M. Tunç et al. / J. Biomedical Science and Engineering 4 (2011) 391-396
Copyright © 2011 SciRes.
396
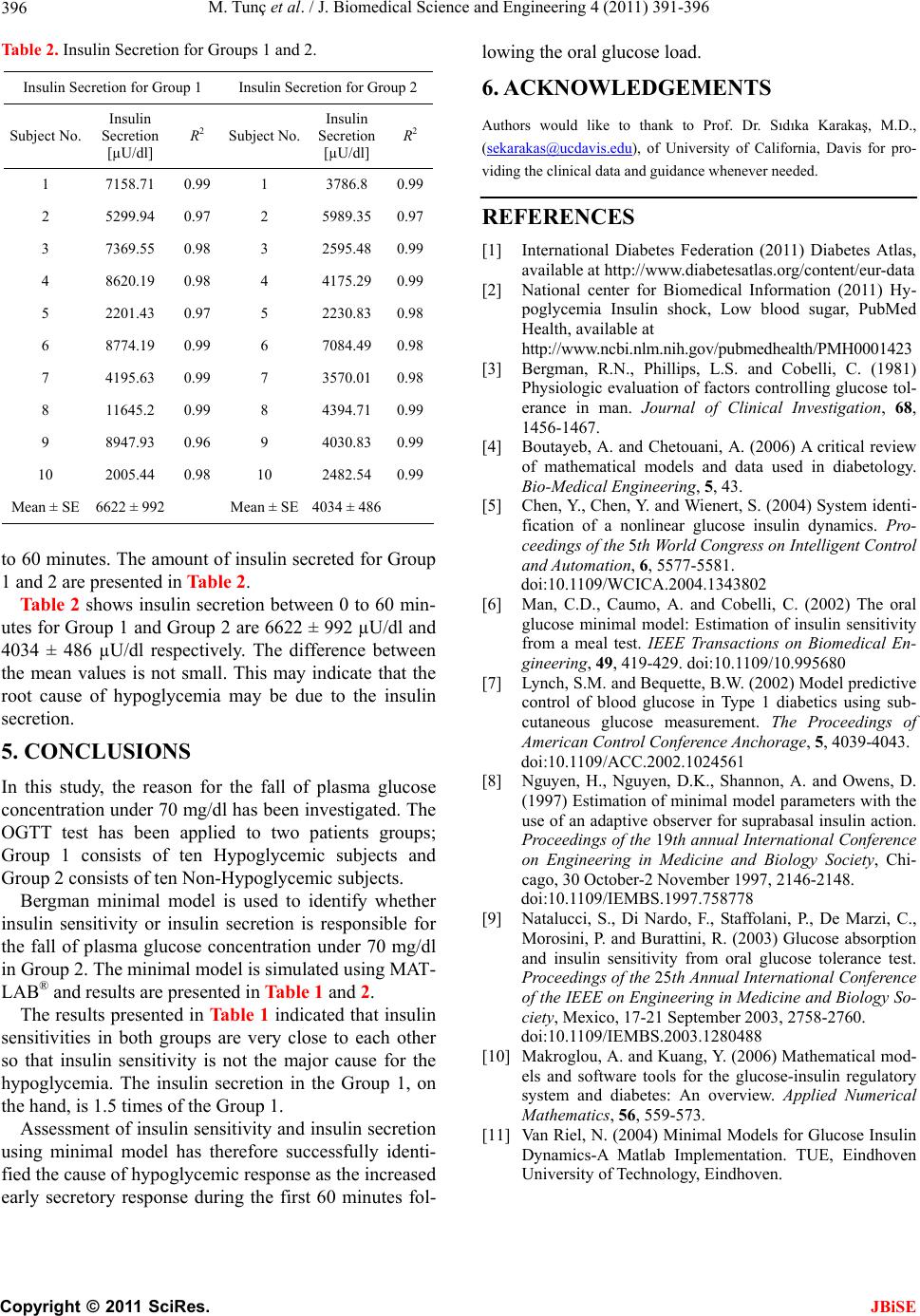
Table 2. Insulin Secretion for Groups 1 and 2.
ion for Group 2 Insulin Secretion for Group 1 Insulin Secret
S
Insulin
S
Insulin
ubject No. Secretion R2
[µU/dl]
ubject No. Secretion R2
[µU/dl]
1 7158.71
JBiSE
0.99 1 3786.8 0.99
2 5299.94 0.97 2 5989.
10 2005.10
Mean ± SE 6622 ± 99Mean ± SE 4034 ± 48
35 0.97
3 7369.55 0.98 3 2595.48 0.99
4 8620.19 0.98 4 4175.29 0.99
5 2201.43 0.97 5 2230.83 0.98
6 8774.19 0.99 6 7084.49 0.98
7 4195.63 0.99 7 3570.01 0.98
8 11645.2 0.99 8 4394.71 0.99
9 8947.93 0.96 9 4030.83 0.99
44 0.98 2482.54 0.99
2 6
tount ofGroup
and 2 are presented in Table 2.
