 Journal of Environmental Protection, 2011, 2, 298-303 doi:10.4236/jep.2011.23033 Published Online May 2011 (http://www.scirp.org/journal/jep) Copyright © 2011 SciRes. JEP 1 Toxicity and Antimicrobial Activities of Ionic Liquids with Halogen Anion You Yu, Yi Nie College of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, China. Email: ny1968@163.com. Received January 2nd, 2011; revised February 18th, 2011; accepted March 24th, 2011. ABSTRACT To investigate the eco-toxicity of ionic liquids (ILs), experiments on growth of three kinds of bacteria were carried out for six common ILs with halogen anion b y a micro-calorimetric method at 310 K. The results indicate that the growth of all the bacteria was inh ibited in the presence o f ILs. In addition, all ILs at definite concentrations show some toxicity to Escherichia coli, Staphylococcus aureus and Bacillus subtilis. Anti-microbial activities of the ILs with halogen anion are strongly related to structures of the ILs. An increase in alkyl group chain length corresponds with an increase in toxicity, and the ILs with pyridinium cation exhibit stro ng er restraining effect than the same series ILs with imida zolium cation. Keywords: Toxicity, Ionic Liquids, Inhibition 1. Introduction Ionic liquids (ILs) were applied in many fields such as biological and chemical reactions for they are non- flammable, sparsely volatile and thermally stable. For these properties, air pollution was prevented greatly. However, release of ILs into aquatic environments may lead to water pollution because of their high solubilities. In recent years, some reports that risks of ionic liquids have been available in environment and organisms. Li Xiaoy et al. [1-5] studied the toxicity of ionic liquids to Daphnia magna. The results showed that the toxicity to Daphnia magna increased with the increasing of n-alkyl chain length. Zhang Feng et al. [6] carried out standard test methods for evaluating the toxicity of chemicals to three aquatic organisms. The results indicated that the inhibiting effects increased significantly with increasing concentrations of ILs. In addition, large-scale application, ILs released into the environments will be inevitable. These cause environmental and agricultural pollution. Some reports [7,8] on the germination rate of seeds and growth of seedlings have indicated different seeds have different reaction to ILs which shown eco-toxicity. Moreover, it is dangerous that poisoning organisms can be increased by increasing the food chain. The food chain of primary producers, primary consumers and predators cause acute and chronic toxicity or cancer. So studying the toxicity of ILs is important. Microcalorimetry was confirmed to be valid as an al- ternative method in the study of metabolism of the cell. [9]. It is a useful tool for investigating the biological processes because it permits the continuous monitoring