Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 201-206 doi:10.4236/jbnb.2011.23025 Published Online July 2011 (http://www.SciRP.org/journal/jbnb) Copyright © 2011 SciRes. JBNB 201 Self-Assembled Core-Shell Poly(Ethylene Glycol)-POSS Nanocarriers for Drug Delivery Kyu-Oh Kim1, Byoung-Suhk Kim2, Ick-Soo Kim2 1Department of Bioscience and Textile Technology, Shinshu University, Ueda, Japan; 2Nano Fusion Technology Research Group, Faculty of Textile Science and Technology, Shinshu University, Ueda, Japan. Email: kbsuhk@yahoo.com, kim@shinshu-u.ac.jp Received April 18th, 2011; revised May 5th, 2011; accepted May 15th, 2011. ABSTRACT In this work, novel nanostructured core-shell poly (ethylene glycol) (PEG)-polyhedral oligosilsesquioxane (POSS) nanoparticles were used to encapsulate insulin as new drug delivery carriers. The morphologies, particle size and potential of th e pure nanostructured core-shell PEG-POSS and th e corresponding insulin-load ed PEG-POSS nanopar- ticles were investigated by transmission electron microscopy (TEM) and laser diffraction particle sizer. TEM analysis demonstrated that pure and insulin-loaded self-assembled PEG-POSS nanoparticles were of spherical shape with core-shell nanostructure, and were well-dispersed and uniform in size distribution. Insulin release test showed that in- sulin was well-protected in side PEG-POSS nanoparticles at gastric pH for 2 hrs, and was released at intestinal pH (pH 6 - 7) where the absorption and activation of the drug are necessary. We therefore believe that such nanostructured PEG-POSS nanoparticles could be useful as a potential carrier for insulin drug delivery systems. Keywords: Self-Assembly, Amphiphilic, Nanoparticles, POSS, Insulin, Oral Delivery 1. Introduction In a recent year, nanotechnology has been utilized to de- velop new therapies and next generation nanosystems for “smart” drug delivery [1]. A variety of organic/inor- ganic nanomaterials and devices have been often used as delivery vehicle to enhance the therapeutic activity by prolonging drug half-life, improving solubility of hydro- phobic drugs, reducing potential immunogenicity, and/or releasing dugs in a sustained or stimuli-triggered fashion. Insulin was known to treat diabetic. Current treatment methods involve regular injections of insulin, which can be both painful and inconvenient, thus leading to low patient compliance [2]. In order to overcome this prob- lem, the oral route is considered to be the most conven- ient and comfortable means of drug administration for pa- tients. However, oral administration of hydrophilic mac- romolecules such as peptide/protein drugs is encountered with many difficulties as the drugs have to confront va- rious major barriers in the gastrointestinal (GI) tract. Firstly, peptide/protein drugs get denatured readily by low pH of gastric medium in the stomach. Secondly, dif- ferent digestive enzymes in the stomach and small intes- tine may lead to degradation of peptide/protein drugs [3]. A new carrier system is therefore required to protect pep- tide/protein drugs from the harsh environment in the GI tract. We have recently developed the organic/inorganic hy- brid materials, such as amphiphilic poly(ethylene glycol) (PEG)-polyhedral oligosilsesquioxane (POSS) and poly (vinyl alcohol) (PVA)-POSS hybrids incorporating POSS macromers onto chain-ends or polymer backbone as pendent groups, respectively [4-7]. Thanks to their am- phiphilic properties (here, POSS is hydrophobic, PEG and PVA hydrophilic), it can be expected that those POSS-containing polymers can form the micelles by tai- loring the composition ratio in polymers and solvent po- larity, and this has important implications for drug deliv- ery systems. For instance, we reported [8] that PVA- POSS hybrid showed unagglomerated nanoparticles within a diameter range of 60 - 90 nm, as confirmed by atomic force microscopy (AFM) and bio-transmission electron microscope (bio-TEM). The prepared nanoparti- cles were found to improve the control release of anti- cancer drug; paclitaxel as a model drug. However, there are few reports on the solution properties and mi- celles/nanoparticles formation of POSS-based polymeric materials for drug delivery system [9-11]. In this report, we prepare the hybrid core-shell nanostructured particles  Self-Assembled Core-Shell Poly(Ethylene Glycol)-POSS Nanocarriers for Drug Delivery Copyright © 2011 SciRes. JBNB 202 composed of POSS as a hydrophobic inner core and PEG as a hydrophilic outer shell by using dialysis approach, and propose novel drug delivery carriers for protein drugs. 2. Experimental Section 2.1. Materials Poly(ethylene glycol) (PEG) (molecular weight = 3.4 kDa, Aldrich) was purified by repeating twice the process of precipitation into n-hexane from chloroform solution. Isocyanatopropyldimethylsilylcyclohexyl-polyhedral oli- gosilsesquioxane (POSS macromer) was purchased from Tomen Plastics Co., Japan. Dibutyl tin dilaurate (DBTDL; Aldrich, 95% purity) as a catalyst for urethane formation was used as received. Amphiphilic PEG3.4k- POSS tel- echelic studied herein was synthesized by direct urethane linkage between the diol end groups of PEG homopoly- mers and the monoisocyanate group of POSS macromers as catalyzed by DBTDL. 1H-NMR results confirmed that the amphiphilic PEG3.4k-POSS telechelic was success- fully prepared. Evidence for the formation of urethane linking groups comes from the emergence of a weak proton signal at about 4.26 ppm, accompanied by the disappearance of a proton signal of the -CH2-NCO group (3.15 ppm). That is, the level of incorporation of POSS macromers in the amphiphilic PEG3.4k-POSS telechelic could be determined quantitatively by the monitoring of the resonances for the cyclohexyl groups of POSS mac- romers. A degree of end functionalization was found to about 2.1, indicating quite consistent with the feed ratio. The detailed synthesis and characterization are described in our previous reports [4,12]. Toluene was dried over CaH2 and then distilled under nitrogen prior to use. A sample of 100 mg of insulin, from bovine pan- creas (ac- tivity: ≥25 USP units/mg, secondary activity: 2500 units) were purchased from Sigma-Aldrich. All chemicals were of analytical purity or higher quality and were used without further purification. 