogen yield (mL), Rs the maximum hy- drogen yield rate (mL.h-1), and λ the lag time of hydro- gen production (h). 3. RESULTS AND DISCUSSIONS 3.1. Effect of Acid and Alkali Treatment on Hydrogen Production Under normal temperature, the SCOD value of different sludge changed. 2#, 3#, 4# were used to mark the sludge pH = 10, 11, 12, respectively. 1# is control group, it is neutral. Fig. 2 showed the changes of SCOD value of sludge treated by acid alkali treatment. Fig. 3 showed the state of sludge anaerobic digestion bio-hydrogen production. Fig. 2 showed that the SCOD value of 2#, 3#, 4# changed with time in the same regular but in dif- ferent level: 9,266, 9,477, 10,624.5 mg.L-1. Compared with 1#, The SCOD value of 2#, 3#, 4# were separately increased 2.47, 2.53, 2.83 times, The data indicated the dissolution of organic increased because of acid alkali treatment. The value of SCOD reached maximum at 24th hour, beginning to decrease at about 24-28th hour. The degradation rate was up to 72.04% from 60.4%, increased by 12%. The reason was that after the sludge was treated by acid alkali treatment, most of the organic has been dissolved, some of the difficult dissolved or- Figure 2. Change of SCOD about Sludge for anaerobic digestion. 20 40 60 80100120140160 0 50 100 150 200 250 300 Hydrogen yield (mL) Time (h) 1# 2# 3# 4# Figure 3. Change of hydrogen production about Sludge for anaerobic digestion ed and modified , and then solidified. 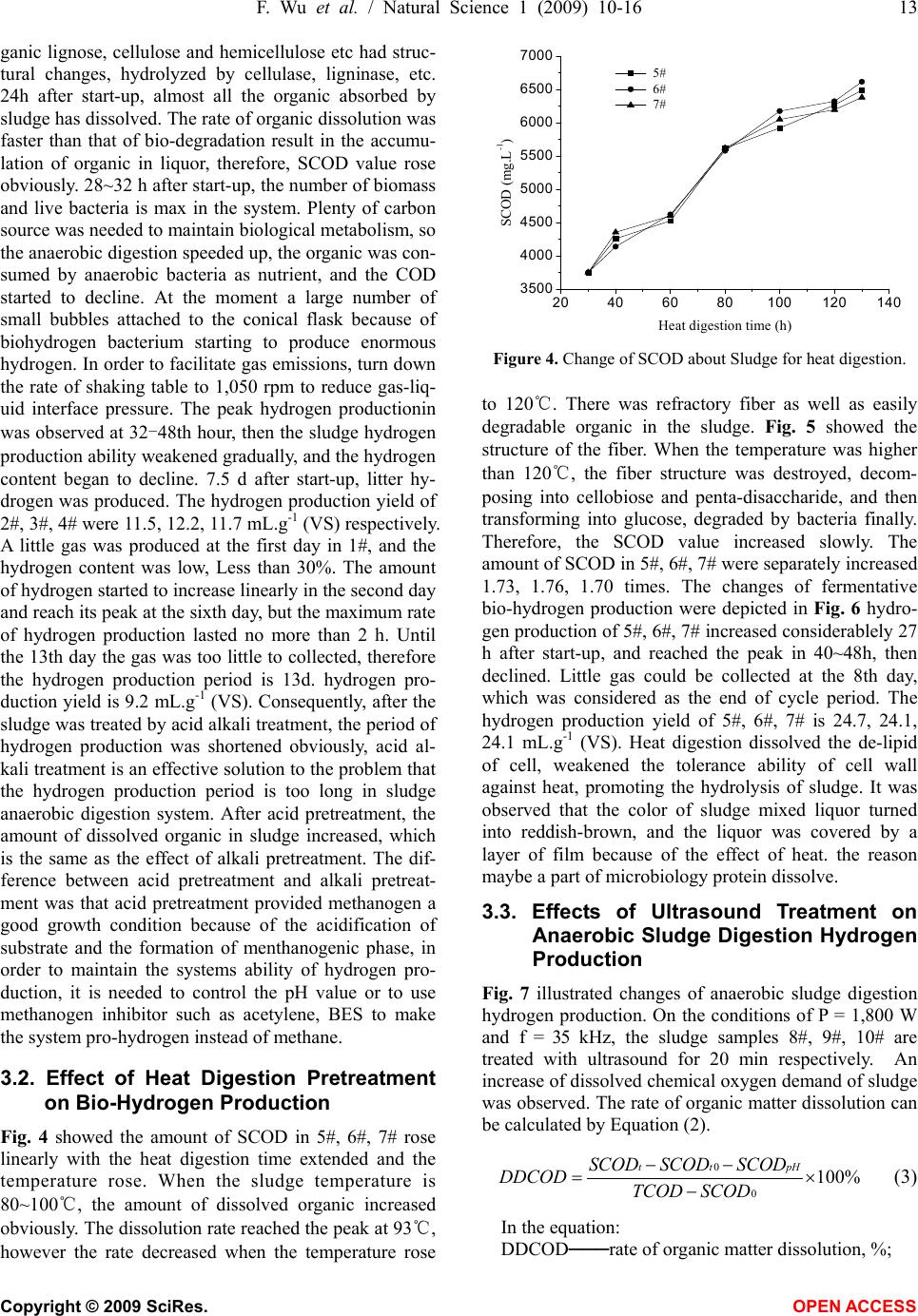 F. Wu et al. / Natural Science 1 (2009) 10-16 13 Copyright © 2009 SciRes. OPEN ACCESS ganic lignose, cellulose and hemicellulose etc had struc- tural changes, hydrolyzed by cellulase, ligninase, etc. 24h after start-up, almost all the organic absorbed by sludge has dissolved. The rate of organic dissolution was faster than that of bio-degradation result in the accumu- lation of organic in liquor, therefore, SCOD value rose obviously. 28~32 h after start-up, the number of biomass and live bacteria is max in the system. Plenty of carbon source was needed to maintain biological metabolism, so the anaerobic digestion speeded up, the organic was con- sumed by anaerobic bacteria as nutrient, and the COD started to decline. At the moment a large number of small bubbles attached to the conical flask because of biohydrogen bacterium starting to produce enormous hydrogen. In order to facilitate gas emissions, turn down the rate of shaking table to 1,050 rpm to reduce gas-liq- uid interface pressure. The peak hydrogen productionin was observed at 32-48th hour, then the sludge hydrogen production ability weakened gradually, and the hydrogen content began to decline. 