of the activity of a living process in situ without disturb- ing the system, and the heat evolved or adsorbed is strictly proportional to the rate of the biological proc- esses [10,11]. With its abundant thermodynamic and kinetic information, micro-calorimetry has been widely applied in clinical analysis, pharmacology, ecology, bio- technology and agriculture [12,13]. In this paper, the effects of common ILs with Halogen anion (as shown in Scheme 1) on Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) and Bacillus subtilis (B. sutilis) growth were investigated by micro- calorimetry, and the bacterial growth rate constants at different concentrations (c) of ILs aqueous solutions were calculated via power-time curves along with the generalized Logistic equation. The -c correlation equa- tions were formulated. The results indicate antimicrobial activities are related to the structures of ILs, indicating a potential eco-toxicity of the ILs to the micro-organisms in the water. 2. Theory The growth of bacteria is often limited by some external constraints, including substrate, product concentration, temperature, pH-values and so on. In the logarithmic growth phase, the number of bacteria and time are re- lated according to [14,15]:  Toxicity and Antimicrobial Activities of Ionic Liquids with Halogen Anion 299 Scheme 1. Chemical structures of prepared Ils. 2 tt dN NN dt t (1) where Nt represents the number of bacteria at time (t), is the growth rate constant, is the deceleration rate constant, and t is the experimental time. By integrating Equation (1) with respect to time (t), the following equation was obtained: (1 ) t t NK e (2) KK in Equation (2) represents the maxi- mum bacterial number during the whole bacterial growth, and is the final multiple of the initial bacterial number (being the integral constant). Under the assumption that the heat production rate Pt is proportional to the bacterial number, and P0 represents the heat production rate by one bacterium, then, 00 , ttm PPNP KP Inserting it into Equation (2), the following equation was obtained: (1 ) t tm PPK e (3) Pm in Equation (3) is the maximum heat production rate during the whole bacterial growth. Using the experimental data Pt and t obtained from the power-time curves, the growth rate constant ( ) can be calculated by Equation (3). 3. Experimental and Material 3.1. Instrument A thermometric eight channel Thermal Activity Monitor (3114/3236TAM Air, Sweden) in conjunction with the operating and analytical software was used in this ex- periment. With this instrument, reactions can be studied at any given temperature in the temperature range of 5˚C - 60˚C within ± 2 × 10−2˚C uncertainty. The system is very sensitive, its detection limit is 1 × 10−5 W, and the baseline stability (over a period of 24 h) is 2 × 10−5 W. The measuring range contains between 60 mW and 600 mW. The maximum sample volume