2.2. Preparation of Insulin-Loaded Hybrid PEG-POSS Particles Insulin solution was prepared by dissolving 100 mg of insulin in 10 ml of 0.01 N HCl solution. Insulin encapsu- lation was carried out on the basis of self assembly proc- ess. Briefly, 40 mg of PEG3.4k-POSS was fully dis- solved in 10 ml of THF. Then, 0.5, 0.9, 1.3 ml insulin so- lutions was added dropwise into the PEG3.4k-POSS so- lutions, respectively. The mixture was poured into di- alysis tube (spectra/Por 6, MWCO: 3.5 kD) and dialyzed against distilled water at room temperature under mag- netic stirring for 1 day. Afterwards, the distilled water was exchanged at least 3 times in order to remove the THF and HCl residues. To determine the loading effi- ciency of insulin in hybrid PEG-POSS nanoparticles, the amount of free insulin in supernatants was assayed by UV-vis spectrophotometry. The drug loading efficiency was calculated using the equations listed below [13,14]. Loading Efficiency % total amount of insulin added amount of free insulin100% total amount of insulin added (1) 2.3. Characterization Particle size and potential of pure and insulin-loaded PEG-POSS nanoparticles were measured depending on pH of the mixture by laser diffraction particle sizer (Nano-ZS, Malvern Instrument Ltd., UK). FI-IR analysis of the prepared nanaoparticles and insulin was carried out using an IRPrestige-21 (Shimadzu Co., Japan) Trans- mission Electron Microscopy (TEM) images were taken out on a JEM-2100 LaB6 microscope (JEOL, Japan) op- erating at an accelerating voltage of 160 kV to observe the morphologies of the obtained pure and insulin-loaded PEG-POSS nanoparticles. The insulin-loaded PEG-POSS nanoparticles dispersed in water was directly dropped onto a carbon-coated copper grid (200-A mesh, Nissin EM Co., Ltd.), followed by drying at room temperature. Finally, the samples were dried in vacuum oven. The av- erage particle size was determined from the TEM micro- graphs using an image analysis software (Image J, Na- tional Institutes of Health, Bethesda, U.S.). 2.4. pH Effects on Insulin Release from PEG-POSS Nanoparticles The pH of the insulin-loaded nanoparticle suspensions was varied at the ranging from pH 2.5 to pH 7.4 to study the release behaviors of the insulin from PEG-POSS nano-particles at room temperature. The insulin-loaded PEG-POSS nanoparticles treated with centrifugation 3000 rpm/15 min were placed into 10 ml of PBS buffer solu- tion (pH 2.5) and incubated at 37˚C for 2 hrs. 0.5 mL of the supernatants, which was isolated by centrifugation (2000 rpm/1 min), was taken by every twenty minutes for the measurement of the released amount of insulin from PEG-POSS nanoparticles and replaced by fresh medium. Afterwards, the same insulin-loaded PEG- POSS nanoparticles were again isolated by centrifugation (3500 rpm/15 min) and were transferred to 10 mL of phosphate buffer at pH 7.4 and incubated at 37˚C for 3 hrs. The released amount of insulin from PEG-POSS nanoparticles were monitored by UV-vis spectrophotome- try. A plot of cumulative release with time was reported, where  Self-Assembled Core-Shell Poly(Ethylene Glycol)-POSS Nanocarriers for Drug Delivery Copyright © 2011 SciRes. JBNB 203 Cumulative release %100% t MAX A A (2) where At is the absorbance of the characteristic peak at 275 nm (ε = 5.53 mM–1·cm–1) [15] at time t, and AMAX is the maximum absorbance of this peak. 3. Results and Discussion In our previous paper [4-7], we have reported that the synthesized