6622 ± 992 µU/dl
40
for the fall of plasma glucose
/dl has been investigated. T
for
th
other
so
uccessfully identi-
fie
ENTS
ıdıka Karakaş, M.D.,
o 60 minutes. The am insulin secreted for
1
Table 2 shows insulin secretion between 0 to 60 min-
utes for Group 1 and Group 2 areand
34 ± 486 µU/dl respectively. The difference between
the mean values is not small. This may indicate that the
root cause of hypoglycemia may be due to the insulin
secretion.
5. CONCLUSIONS
In this study, the reason
concentration under 70 mghe
OGTT test has been applied to two patients groups;
Group 1 consists of ten Hypoglycemic subjects and
Group 2 consists of ten Non-Hypoglycemic subjects.
Bergman minimal model is used to identify whether
insulin sensitivity or insulin secretion is responsible
e fall of plasma glucose concentration under 70 mg/dl
in Group 2. The minimal model is simulated using MAT-
LAB® and results are presented in Table 1 and 2.
The results presented in Table 1 indicated that insulin
sensitivities in both groups are very close to each
that insulin sensitivity is not the major cause for the
hypoglycemia. The insulin secretion in the Group 1, on
the hand, is 1.5 times of the Group 1.
Assessment of insulin sensitivity and insulin secretion
using minimal model has therefore s
d the cause of hypoglycemic response as the increased
early secretory response during the first 60 minutes fol-
lowing the oral glucose load.
6. ACKNOWLEDGEM
Authors would like to thank to Prof. Dr. S
(sekarakas@ucdavis.edu), of University of California, Davis for pro-
viding the clinical data and guidance whenever needed.
REFERENCES
[1] International Diabetes Federation (2011) Diabetes Atlas,
ih.gov/pubmedhealth/PMH0001423
. and Chetouani, A. (2006) A critical review
System identi-
02
[6] C. (2002) The oral
redictive
[8] non, A. and Owens, D.
[9] lani, P., De Marzi, C.,
[10] ) Mathematical mod-
l Models for Glucose Insulin
available at http://www.diabetesatlas.org/content/eur-data
[2] National center for Biomedical Information (2011) Hy-
poglycemia Insulin shock, Low blood sugar, PubMed
Health, available at
http://www.ncbi.nlm.n
[3] Bergman, R.N., Phillips, L.S. and Cobelli, C. (1981)
Physiologic evaluation of factors controlling glucose tol-
erance in man. Journal of Clinical Investigation, 68,
1456-1467.
[4] Boutayeb, A
of mathematical models and data used in diabetology.
Bio-Medical Engineering, 5, 43.
[5] Chen, Y., Chen, Y. and Wienert, S. (2004)
fication of a nonlinear glucose insulin dynamics. Pro-
ceedings of the 5th World Congress on Intelligent Control
and Automation, 6, 5577-5581.
doi:10.1109/WCICA.2004.13438
Man, C.D., Caumo, A. and Cobelli,
glucose minimal model: Estimation of insulin sensitivity
from a meal test. IEEE Transactions on Biomedical En-
gineering, 49, 419-429. doi:10.1109/10.995680
[7] Lynch, S.M. and Bequette, B.W. (2002) Model p
control of blood glucose in Type 1 diabetics using sub-
cutaneous glucose measurement. The Proceedings of
American Control Conference Anchorage, 5, 4039-4043.
doi:10.1109/ACC.2002.1024561
Nguyen, H., Nguyen, D.K., Shan
(1997) Estimation of minimal model parameters with the
use of an adaptive observer for suprabasal insulin action.
Proceedings of the 19th annual International Conference
on Engineering in Medicine and Biology Society, Chi-
cago, 30 October-2 November 1997, 2146-2148.
doi:10.1109/IEMBS.1997.758778
Natalucci, S., Di Nardo, F., Staffo
Morosini, P. and Burattini, R. (2003) Glucose absorption
and insulin sensitivity from oral glucose tolerance test.
Proceedings of the 25th Annual International Conference
of the IEEE on Engineering in Medicine and Biology So-
ciety, Mexico, 17-21 September 2003, 2758-2760.
doi:10.1109/IEMBS.2003.1280488
Makroglou, A. and Kuang, Y. (2006
els and software tools for the glucose-insulin regulatory
system and diabetes: An overview. Applied Numerical
Mathematics, 56, 559-573.
[11] Van Riel, N. (2004) Minima
Dynamics-A Matlab Implementation. TUE, Eindhoven
University of Technology, Eindhoven.