7.5 d after start-up, litter hy- drogen was produced. The hydrogen production yield of 2#, 3#, 4# were 11.5, 12.2, 11.7 mL.g-1 (VS) respectively. A little gas was produced at the first day in 1#, and the hydrogen content was low, Less than 30%. The amount of hydrogen started to increase linearly in the second day and reach its peak at the sixth day, but the maximum rate of hydrogen production lasted no more than 2 h. Until the 13th day the gas was too little to collected, therefore the hydrogen production period is 13d. hydrogen pro- duction yield is 9.2 mL.g-1 (VS). Consequently, after the sludge was treated by acid alkali treatment, the period of hydrogen production was shortened obviously, acid al- kali treatment is an effective solution to the problem that the hydrogen production period is too long in sludge anaerobic digestion system. After acid pretreatment, the amount of dissolved organic in sludge increased, which is the same as the effect of alkali pretreatment. The dif- ference between acid pretreatment and alkali pretreat- ment was that acid pretreatment provided methanogen a good growth condition because of the acidification of substrate and the formation of menthanogenic phase, in order to maintain the systems ability of hydrogen pro- duction, it is needed to control the pH value or to use methanogen inhibitor such as acetylene, BES to make the system pro-hydrogen instead of methane. 3.2. Effect of Heat Digestion Pretreatment on Bio-Hydrogen Production Fig. 4 showed the amount of SCOD in 5#, 6#, 7# rose linearly with the heat digestion time extended and the temperature rose. When the sludge temperature is 80~100℃, the amount of dissolved organic increased obviously. The dissolution rate reached the peak at 93℃, however the rate decreased when the temperature rose 20 40 60 80100120140 3500 4000 4500 5000 5500 6000 6500 7000 SCOD (mg.L-1) Heat digestion time (h) 5# 6# 7# Figure 4. Change of SCOD about Sludge for heat digestion. to 120℃. There was refractory fiber as well as easily degradable organic in the sludge. Fig. 5 showed the structure of the fiber. When the temperature was higher than 120℃, the fiber structure was destroyed, decom- posing into cellobiose and penta-disaccharide, and then transforming into glucose, degraded by bacteria finally. Therefore, the SCOD value increased slowly. The amount of SCOD in 5#, 6#, 7# were separately increased 1.73, 1.76, 1.70 times. The changes of fermentative bio-hydrogen production were depicted in Fig. 6 hydro- gen production of 5#, 6#, 7# increased considerablely 27 h after start-up, and reached the peak in 40~48h, then declined. Little gas could be collected at the 8th day, which was considered as the end