organic/inorganic PEG-POSS and PVA- POSS hybrid materials were found to be amphiphilic, and thereby resulted in self-assembling into core-shell nanostructured micelles with hydrophobic inner-core (POSS moieties) and hydrophilic outer-shell (PEG or PVA moieties) in aqueous media. Therefore, hydropho- bic drugs, such as insulin used in this work can be easily entrapped into the core. Scheme 1 shows the schematic illustration of self-assembling process, which leads to self-aggregation into core-shell nanostructured particles (i.e., flower-like micelles) encapsulating insulin inside the hydrophobic core in aqueous solution. Here, it is worth mentioning that the formation of self-assembled nanostructures using POSS-PEG-POSS telechelic (ABA triblock copolymers) depends on two competing forces: the entropy loss due to loop formation of the central block in the micelle corona and the interfacial energy penalty that accompanies the transfer of insoluble block from the micelle corona to the solution [16]. 3.1. Characterization of Insulin-Loaded PEG-POSS Nanoparticles Figure 1 shows TEM images of pure and insulin-loaded self-assembled PEG-POSS nanoparticles. It can be clearly seen that insulin-loaded PEG-POSS nanoparticles were of spherical shape with core-shell nanostructure, and were well-dispersed and uniform in size distribution unlike pure PEG-POSS nanoparticles. The size of pure self- assembled PEG-POSS nanoparticles was about 15.9 ± 1.3 nm, while the insulin-loaded self-assembled PEG- POSS nanoparticles (loading content ~ 0.9 mg) were 330 ± 80 nm, as measured by TEM analysis, suggesting suc- cessful encapsulation of insulin molecules into a hydro- phobic core. In addition, the loading efficiency (LE) of insulin in the insulin-loaded PEG-POSS nanoparticles with an added insulin contents of 5, 9, 13 mg was found to be 52.6%, 70.5% and 76.5%, indicating that PEG- POSS nanoparticles have good loading capability of hy- drophobic drug, insulin [17,18]. In addition, FT-IR analysis demonstrated the existence of insulin in insulin- loaded PEG-POSS nanoparticles (Figure 2). The char- acteristic peaks at 1651 cm–1 and 1531 - 1514 cm–1 re- gion were observed respectively, corresponding to C–N stretching and N-H bending modes of amide II region in pristine insulin [19]. The Si-O-Si peak in the insulin- loaded PEG-POSS nanoparticles was shifted toward higher wavenumber, compared to pure PEG-POSS nano- particles. This suggests that there are strong interact- tions between insulin and PEG-POSS segments [20]. 3.2. pH Effects on Insulin-Loaded PEG-POSS Nanoparticles The -potential and size of the obtained hybrid nanopar- ticles was investigated at various pH and the results were shown in Figure 3. The -potential of pure PEG-POSS nanoparticles was close to constant zero -potential values as increasing pH, whereas the insulin-loaded PEG-POSS nanoparticles obviously exhibited increased negative charges, presumably due to ionized carboxyl groups in an encapsulated insulin (Figure 3(a)) [21]. Moreover, it is also expected that such negative charged nanoparticles may be less aggregated and show good dispersion-sta- bilities because of an electrostatic repulsion. Therefore, the resultant negatively charged insulin-loaded PEG-POSS nanoparticles exhibit well-dispersed nanoparticles, which can help them to be taken up by cells more easily than aggregated ones [8]. As expected, unlike pure PEG-POSS nanoparticles, the size of insulin-loaded PEG-POSS nanoparticles clearly increased as increasing pH (loading content ~ 0.9 mg, Figure 3(b)). 