is 24 mL. 3.2. Materials Standard strains of E. coli, S. aureus and B. subtilis were used as the test organism. The beef culture medium was used, containing 1 g NaCl, 2 g peptone and 1 g beef extract in every 200 mL. The pH of medium was adjusted to 7.2 - 7.4 before auto- claving. The culture medium was sterilized in high pres- sure steam at 121℃ for 30 min before experiments. Ionic liquids with halide anion used in this experiment were synthesized according to the published method [16-17]. Aqueous solutions of ILs at different concentra- tions were prepared using doubly distilled water. 3.3. Experimental Method Ampoule mode was used in this experiment. The bacte- rial suspension with a volume of 8 mL was poured into each 24 mL glass ampoule in sterile conditions. After adding ILs aqueous solution at different concentrations into the ampoule, the ampoules were then sealed with caps and placed into channels. Power-time curves of continuous bacterial growth were recorded by computer. When the baseline was re-established and became stabi- lized, the process of bacterial growth was complete. The micro-calorimeter was controlled at 310 K by thermostat in the whole process, which is the optimum growth temperature. 4. Results and Discussion 4.1. Power-Time Curves of Bacterial Growth All ILs with halogen anion were tested for antimicrobial activities against E. coli, S. aureus and B. subtilis. The power-time curves of bacterial growth at 310 K in the absence and presence of ILs have been determined, and parts of the fit curves in logarithmic growth phase are plotted in Figure 1. It can be seen that the slopes of ex- ponential growth phase at different IL concentrations are different. It can be concluded that the bacterial growth phase changes with adding the ILs aqueous solution into the culture medium. 4.2. Bacterial Growth Rate Constants The data of Pt and t were obtained from Figure 1. Ac- cording to Equation (3), the bacterial growth rate con- stants ( ) were calculated, and shown in Tables 1-3. The growth rate constants ( ) of the E. coli, S. aureus and B. subtilis gradually decrease with the increase of the IL concentration (c). This is mainly attributed to the inhibi- tory effect of the halide ILs to some cells in the bacteria suspension. The survivors remain metabolizing continu- ously at a lower level of heat production rate, depending on the concentration of IL in the solution. 4.3. Growth Rate Constants VS Concentrations and Structure of ILs The growth rate constants decrease with the increase of L concentrations. The results indicate that the ILs with I Copyright © 2011 SciRes. JEP  Toxicity and Antimicrobial Activities of Ionic Liquids with Halogen Anion Copyright © 2011 SciRes. JEP 300 Table 1. Growth rate constants μ/min−1 of E. Coli at different concentrations of ILs at 310 K. ILs c(mmol/L) 4.985 7.431 16.924 25.123 30.001 39.617 EMIMCl (min−1) 