of cycle period. The hydrogen production yield of 5#, 6#, 7# is 24.7, 24.1, 24.1 mL.g-1 (VS). Heat digestion dissolved the de-lipid of cell, weakened the tolerance ability of cell wall against heat, promoting the hydrolysis of sludge. It was observed that the color of sludge mixed liquor turned into reddish-brown, and the liquor was covered by a layer of film because of the effect of heat. the reason maybe a part of microbiology protein dissolve. 3.3. Effects of Ultrasound Treatment on Anaerobic Sludge Digestion Hydrogen Production Fig. 7 illustrated changes of anaerobic sludge digestion hydrogen production. On the conditions of P = 1,800 W and f = 35 kHz, the sludge samples 8#, 9#, 10# are treated with ultrasound for 20 min respectively. An increase of dissolved chemical oxygen demand of sludge was observed. The rate of organic matter dissolution can be calculated by Equation (2). 0 0 100% tt pHSCOD SCODSCOD DDCOD TCOD SCOD (3) In the equation: DDCOD───rate of organic matter dissolution, %; 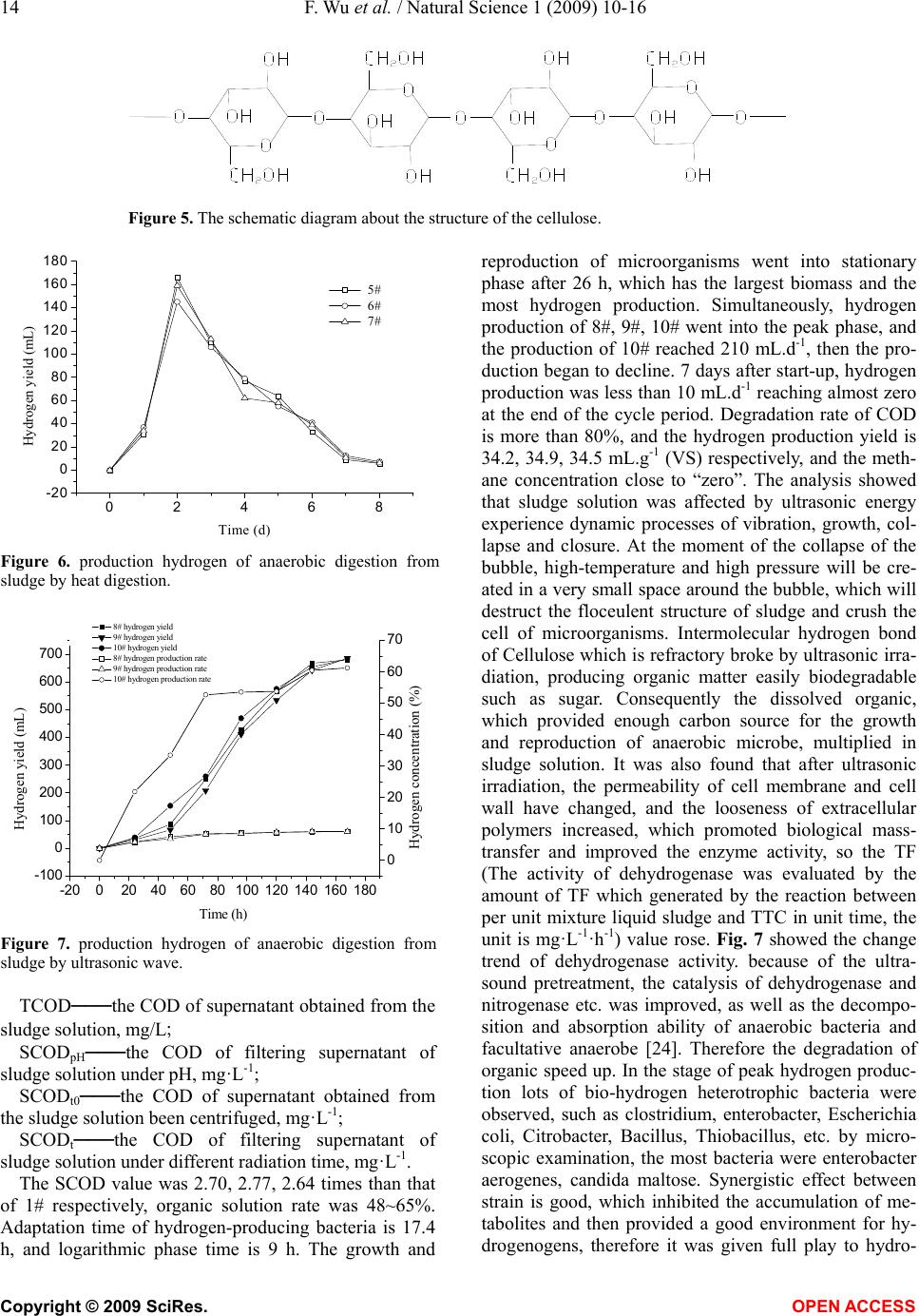 14 F. Wu et al. / Natural Science 1 (2009) 10-16 Copyright © 2009 SciRes. OPEN ACCESS Figure 5. The schematic diagram about the structure of the cellulose. 02468 -20 0 20 40 60 80 100 120 140 160 180 Hydrogen yield (mL) Time (d) 5# 6# 7# Figure 6. production hydrogen of anaerobic digestion from sludge by heat digestion. -20020 40 60 80100120140160180 -100 0 100 200 300 400 500 600 700 8# hydrogen yield 9# hydrogen yield 10# hydrogen yield 8# hydrogen production rate 9# hydrogen production rate 10# hydrogen production rate Time (h) Hydrogen yield (mL) 0 10 20 30 40 50 60 70 Hydrogen concentration (%) Figure 7. production hydrogen of anaerobic digestion from sludge by ultrasonic wave. TCOD───the COD of supernatant obtained from the sludge solution, mg/L; SCODpH───the COD of filtering supernatant of sludge solution under pH, mg·L-1; SCODt0───the COD of supernatant obtained from the sludge solution been centrifuged, mg·L-1; SCODt───the COD of filtering supernatant of sludge solution under different radiation time, mg·L-1. The SCOD value was 2.70, 2.77, 2.64 times than that of 1# respectively, organic solution rate was 48~65%. Adaptation time of hydrogen-producing bacteria is 17.4 h, and logarithmic phase time is 9 h. The growth and reproduction of microorganisms went into stationary phase after 26 h, which has the largest biomass and the most hydrogen production. Simultaneously, hydrogen production of 8#, 9#, 10# went into the peak phase, and the production of 10# reached 210 mL.d-1, then the pro- duction began to decline. 