3.3. Insulin-Release Study Figure 4 shows insulin release behavior from insulin- loaded PEG-POSS nanoparticles at different pH values of 2.0 and 7.4. At pH = 2.0, even though a tiny release of insulin was observed, probably due to a weakly physical- adsorbed insulin, PEG-POSS nanoparticles appeared to have higher insulin retention capacity at low pH. Af- terwards, following a pH change to 7.4, a dramatic re- lease of insulin was observed during the first hour fol- lowed by a relatively sustained release, due to a fast dis- sociation of insulin from PEG-POSS nanoparticles at higher pH. This suggests that insulin was well-protected inside PEG-POSS nanoparticles at gastric pH for 2 hrs, and was released at intestinal pH (pH 6-7) where the ab- sorption and activation of the drug are necessary. We believe that such PEG-POSS nanoparticles could be used as a potential carrier for insulin drug delivery systems. The release data have been further studied [22] by fitting the cumulative fraction release data, Mt/M to an empiri- cal Equation (1). The drug release behavior according to diffusion controlled mechanism is usually governed by the following Equation (3), n t M Mkt (3) where M is the total amount of insulin in dosage form, 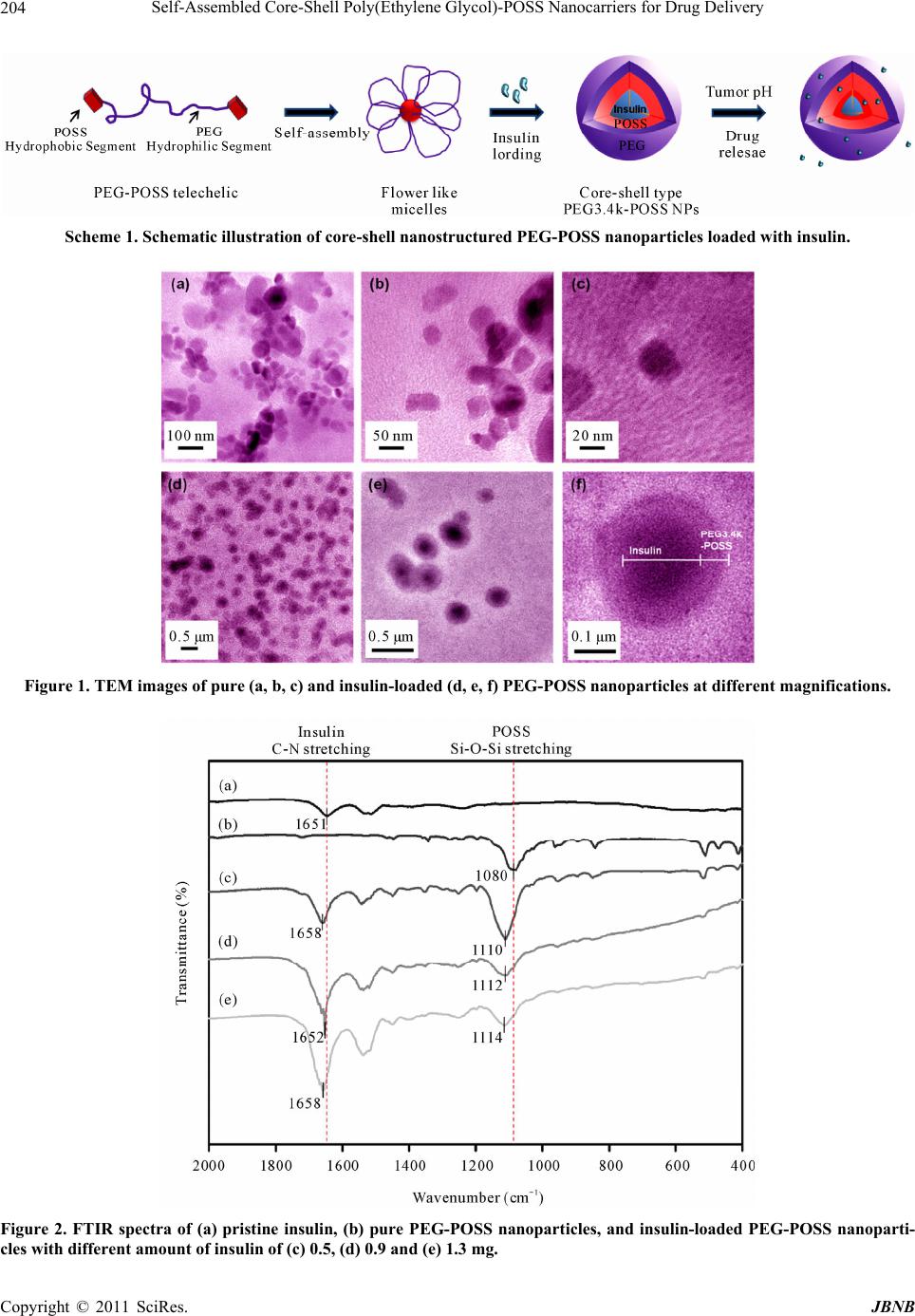 Self-Assembled Core-Shell Poly(Ethylene Glycol)-POSS Nanocarriers for Drug Delivery Copyright © 2011 SciRes. JBNB 204 Scheme 1. Schematic illustration of core-shell nanostructured PEG-POSS nanoparticles loaded with insulin. Figure 1. TEM images of pure (a, b, c) and insulin-loaded (d, e, f) PEG-POSS nanoparticles at different magnifications. Figure 2. FTIR spectra of (a) pristine insulin, (b) pure PEG-POSS nanoparticles, and insulin-loaded PEG-POSS nanoparti- cles with different amount of insulin of (c) 0.5, (d) 0.9 and (e) 1.3 mg.  Self-Assembled Core-Shell Poly(Ethylene Glycol)-POSS Nanocarriers for Drug Delivery Copyright © 2011 SciRes. JBNB 205 (a) (b) Figure 3. Zeta-potential (a) and particle size (b) of pure (●) and insulin-loaded () PEG-POSS nanoparticles with re- spect to the pH. Figure 4. Insulin release from insulin-loaded PEG-POSS nanoparticles produced with an insulin mass of 0.9 ml in gas- tric pH 2 simulated fluids for 2 hrs followed by additional 16 hrs in intestinal pH 7.4 simulated fluids at 37˚C. Mt is the amount of insulin released at time t, k is kinetic constant, and n is diffusion or release exponent constant. Using the least-squares procedure, n value was estimated to about 0.87, suggesting anomalous diffusion or non- fickian diffusion. This finding refers to combination of both diffusion and erosion controlled rate release. Ac- cordingly, it is expected that insulin release from PEO- POSS nanostructured nanoparticles was pH-controlled, accompanying the swelling of insulin-loaded PEG-POSS nanoparticles. 