0.06029 0.05459 0.045 0.04277 0.03509 0.02689 c(mmol/L) 1.44 9.132 11.133 13.784 18.084 BMIMCl (min−1) 0.06456 0.05515 0.04788 0.04211 0.03701 c(mmol/L) 0.537 0.892 1.422 1.773 2.122 2.643 HMIMCl (min−1) 0.05583 0.04861 0.04152 0.03558 0.02063 0.01481 c(mmol/L) 0 0.646 1.288 1.928 3.197 HPyCl (min−1) 0.09126 0.0768 0.04322 0.03798 0.01876 c(mmol/L) 0 0.235 0.352 0.584 0.699 OPyCl (min−1) 0.09126 0.07036 0.04481 0.0341 0.02268 c(mmol/L) 0 3.491 6.947 13.757 20.435 26.985 BMIMBr (min−1) 0.09126 0.08043 0.07743 0.06148 0.0582 0.03734 Table 2. Growth rate constants μ/min−1 of S. aureus at different concentrations of ILs at 310 K. ILs c(mmol/L) 0 1.508 2.508 3.998 4.985 5.967 8.885 EMIMCl (min−1) 0.10048 0.07313 0.06869 0.05495 0.04731 0.03729 0.0259 c(mmol/L) 0 1.44 2.155 2.866 3.574 4.278 BMIMCl (min−1) 0.10048 0.07859 0.05113 0.04211 0.04367 0.03254 c(mmol/L) 0 0.178 0.529 0.701 0.87 1.038 HMIMCl (min−1) 0.10048 0.09172 0.07211 0.06802 0.03621 0.02643 c(mmol/L) 0 0.0646 0.129 0.256 0.32 0.477 HPyCl (min−1) 0.10048 0.09299 0.0827 0.0677 0.05613 0.03075 c(mmol/L) 0 0.0235 0.0352 0.0468 0.0584 OPyCl (min−1) 0.10048 0.05 0.03573 0.03095 0.0271 c(mmol/L) 0 4.358 6.947 8.662 12.913 BMIMBr (min−1) 0.10048 0.09307 0.07162 0.06862 0.05074 Table 3. Growth rate constants μ/min−1 of B. subtilis at different concentrations of ILs at 310 K. ILs c(mmol/L) 0 2.508 4.985 9.848 14.594 EMIMCl (min−1) 0.11248 0.09984 0.0799 0.06637 0.0472 c(mmol/L) 0 1.798 3.574 5.327 7.06 8.771 BMIMCl (min−1) 0.11248 0.10884 0.09289 0.07786 0.06444 0.04914 c(mmol/L) 0 0.716 2.844 5.286 7.005 8.703 HMIMCl (min−1) 0.11248 0.09693 0.0766 0.04609 0.03397 0.01186 c(mmol/L) 0 0.646 1.288 1.928 2.564 3.197 HPyCl (min−1) 0.11248 0.10393 0.0887 0.07373 0.06781 0.05921 c(mmol/L) 0 0.59 1.177 1.693 2.342 OPyCl (min−1) 0.11248 0.09185 0.08533 0.0607 0.03813 c(mmol/L) 0 1.625 3.229 4.814 6.379 9.454 BMIMBr (min−1) 0.112 48 0.091 81 0.079 63 0.0712 0.064 19 0.039 72 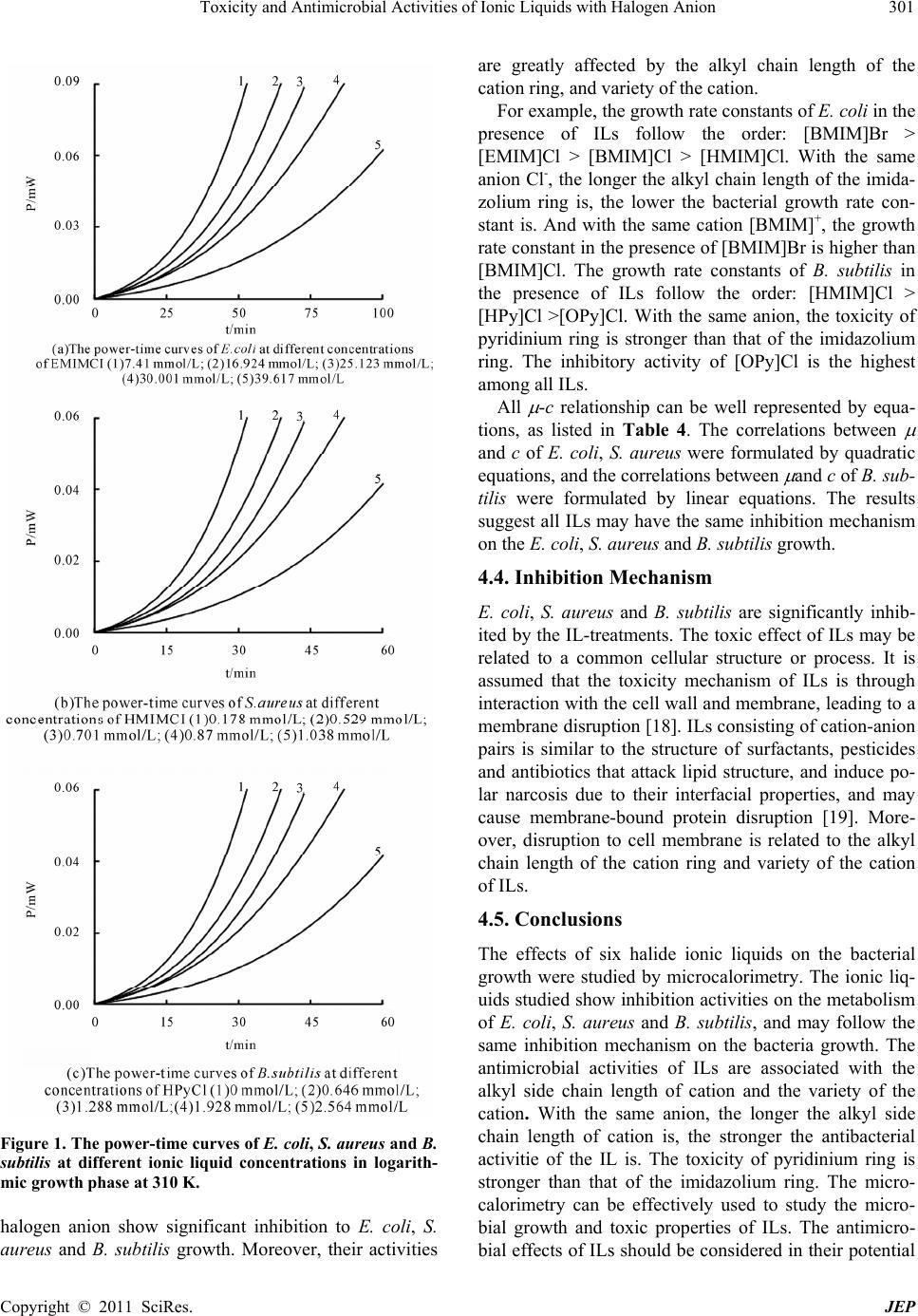 Toxicity and Antimicrobial Activities of Ionic Liquids with Halogen Anion 301 Figure 1. The power-time curves of E. coli, S. aureus and B. subtilis at different ionic liquid concentrations in logarith- mic growth phase at 310 K. halogen anion show significant inhibition to E. coli, S. aureus and B. subtilis growth. Moreover, their activities are greatly affected by the alkyl chain length of the cation ring, and variety of the cation. For example, the growth rate constants of E. coli in the presence of ILs follow the order: [BMIM]Br > [EMIM]Cl > [BMIM]Cl > [HMIM]Cl. With the same anion Cl-, the longer the alkyl chain length of the imida- zolium ring is, the lower the bacterial growth rate con- stant is. And with the same cation [BMIM]+, the growth rate constant in the presence of [BMIM]Br is higher than [BMIM]Cl. The growth rate constants of B. subtilis in the presence of ILs follow the order: [HMIM]Cl > [HPy]Cl >[OPy]Cl. With the same anion, the toxicity of pyridinium ring is stronger than that of the imidazolium ring. The inhibitory activity of [OPy]Cl is the highest among all ILs. All -c relationship can be well represented by equa- tions, as listed in Table 4. The correlations between and c of E. coli, S. aureus were formulated by quadratic equations, and the correlations between and c of B. sub- tilis were formulated by linear equations. The results suggest all ILs may have the same inhibition mechanism on the E. coli, S. a ureus and B. subtilis growth . 4.4. Inhibition Mechanism E. coli, S. aureus and B. subtilis are significantly inhib- ited by the IL-treatments. The toxic effect of ILs may be related to a common cellular structure or process. It is assumed that the toxicity mechanism of ILs is through interaction with the cell wall and membrane, leading to a membrane disruption [18]. ILs consisting of cation-anion pairs is similar to the structure of surfactants, pesticides and antibiotics that attack lipid structure, and induce po- lar narcosis due to their interfacial properties, and may cause membrane-bound protein disruption [19]. More- over, disruption to cell membrane is related to the alkyl chain length of the cation ring and variety of the cation of ILs. 4.5. Conclusions The effects of six halide ionic liquids on the bacterial growth were studied by microcalorimetry. The ionic liq- uids studied show inhibition activities on the metabolism of E. coli, S. aureus and B. subtilis, and may follow the same inhibition mechanism on the bacteria growth. The antimicrobial activities of ILs are associated with the alkyl side chain length of cation and the variety of the cation. With the same anion, the longer the alkyl side chain length of cation is, the stronger the antibacterial activitie of the IL is. The toxicity of pyridinium ring is stronger than that of the imidazolium ring. The micro- calorimetry can be effectively used to study the micro- bial growth and toxic properties of ILs. The antimicro- bial effects of ILs should be considered in their potential Copyright © 2011 SciRes. JEP  Toxicity and Antimicrobial Activities of Ionic Liquids with Halogen Anion 302 Table 4. μ-c correlation equations. bacteria Ionic liquids c equations [EMIM]Cl 62 4 1.74 109.76 100.0633cc 20.9738R [BMIM]Cl 52 3 1.22 101.50 100.0672cc 20.9708R [HMIM]Cl 42 5.70 100.01790.0650cc 20.9974R [BMIM]Br 62 3 5.16 101.69 100.0890cc 20.9662R [HPy]Cl 32 5.30 100.04030.0936cc 20.9679R E.coli [OPy]Cl 22 4.70 100.1320.0924cc 20.9684R [EMIM]Cl 42 6.30100.01370.0979 cc 20.9873R [BMIM]Cl 32 2.24 100.02580.102cc 20.9519R [HMIM]Cl 22 4.78100.02260.0993 cc 20.9702R [BMIM]Br 523 6.52103.22100.102 cc 20.9575R [HPy]Cl 22 5.93100.1180.100 cc 20.9986R S. aureus [OPy]Cl 2 24.92.690.100 cc 20.9983R [EMIM]Cl 5 2.99100.109 c 20.973R [BMIM]Cl 3 7.57100.118 c 20.981R [HMIM]Cl 2 1.11100.108 c 20.9929R [BMIM]Br 3 7.20100.107 c 20.9769R [HPy]Cl 2 1.73100.112 c 20.9673R B. subtilis [OPy]Cl 2 3.06100.114 c 20.9716R industrial applications and overall risk assessment. 