7 days after start-up, hydrogen production was less than 10 mL.d-1 reaching almost zero at the end of the cycle period. Degradation rate of COD is more than 80%, and the hydrogen production yield is 34.2, 34.9, 34.5 mL.g-1 (VS) respectively, and the meth- ane concentration close to “zero”. The analysis showed that sludge solution was affected by ultrasonic energy experience dynamic processes of vibration, growth, col- lapse and closure. At the moment of the collapse of the bubble, high-temperature and high pressure will be cre- ated in a very small space around the bubble, which will destruct the floceulent structure of sludge and crush the cell of microorganisms. Intermolecular hydrogen bond of Cellulose which is refractory broke by ultrasonic irra- diation, producing organic matter easily biodegradable such as sugar. Consequently the dissolved organic, which provided enough carbon source for the growth and reproduction of anaerobic microbe, multiplied in sludge solution. It was also found that after ultrasonic irradiation, the permeability of cell membrane and cell wall have changed, and the looseness of extracellular polymers increased, which promoted biological mass- transfer and improved the enzyme activity, so the TF (The activity of dehydrogenase was evaluated by the amount of TF which generated by the reaction between per unit mixture liquid sludge and TTC in unit time, the unit is mg·L-1·h-1) value rose. Fig. 7 showed the change trend of dehydrogenase activity. because of the ultra- sound pretreatment, the catalysis of dehydrogenase and nitrogenase etc. was improved, as well as the decompo- sition and absorption ability of anaerobic bacteria and facultative anaerobe [24]. Therefore the degradation of organic speed up. In the stage of peak hydrogen produc- tion lots of bio-hydrogen heterotrophic bacteria were observed, such as clostridium, enterobacter, Escherichia coli, Citrobacter, Bacillus, Thiobacillus, etc. by micro- scopic examination, the most bacteria were enterobacter aerogenes, candida maltose. Synergistic effect between strain is good, which inhibited the accumulation of me- tabolites and then provided a good environment for hy- drogenogens, therefore it was given full play to hydro-  F. Wu et al. / Natural Science 1 (2009) 10-16 15 Copyright © 2009 SciRes. OPEN ACCESS genogens, the hydrogen production yield rose. 3.4. Effect of Dehydrogenase Activity by Pretreatment Sludge Dehydrogenase activity is defined as the TF volume per unit time, with the unit mg·L-1·h-1 [25]. From Fig. 8, the initial TF was 60.6 mg·L-1·h-1, after pretreatment TF were 71.2, 69.9, 78.8 mg·L-1·h-1 respectively.12h later, TF declined to 32, 31.2, 27.7 mg·L-1·h-1 respectively. The result showed Dehydrogenase activity was increased with pretreatment. the permeability of cell membrane and cell wall changed because of pretreatment, which promoted the mass transfer, the production and activity of cell enzyme, so the metabolism speeded up. Moreover, NAD+ or NADP+ regenerated by cell can absorb and transport substrate or TTC effectively, therefore the amount of TF increased [26]. 