C. Alur et al. / Natural Science 3 (2011) 414-418
Copyright © 2011 SciRes. OPEN ACCESS
415
having the elements in the region 57 ≤ Z ≤ 70.
2. EXPERIMENTAL ARRANGEMENT
The good-geometry experimental arrangement used
for the determination of the mass attenuation coefficient
is similar to the one described in detail by us earlier [2];
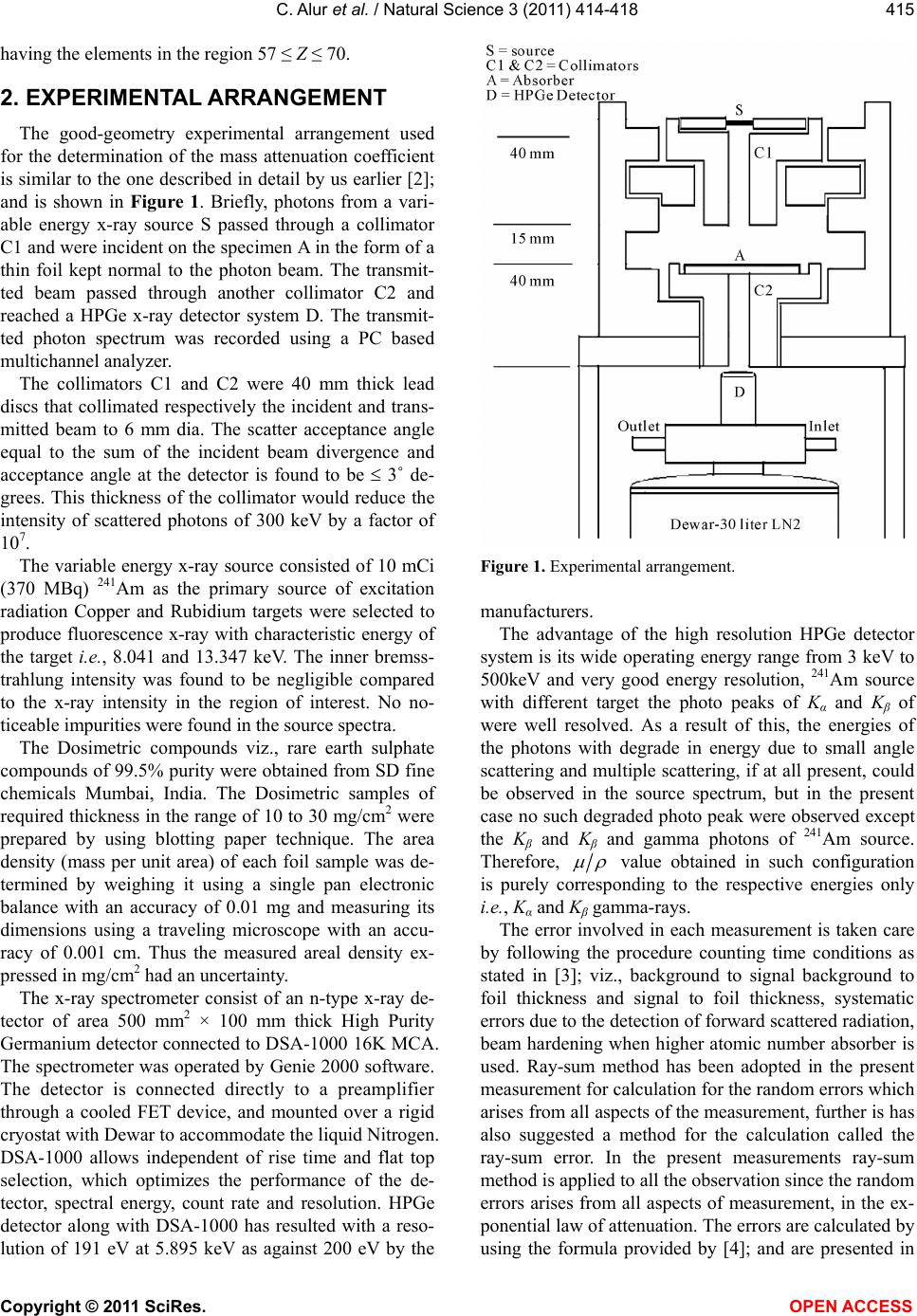
and is shown in Figure 1. Briefly, photons from a vari-
able energy x-ray source S passed through a collimator
C1 and were incident on the specimen A in the form of a
thin foil kept normal to the photon beam. The transmit-
ted beam passed through another collimator C2 and
reached a HPGe x-ray detector system D. The transmit-
ted photon spectrum was recorded using a PC based
multichannel analyzer.