5. Acknowledgements Shandong Province Natural Scientific Foundation of China (ZR2009BM035), and Shandong Province Science Research Reward Foundation for Excellent Young and Middle-aged Scientists (2008BS02021). REFERENCES [1] X. Li, Y. Luo, L. Li, J. Wang and Z. Sun, “Effects of the Ionic Liquid 1-Methyl 3-Octylimidazolium Bromide on the Feeding Intensity of Daphnia Magna,” Acta Scientiae Circumstantiae, Vol. 28, No. 11, November 2008, pp. 2331-2335. [2] Y. Luo, X. Li, P. Huang, J. Wang and Z. Sun, “Effect of Ionic Liquids with Different Alkyl Chain Lengths on Feeding Behavior of Daphnia Magna,” Chinese Journal of Applied and Environmental Biology, Vol. 14, No. 3, June 2008, pp. 383-387. [3] B. Zhang, Y. Luo and H. Fang, “The Acute Toxicity of 1 Methyl-3-Octylimidazolium Bromide to Daphnia Magna at Different Developmental Stages,” Ecology and Envir- ronment, Vol. 17, No. 3, May 2008, pp. 1021-1023. [4] R. J. Bernot, M. A. Brueseke, M. A. Evans-White and G. A. Lamberti, “Acute and Chronic Toxicity of Imida- zolium-Based Ionic Liquids on Daphnia Magna,” Envi- ronmental Toxicology and Chemistry, Vol. 24, No.1, January 2005, pp. 87-92 doi:10.1897/03-635.1 [5] T. P. T. Pham, C. W. Cho, K. Vijayaraghavan, J. Min and Y. S. Yun, “Effect of Imidazolium-Based Ionic Liquids on the Photosynthetic Activity and Growth Rate of Sele- nastrum Capricornutum,” Environmental Toxicology and Chemistry, Vol. 27, No.7, July 2008, pp. 1583-1589. doi:10.1897/07-415.1 [6] H. Mu, X. Peng and F. Zhang, “Toxicity of [C8mim]PF6 to Aquatic Organisms,” China Environmental Science, Vol. 29, No. 11, November 2009, pp. 1196-1201 [7] F. Yang, H. Meng, C. Li and Z. Wang, “Ecotoxicity of Ionic Liquids on the Germination and Growth of Three Seeds,” Chinese Journal of Environmental Engineering, Vol. 3, No. 4, April 2009, pp. 751-754 [8] P. Liu, L. Sun, H. Liu, K. Xu, Y. Ding, X. Li and J. Wang, “Effects of 1-Octyl-3-Methyl Imidazolium Bromide Ionic Liquid on the Germination and Growth of Wheat Seed- lings,” Journal of Agro-Environment Science, Vol. 27, No. 2, March 2008, pp. 425-429. [9] L. N. Yang, F. Xu, L. X. Sun, Z. C. Tan, H. D. Tan, Z. B. Zhao and J. G. Liang, “”Study on Interaction between Copyright © 2011 SciRes. JEP  Toxicity and Antimicrobial Activities of Ionic Liquids with Halogen Anion 303 Antibiotics and Escherichia Coli DH5Α by Microcalo- rimetric Method,” Journal of Thermal Analysis and Calorimetry, Vol. 85, No. 3, September 2006, pp. 807-810.doi:10.1007/s10973-006-7507-4 [10] L. Núñez-Regueira, J. A. Rodríguez-Añón, J. Proupín- Castiñeiras and O. Núñez-Fernández, “Microcalorimetric Study of Changes in the Microbial Activity in a Humic Cambisol after Reforestation with Eucalyptus in Galicia (NW Spain),” Soil Biology