4. Conclusions We have successfully prepared the pure and insulin- loaded nanostructured core-shell poly(ethylene glycol) (PEG)-polyhedral oligosilsesquioxane (POSS) nanopar- ticles via self-assembly process. TEM analysis demon- strated that pure and insulin-loaded self-assembled PEG- POSS nanoparticles were of spherical shape with core- shell nanostructure, and were well-dispersed and uniform in size distribution. Such PEG-POSS nanoparticles showed a good loading capability of hydrophobic drug, insulin. It was found that insulin was well-protected in- side PEG-POSS nanoparticles at gastric pH for 2 hrs, and was released at intestinal pH (pH 6 - 7) where the ab- sorption and activation of the drug are necessary. As a result, insulin release from PEG-POSS nanoparticles was pH-dependent. 5. Acknowledgements The authors acknowledge the support of Shinshu Univer- sity Global COE Program “International Center of Ex- cellence on Fiber Engineering”. This paper is dedicated to the first principal, Chotaro Harizuka, on the occasion of 100th anniversary of Faculty of Textile Science and Technology, Shinshu University REFERENCES [1] J. J. Shi, A. R. Votruba, O. C. Farokhzad and R. Langer, “Nanotechnology in Drug Delivery and Tissue Engineer- ing: From Discovery to Applications,” Nano Letters, Vol. 10, No. 9, 2010, pp. 3223-3230. doi:10.1021/nl102184c [2] J. H. Kwon, B. H. Lee, J. J. Lee and C. W. Kim, “Insulin Microcrystal Suspension as a Long-Acting Formulation for Pulmonary Delivery,” European Journal of Pharma- ceutical Science, Vol. 22, No. 2-3, 2004, pp. 107-116. doi:10.1016/j.ejps.2004.02.007 [3] M. Goldberg and I. Gomez-Orellana, “Challenges for the Oral Delivery of Macromolecules,” Nature Reviews Drug Discovery, Vol. 2, No. 4, 2003, pp. 289-295. doi:10.1038/nrd1067 [4] B. S. Kim and P. T. Mather, “Amphiphilic Telechelics In- corporating Polyhedral Oligosilsesquioxane: 1. Synthesis  Self-Assembled Core-Shell Poly(Ethylene Glycol)-POSS Nanocarriers for Drug Delivery Copyright © 2011 SciRes. JBNB 206 and Characterization,” Macromolecules, Vol. 35, No. 22, 2002, pp. 8378-8384. doi:10.1021/ma020226h [5] B. S. Kim and P. T. Mather, “Morphology, Microstructure, and Rheology of Amphiphilic Telechelics Incorporating Polyhedral Oligosilsesquioxane,” Macromolecules, Vol. 39, No. 26, 2006, pp. 9253-9260. doi:10.1021/ma062069i [6] W. J. Lee, S. Ni, J. Deng, B. S. Kim, S. K. Satija, P. T. Mather and A. R. Esker, “Telechelic Poly(ethylene gly- col)-POSS Amphiphiles at the Air/Water Interface,” Ma- cromolecules, Vol. 40, No. 3, 2007, pp. 682-688. doi:10.1021/ma0618171 [7] B. S. Kim and P. T. Mather, “Amphiphilic Telechelics with Polyhedral Oligosilsesquioxane(POSS) End-groups: Dilute Solution Viscometry,” Polymer, Vol. 47, No. 17, 2006, pp. 6202-6207. doi:10.1016/j.polymer.2006.06.050 [8] C. K. Kim, B. S. Kim, F. A. Sheikh, U. S. Lee, M. S. Khil and H. Y. Kim, “Amphiphilic Poly(Vinyl Alcohol) Hybrids and Electrospun Nanofibers Incorporating Polyhedral Oli- gosilsesquioxane,” Macromolecules, Vol. 40, No. 14, 2007, pp. 4823-4828. doi:10.1021/ma070056e [9] F. A. Sheikh, N. A. M. Barakat, B. S. Kim, S. Aryal, M. S. Khil and H. Y. Kim, “Self-Assembled Amphiphilic Poly- hedral Oligosilsesquioxane(POSS) Grafted Poly(Vinyl Al- cohol) (PVA) Nanoparticles,” Materials Science and En- gineering C, Vol. 29, No. 3, 2009, pp. 869-876. doi:10.1016/j.msec.2008.07.029 [10] W. Zhang, B. Fang, A. Walther