Paper Menu >>
Journal Menu >>
 Vol.3, No.4, 245-248 (2011) Health doi:10.4236/health.2011.34043 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Massive carcinoma of the cornea in an immunocompetent patient Irene Pecorella1, Pierluigi Grenga2, Enzo Maria Vingolo2 1Department of Experimental Medicine, University of Rome “Sapienza” Viale Regina Elena, Rome, Italy; *Corresponding Author: irene.pecorella@uniroma1.it 2Department of Ophthalmologic Science, University of Rome “Sapienza” Viale Regina Elena, Rome, Italy; pigig@tiscalinet.it; e.vingolo@rdn.it Received 11 February 2011; revised 7 March 2011; accepted 29 March 2011. ABSTRACT A case of massive well-differentiated, superfi- cially invasive, squamous cell carcinoma of the cornea is described. This patient did not have any common identifiable risk factors in the de- velopment of SCC of th e oc ular surfa ce, such as excessive solar exposure, HPV infection, im- munocompromised state, or chronic ocular ir- ritation. A perforating injury in the cornea of the same eye had occurred 11 years before. The possible role of trauma in causing subsequent neoplastic development is discus sed, as well as the immunohistochemical results for p53 and p63. Keywords: Squamous Cell Carcinoma; Cornea; HPV; Trauma; Solar Elastosis 1. INTRODUCTION Squamous cell carcinoma (SCC) is the most common primary malignancy of the ocular surface, making up 14% of all primary ocular and orbital tumors. It pre- dominantly affects male patients with an average age of 56 years (range: 4 to 91 yrs). SCC most commonly oc- curs in the limbus (87.8%), where the corneal epithelial stem cells are located [1], and in the interpalpebral space, where the ocular tissues are mostly exposed to ultravio- let irradiation. From this location, the tumour may spread onto the bulbar conjunctiva and/or the cornea. SCC of the cornea without involvement of the conjunc- tiva and limbus is a very rare condition. The corneal intrastromal infiltration is clinically described as corneal cystic gelatinous lesion [1]. Intraocular invasion is often heralded by the onset of low-grade inflammation and secondary glaucoma. Metastasis is uncommon and, when present, usually occurs in the preauricular, sub- mandibular or cervical lymph nodes, the parotid gland, the lungs or the bones. Its origin is debated and some investigators believe that the corneal epithelium may undergo dysplastic and cancerous changes, whereas the limbal transition zone/ stem cell theory has been proposed for the development of corneal intraepithelial n eoplasia by Lee and Hirst [1 ]. Recently, Chikama et al demonstrated in transgenic mice lines that upregulation of FGF-7 signalling pathway(s) may be common in tumorigenesis derived from limbal stem cells that undergo oncogenic transformation by insults, such as lo ng-term ultraviolet B (UVB) exposure, infection of human papilloma virus (HPV) and human immune deficiency virus (HIV), and cigarette smoking, etc. [2]. We present herein a case of massive SCC of the lim- bus and cornea in an immunocompetent patient and dis- cuss the possible etiological role of a preceding trau- matic event to the same eye in causing the occurrence of the tumor. 2. CLINICAL HISTORY A 67-years-old male patient presented with a painful eye and a massive, protuberant lesion covering the nasal half of the left co rnea (Figure 1). The lesion had initially appeared in the nasal limbal area and had been slowly enlar ging to it s present size for approxim ately 18 m ont hs. Eleven years before a perforating injury in the left cornea of the same eye, had caused progression to bulbar phthisis. His work history did not involve exposure to radiation, chemicals, or the sun. The patient was other- wise in good health. Ophthalmological examination showed a white, fleshy papillomatous mass, hang ing from the surface of the left cornea. The neighbouring conjunctiva was markedly hy- peraemic. A clinical diagnosis of squamous cell carci- noma of the ocular surface was made. Visual acuity was of hand motion