3.5. Analyzing Kinetic Model of Hydrogen Production Bacteriaons Fig. 3, Fig. 6, and Fig. 7 showed hydrogen production closely related to the microbial growth regularity. The change of hydrogen yield contain four phases: lag phase, beginning of hydrogen production, continuous hydrogen production and attenuation of hydrogen production. The lag phase was short (0~11 h). There was no hydrogen production until a stable hydrogen production flora formed after acclimation, cultivation and propagation. Subsequently, hydrogen yield increased gradually with the exponential increase of bacteria. Hydrogen content rose when the growth rate of bacteria was maximum. About 27 h later, the organic content of substrate de- clined because of the rapid bacteria propagation con- suming considerable organic material. Meanwhile, the accumulation of metabolites poisoned bacteria, and bac- teria death rate rose. When the growth rate balanced the death rate, the amount of bacteria in the system was maximum. After 80h nutrition was exhausted, the bacte- 0 102030405060 20 30 40 50 60 70 80 TF/mg.L-1.h-1 tim e (m in ) sample1 sample2 sample3 Figure 8. Variation of dehydrogenase activity with pretreat- ment. ria performed endogenous respiration and even formed spore, hydrogen yield declined until the end. The reac- tion period was about 160 h. No methane was observed during the reaction. Gompertz model curve fitting on hydrogen production was carried out. All the values of correlation factor R2 were more than 0.97. Therefore the fitting effect of Gompertz model on describing the bio- hydrogen production process was good. 4. CONCLUSIONS The sludge been treated by acid and alkali, heat diges- tion, ultrasonic treatment, most of the refractory organic transformed into easily degradable carbohydrate. Com- pared with control group, the dehydrogenase activity was increased, the SCOD was increased 2.47~2.83, 1.70~1.76, 2.6~2.77 times respectively, the hydrogen production yield were 11.5~12.2, 24.1~24.7, 34.2~34.9 mL.g-1(VS) respectively, the period of hydrogen produc- tion was shorten to 7.5, 8.0, 6.5 d respectively. Remove of the COD was up to 72.04%, 81.4%, 80% respectively. the methane concentration in the gas was close to “zero”. The hydrogen concentration can reach 99.3% after the bio-gas was purified by Ca(OH)2 saturated solution. Gompertz model