and Biochemistry, Vol. 38, No. 1, January 2006, pp. 115-124. doi:10.1016/j.soilbio.2005.04.031 [11] J. C. Zhu, C. H. Li, Y. Liu, Z. H. Zhang, A. X. Hou and S. S. Qu, “A Microcalorimetric Study of the Action of Mercuric Chloride on the Metabolism of Mitochondria Isolated from Cyprinus Carpio Liver Tissue,” Journal of Thermal Analysis and Calorimetry, Vol. 83, No. 1, Janu- ary 2006, pp. 181-186. doi:10.1007/s10973-005-7020-1 [12] J. Ma, W. T. Qi, L. N. Yang, W. T. Yu, Y. B. Xie, W. Wang, X. J. Ma, F. Xu and L. X. Sun, “Microcalorimetric Study on the Growth and Metabolism of Microencapsu- lated Microbial Cell Culture,” Journal of Microbiological Methods, Vol. 68, No. 1, January 2007, pp. 172-177. doi:10.1016/j.mimet.2006.07.007 [13] H. K. Koblish, S. Zhao, C. F. Franks, R. R. Donatelli, R. M. Tominovich, L. V. LaFrance, K. A. Leonard, J. M. Gushue, D. J. Parks, R. R. Calvo, K. L. Milkiewicz, J. J. Marugán, P. Raboisson, M. D. Cummings, B. L. Gras- berger, D. L. Johnson, T. Lu, C. J. Molloy and A. C. Ma- roney, “Benzodiazepinedione Inhibitors of the Hdm2:P53 Complex Suppress Human Tumor Cell Proliferation in Vitro and Sensitize Tumors to Doxorubicin in Vivo,” Molecular Cancer Therapeutics, Vol. 5, No. 1, January 2006, pp. 160-169. doi:10.1158/1535-7163.MCT-05-0199 [14] H. L. Zhang, H. T. Sun, Z. D. Nan and Y. J. Liu, “Estab- lishment of Experimental Model of Bacterial Growth un- der Inhibitory Conditions and Study of Optimum Growth Temperature,” Journal of Thermal Analysis, Vol. 44, No. ochimica 004, pp. 71-74. 1, January 1995, pp. 105-109. [15] H. L. Zhang, X. F. Yu, X. X. Li and X. R. Pan, “A Study of Promotive and Fungistatic Actions of Steroidal Saponin by Microcalorimetric Method,” Therm Acta, Vol. 416, No. 1-2, June 2 doi:10.1016/j.tca.2003.11.033 [16] T. Nishida, Y. Tashiro and M. Yamamoto, “Physical and Electrochemical Properties of 1-Alkyl-3-Methylimidazolium Tetrafluoroborate for Elec- trolyte,” Journal of Flu- orine Chemistry, Vol. 120, No. 2, April 2003, pp. 135-141.doi:10.1016/S0022-1139(02)00322-6 [17] Y. H. Zhu, C. B. Ching, K. Carpenter, R. Xu, S. Selva- ratnam, N. S. Hosmane and J. A. Maguire, “Synthesis of the Novel Ionic Liquid [N-pentylpyridinium]+ [Closo-CB11H12]− and Its Usage as a Reaction Medium in Cata- lytic Dehalogenation of Aromatic Halides,” Applied Organometallic Chemistry, Vol. 17, No. 6-7, June-July 2003, pp. 346-350.doi:10.1002/aoc.407 [18] K. M. Docherty and C. F. Kulpa. “Toxicity and Antim- icrobial Activity of Imidazolium and Pyridinium Ionic Liquids,” Green Chemistry, Vol. 7, No. 4, March 2005, pp. 185-189. doi:10.1039/b419172b [19] R. J. Bernot, E. E. Kennedy and G. A. Lamberti, “Effects of Ionic Liquids on the Survival, Movement, and Feeding Behavior of the Freshwater Snail, Physa Acuta,” Envi- ronmental Toxicology and Chemistry, Vol. 24, No. 7, July 2005, pp. 1759-1765. doi:10.1897/04-614R.1 Copyright © 2011 SciRes. JEP
|