The collimators C1 and C2 were 40 mm thick lead
discs that collimated respectively the incident and trans-
mitted beam to 6 mm dia. The scatter acceptance angle
equal to the sum of the incident beam divergence and
acceptance angle at the detector is found to be 3˚ de-
grees. This thickness of the collimator would reduce the
intensity of scattered photons of 300 keV by a factor of
107.
The variable energy x-ray source consisted of 10 mCi
(370 MBq) 241Am as the primary source of excitation
radiation Copper and Rubidium targets were selected to
produce fluorescence x-ray with characteristic energy of
the target i.e., 8.041 and 13.347 keV. The inner bremss-
trahlung intensity was found to be negligible compared
to the x-ray intensity in the region of interest. No no-
ticeable impurities were found in the source spectra.
The Dosimetric compounds viz., rare earth sulphate
compounds of 99.5% purity were obtained from SD fine
chemicals Mumbai, India. The Dosimetric samples of
required thickness in the range of 10 to 30 mg/cm2 were
prepared by using blotting paper technique. The area
density (mass per unit area) of each foil sample was de-
termined by weighing it using a single pan electronic
balance with an accuracy of 0.01 mg and measuring its
dimensions using a traveling microscope with an accu-
racy of 0.001 cm. Thus the measured areal density ex-
pressed in mg/cm2 had an uncertainty.
The x-ray spectrometer consist of an n-type x-ray de-
tector of area 500 mm2 × 100 mm thick High Purity
Germanium detector connected to DSA-1000 16K MCA.
The spectrometer was operated by Genie 2000 software.
The detector is connected directly to a preamplifier
through a cooled FET device, and mounted over a rigid
cryostat with Dewar to accommodate the liquid Nitrogen.
DSA-1000 allows independent of rise time and flat top
selection, which optimizes the performance of the de-
tector, spectral energy, count rate and resolution. HPGe
detector along with DSA-1000 has resulted with a reso-
lution of 191 eV at 5.895 keV as against 200 eV by the
Figure 1. Experimental arrangement.
manufacturers.
The advantage of the high resolution HPGe detector
system is its wide operating energy range from 3 keV to
500keV and very good energy resolution, 241Am source
with different target the photo peaks of Kα and Kβ of
were well resolved. As a result of this, the energies of
the photons with degrade in energy due to small angle
scattering and multiple scattering, if at all present, could
be observed in the source spectrum, but in the present
case no such degraded photo peak were observed except
the Kβ and Kβ and gamma photons of 241Am source.
Therefore,
value obtained in such configuration
is purely corresponding to the respective energies only
i.e., Kα and Kβ gamma-rays.
The error involved in each measurement is taken care
by following the procedure counting time conditions as
stated in [3]; viz., background to signal background to
foil thickness and signal to foil thickness, systematic
errors due to the detection of forward scattered radiation,
beam hardening when higher atomic number absorber is
used. Ray-sum method has been adopted in the present
measurement for calculation for the random errors which
arises from all aspects of the measurement, further is has
also suggested a method for the calculation called the
ray-sum error. In the present measurements ray-sum
method is applied to all the observation since the random
errors arises from all aspects of measurement, in the ex-
ponential law of attenuation. The errors are calculated by
using the formula provided by [4]; and are presented in