curve fitting on hydrogen production was carried out. All the values of correlation factor R2 were more than 0.97. ACKNOWLEDGEMENTS The authors wish to thank the New Century Outstanding Young Teacher Grant of the Ministry of Education of China for financial support (NCET-04-0819). REFERENCES [1] Liliana, M., Alzate-Gaviria, Sebastian, P. J., et al. (2007) Comparison of two anaerobic systems for hydrogen pro- duction from the organic fraction of municipal solid waste and synthetic wastewater [J]. Hydrogen Energy, 3(4), 1-6. [2] Zabut, B., Kahlout, K. E., Yücel, M., et al. (2006) Hy- drogen gas production by combined systems of Rhodo- bacter sphaeroides O. U. 001 and Halobacterium salina- rum in a photobioreactor [J]. Hydrogen Energy, 31(11), 1553- 1562. [3] Venkata, M. S., Lalit, B. V. and Sarma, P. N. (2007) An- aerobic biohydrogen production from dairy wastewater treatment in sequencing batch reactor (AnSBR): effect of organic loading rate [J]. Enz Micro Technology, 3(3), 1-10. [4] Tan, T. W., Wang, F. and Deng, L. (2003) Present situa- tion and prospect for bioenergy [J]. Modern chemical industry, 23(9), 8-14. [5] Wang, Q. H., Ma, H. Z., Wang, X. M., et al. (2004) Re- source recycling technology of foodwastes [J]. Modern Chemical Industry, 24(7), 56-59. [6] Ren, N. Q., Lia, J. Z., Lib, B. K., et al. (2006) Bio-hy- drogen production from molasses by anaerobic fermenta-  16 F. Wu et al. / Natural Science 1 (2009) 10-16 Copyright © 2009 SciRes. OPEN ACCESS tion with a pilot-scale bioreactor system [J]. International Journal of Hydrogen Energy, 31, 2147-2157. [7] Li, Y. J., Li, A. M. and Gao, N. B. (2002) An approach to a subject of innocuous in cineration of municipal sold- waste [J]. Journal of Shenyang Institute of Aeronautical Engineering, 19(3), 57-60. [8] Gomez, X., Moran, A., Cuetos, M. J., et al. (2006) The production of hydrogen by dark fermentation of munici- pal solid wastes and slaughterhousewaste: A two-phase process [J]. Sour, 157, 727-732. [9] Levin, D. B., Islam, R., Cicek, N. and Sparling, R. (2006) Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates [J]. Hydrogen Energy, 31(11), 1496-1503. [10] Zuo, Y., Zuo, J. N., Zhang, W., et al. (2004) A study on steady anaerobic bio-hydrogen production using river sediments as seed sludge [J]. China biogas, 22(4), 22-25. [11] Tai, M. L. and Liu J. X. (2005) Factors of effecting hy- drogen production from anaerobic fermentation of excess seweage sludge [J]. Environmental Science, 26(2), 98- 111. [12] Ginkel, S. W., Oha, S.