near to face. Digital tonometry revealed 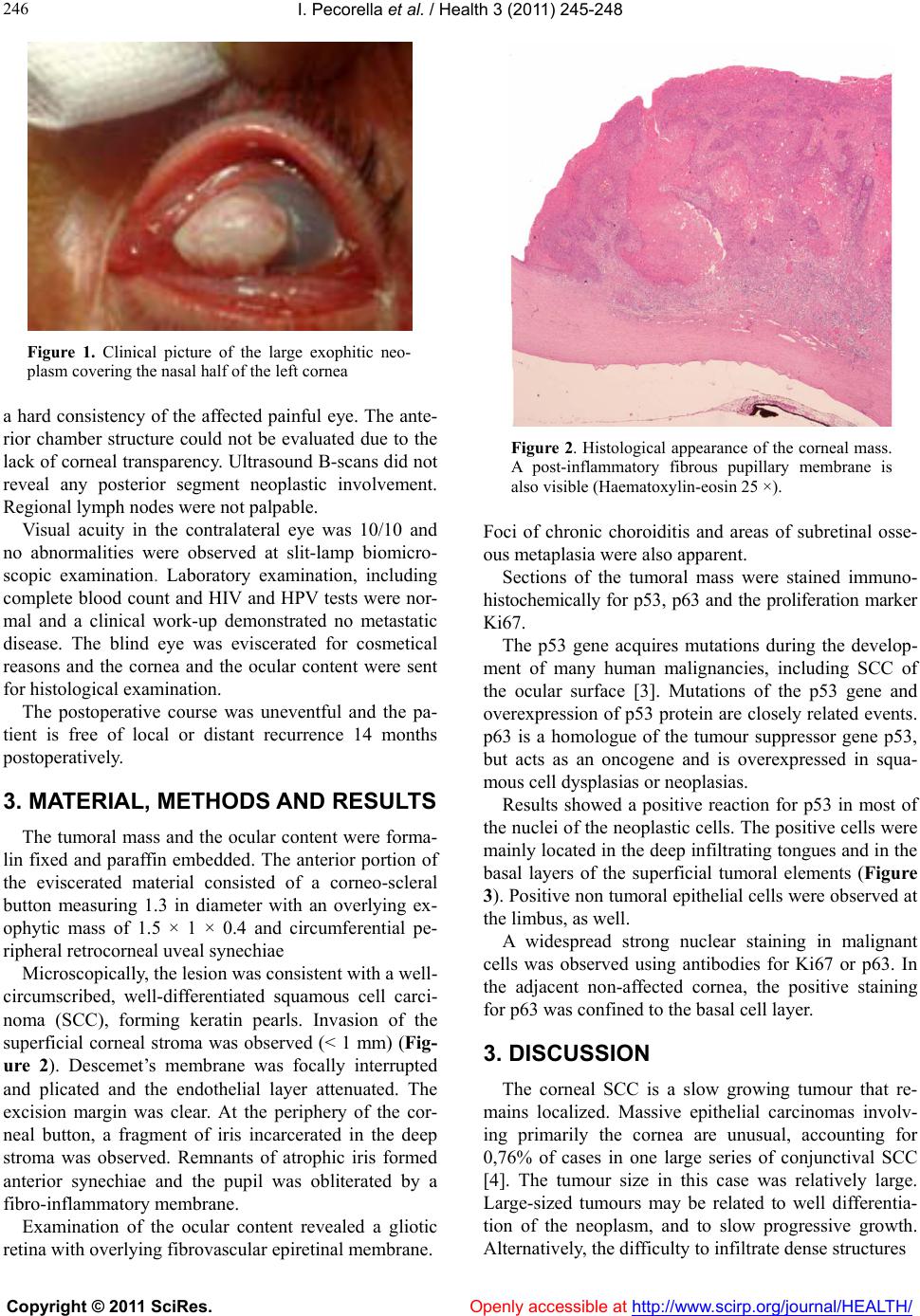 I. Pecorella et al. / Health 3 (2011) 245-248 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 246 Figure 1. Clinical picture of the large exophitic neo- plasm covering the nasal half of the left cornea a hard consistency of the affected painful eye. The ante- rior chamber structure could not be evaluated due to the lack of co rneal tran sparency. Ultrasound B-scans did not reveal any posterior segment neoplastic involvement. Regional lymph nodes were not palpable. Visual acuity in the contralateral eye was 10/10 and no abnormalities were observed at slit-lamp biomicro- scopic examination. Laboratory examination, including complete blood count and HIV and HPV tests were nor- mal and a clinical work-up demonstrated no metastatic disease. The blind eye was eviscerated for cosmetical reasons and the cornea and the ocular content were sent for histological examination. The postoperative course was uneventful and the pa- tient is free of local or distant recurrence 14 months postoperatively. 3. MATERIAL, METHODS AND RESULTS The tumoral mass and the ocular content were forma- lin fixed and paraffin embedded. The anterior portion of the eviscerated material consisted of a corneo-scleral button measuring 1.3 in diameter with an overlying ex- ophytic mass of 1.5 × 1 × 0.4 and circumferential pe- ripheral retrocorneal uveal synechiae Microscopically, the lesion was consistent with a well- circumscribed, well-differentiated squamous cell carci- noma (SCC), forming keratin pearls. Invasion of the superficial corneal stroma was observed (< 1 mm) (Fig- ure 2). Descemet’s membrane was focally interrupted and plicated and the endothelial layer attenuated. The excision margin was clear. At the periphery of the cor- neal button, a fragment of iris incarcerated in the deep stroma was observed. Remnants of atrophic iris formed anterior synechiae and the pupil was obliterated by a fibro-inflammatory membrane. Examination of the ocular content revealed a gliotic retina with overlying fibrovascular epiretinal membrane. Figure 2. Histological appearance of the corneal mass. A post-inflammatory fibrous pupillary membrane is also visible (Haematoxylin-eosin 25 ×). Foci of chronic choroiditis and areas of subretinal osse- ous metaplasia were also apparent. Sections of the tumoral mass were stained immuno- histochemically for p53, p63 and the proliferation marker Ki67. The p53 gene acquires mutations during the develop- ment of many human malignancies, including SCC of the ocular surface [3]. Mutations of the p53 gene and overexpression of p53 protein are closely related events. p63 is a homologue of the tumour suppressor gene p53, but acts as an oncogene and is overexpressed in squa- mous cell dysplasias or neoplasias. Results showed a positive reaction for p53 in most of the nuclei of the neoplastic cells. The po sitive cells were mainly located in the deep infiltratin g tongues and in the basal layers of the superficial tumoral elements (Figure 3). Positive non tumoral epithelial cells were ob served at the limbus, as well. A widespread strong nuclear staining in malignant cells was observed using antibodies for Ki67 or p63. In the adjacent non-affected cornea, the positive staining for p63 was confined to the basal cell layer. 3. DISCUSSION The corneal SCC is a slow growing tumour that re- mains localized. Massive epithelial carcinomas involv- ing primarily the cornea are unusual, accounting for 0,76% of cases in one large series of conjunctival SCC [4]. The tumour size in this case was relatively large. Large-sized tumours may be related to well differentia- tion of the neoplasm, and to slow progressive growth. Alternatively, the difficulty to infiltrate dense structures  I. Pecorella et al. / Health 3 (2011) 245-248 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 247 Figure 3. Immunohistochemical detection of p53 in the nuclei (brown color) of the malignant cells. Diffuse nu- clear positivity in the