and A. H. E. Muller, “Synthesis via RAFT polymerization of Tadpole-shaped Organic/inorganic Hybrid PAA Containing POSS and Their Self-Assembly in Water,” Macromolecules, Vol. 42, No. 7, 2009, pp. 2563-2569. doi:10.1021/ma802803d [11] W. Zhang, L. Liu, X. D. Zhuang, X. Li, J. Bai and Y. Chen, “Synthesis and Self-assembly of Tadpole-Shaped Organic/ inorganic Hybrid Poly(N-isopropylacrylamide) Containing Polyhedral Oligomeric Silsesquioxane via RAFT Polym- erization,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 46, No. 21, 2008, pp. 7049-7061. doi:10.1002/pola.23010 [12] H. Hussain, B. H. Tan, G. L. Seah, Y. Liu, C. B. He and T. P. Davis, “Micelle Formation and Gelation of (PEG- P(MA-POSS)) Amphiphilic Block Copolymers via Asso- ciative Hydrophobic Effects,” Langmuir, Vol. 26, No. 14, 2010, pp. 11763-11773. doi:10.1021/la101686q [13] B. H. Tan, H. Hussain and C. B. He, “Tailoring Micelle Formation and Gelation in (PEG-P(MA-POSS)) Amphi- philic Hybrid Block Copolymers,” Macromolecules, Vol. 44, No. 3, 2011, pp. 622-631. doi:10.1021/ma102510u [14] K. O. Kim, Y. A. Seo, B. S. Kim, K. J. Yoon, M. S. Khil, H. Y. Kim and I. S. Kim, “Transition Behaviors and Hy- brid Nanofibers of Poly(vinyl alcohol) and polyethylene- glycol–POSS Telechelic Blends,” Colloid and Polymer Science, Vol. 289, No. 8, 2011, pp. 863-870. doi:10.1007/s00396-011-2407-y [15] Y. H. Lin, F. L. Mi, C. T. Chen, W. C. Chang, S. F. Peng, H. F. Liang and H. W. Sung, “Preparation and Charac- terization of Nanoparticles Shelled with Chitosan for Oral Insulin Delivery,” Biomacromolecules, Vol. 8, No. 1, 2007, pp. 146-152. doi:10.1021/bm0607776 [16] Y. H. Lin, C. T. Chen, H. F. Liang, A. R. Kulkarni, P. W. Lee, C. H. Chen and H. W. Sung, “Novel Nanoparticles for Oral Insulin Delivery via the Paracellular Pathway,” Nanotechnology, Vol. 18, No. 10, 2007, pp. 105-102. doi:10.1088/0957-4484/18/10/105102 [17] V. Sluzky, J. A. Tamada, A. M. Klibanovt and R. Langer, “Kinetics of Insulin Aggregation in Aqueous Solutions upon Agitation in the Presence of Hydrophobic Surfaces,” Applied Biological Sciences, Vol. 88, No. 21, 1991, pp. 9377-9381. [18] C. L. Lawson and R. J. Hanson, “Solving Least Squares Problem,” Prentice-Hall, Englewood Cliffs, 1974. [19] B. Sarmento, A. Ribeiro, F. Veiga, D. Ferreira and R. Neufeld, “Oral Bioavailability of Insulin Contained in Polysaccharide Nanoparticles,” Biomacromolecules, Vol. 8, No. 10, 2007, pp. 3054-3060. doi:10.1021/bm0703923 [20] K. Sonaje, K. J. Lin, J. J. Wang, F. L. Mi, C. T. Chen, J. H. Juang and H. W. Sung, “Self-Assembled pH-Sensitive Nanoparticles: A platform for Oral Delivery of Protein Drugs,” Advanced Functional Materials, Vol. 20, No. 21, 2010, pp. 3695-3700. doi:10.1002/adfm.201001014 [21] S. Gelinas, C. Chapados, M. Beauregard, I. Gosselin and M. G. Martinoli, “Effect of Oxidative Stress on Stability and Structure of Neurofilament Proteins,” Biochemistry and Cell Biology, Vol. 78, No. 6, 2000, pp. 667-674. doi:10.1139/o00-070 [22] W. P. Wang, X. X. Jie, M. Fei and H. Jiang, “Synthesis of Core-shell Particles by Batch Emulsion Polymerization of Styrene and Octavinyl Polyhedral Oligomeric Silsesqui- oxane,” Journal of Polymer Reseraches, Vol. 18, No. 1, 2011, pp. 13-17. doi:10.1007/s10965-009-9385-5 |