-E. and Logan, B. E. (2005) Bio- hydrogen gas production from food processing and do- mestic wastewaters [J]. International Journal of Hydrogen Energy, 30, 1535-1542. [13] Zhu, H. G., Parker, W., Basnar R., et al. (2008) Biohy- drogen production by anaerobic co-digestion of munici- pal food waste and sewage sludges [J]. International Journal of Hydrogen Energy, 33, 3651-3659. [14] Krupp, M. and Widmann, R. (2008) Biohydrogen pro- duction by dark fermentation: Experiences of continuous operation in large lab scale [J]. International Journal of Hydrogen Energy, 28, 1-8. [15] Ela Eroglu, Inci Erolu, Ufuk Gündüzb, et al. (2006) Bio- logical hydrogen production from olive mill wastewater with two-stage processes [J]. International Journal of Hydrogen Energy, 31, 1527-1535. [16] Li, D. M. and Chen, H. Z. (2007) Biological hydrogen production from steam-exploded strawby simultaneous saccharification and fermentation [J]. International Jour- nal of Hydrogen Energy, 32, 1742-1748. [17] Zabut, B., Kahlout, K. E., Yücel M., et al. (2006) Hy- drogen gas production by combined systems of Rhodo- bacter sphaeroides O. U. 001 and Halobacterium salina- rum in a photobioreactor [J]. International Journal of Hydrogen Energy, 31(11), 1553-1562. [18] State Environmental Protection Administration of China (2002) Detecting methods of water and wastewater (Edi- tion )Ⅳ [M]. Beijing, China Environmental Science Press. [19] Zhu, N. W., Min, H., Cheng, M. C., et al. (1996) The study of Determination on TTC-Dehydrogenase Activity [J]. China Biogas, 14(2), 3-5. [20] An, L. C., Niu, H., Zeng C., et al. (1996) A Study on the Measurement of dehydrogenase activity in activated sludge [J]. Pollution control Technology, 9(3), 186-188. [21] Pan, C. M., Fan, Y. T., Xing, Y., et al. (2008) Statistical optimization of process parameters on bio-hydrogen pro- duction from glucose by Clostridium sp. Fanp2 [J]. Bio- resour Biotechchnol, 99, 3146-3154. [22] Lay, J. J., Lee, Y. J. and Noike, T. (1999) Feasibility of biological hydrogen production from organic fraction of municipal solid waste [J]. Wat. Res, 33(11), 2579-2586. [23] Cai, M. L. and Liu, J. X. (2005) Factors of effecting hy- drogen production from anaerobic fermentation of excess sewage sludge [J]. Environmental Science, 26(2), 97-100. [24] Bartons, Bullock, C. and Weir, D. (1996) The effects of uktrasound on the activities of some glycosidase en- zymes of industrial importance [J]. Enzyme Microbe Technology, 18(3), 190-194. [25] Tang, N., Chai, L. Y., Min X. B., et al. (2005) Character- istic bacterial number of anaerobic activated sludge with dehydrogenase activity as indicator [J]. Journal of Mi- crobiology, 25(2), 31-34. [26] Jeffcres T. W. and Kurtzman C. P. (1994) Strain selection, taxonony, and genetics of xylose-fermentation yeast [J]. Enzyme Microb Technology, 16, 922-931.
|