infiltrating tongue of cancer cells in- vading the anterior corneal stroma (asterisk) (Streptavidin- biotin- DAB staining 100 ×). like the cornea and the sclera may favour a superficial growth for a long time and an exoph ytic pattern in long- standing lesions. Our patient did not have any of the several identifiable risk factors in the development of SCC of the ocular surface, such as excessive solar exposure, HPV infection, immunocompromised state such as in HIV, a history of smoking, or chronic irritation from dust, wind petroleum exposure. Mechanical trauma to the ocular surface, including thermal burns, is occasionally mentioned among the risk factors for SCC of the eye. Two of the 17 cases of con- junctival SCC listed by Auw-Haedrich and coll. had suffered from ocular injury many years before the oc- currence of the neoplasm [5]. Three cases were reported in an orbital socket after long wear ing of ocular pro sthe- ses [6]. Scars can promote malignant growth of tumour cells. Thick, dense scars are the type that usually gives rise to squamous cell carcinoma of the skin and the cica- trix may be caused by any traumatic event. Surgical in- cisions have also been claimed to cause skin cancer. Trauma is considered not to act as initiator, but to act as promoter of carcinogenesis. There are two theories as for the induction of scar cancer. One is that the cells com- posing scar tissues convert themselves directly into tu- morigenic ones; the other is that scar tissues secrete soluble factors that stimulate carcinogenic conversion or proliferation of tumour cells pre-ex isting around the scar [7]. Whether fibrosis is accompanied with tumour or not depends on the types of collagen it produces. Tumour- associated fibrosis is characterized by increased collagen Type III content [8]. According to Martins-Green and coll., during the inflammatory response caused by wound- ing, a plethora of cytokines are released that cause blood vessel leakage and stimulate cell division which are key elements in the process leading to possible viral onco- gene integration and activation and then to tumour de- velopment. In their experimental work, wounding at 4 or 5 days after inoculation of Rous sarcoma virus resulted in 100% of the chickens developing wound tumours [7]. Their findings casts a new light on the possible role of viruses in tumour development and, possibly, on pre- existing HPV ocular infection and post-traumatic neo- plasm. We believe that the previous injury to the eye may have played a role in the development of the corneal neoplasia in this patient. However, we cannot dismiss the possibility that an actinic damage not related to out- door working might have contributed to the development of the corneo/conjunctival neoplasia, as pointed out by the diffuse immunopositivity to p53. Normally, p53 is undetectable immunohistochemically and negative in normal human conjunctiva. Mutations of p53 are char- acteristic of solar damage and are detected in the major- ity of SCC of the skin and in around 50% of the con- junctival SCC. Also Toth and coll. recently repo rted p53 gene overexpression in 78% of conjunctival SCC and found no relationship with HPV type 16 or 18 infection within the tumour tissue [4]. In a bilateral SCC of the conjunctiva, p53 positivity was found in only one of the eyes [9], perhaps indicating that the presence of mutant p53 is rather a marker for tumour aggressiveness. Inter- estingly, also the limbal cells overexpressed this tumour marker in our specimen. Its detection may be related to its mutation or, alternatively to its angiogenic activity in an eye with a previous penetrating injury and ocular surface remodelling. Our immunohistochemical results also confirm the previously reported p63 expression in the neoplastic cells of the conjunctival SCC and its distribution in all layers of this invasive carcinoma [6]. Its detection may prove useful in the differential diagnosis between reac- tive and malignant neoplastic lesions of the conjunctiva. REFERENCES [1] Lee, G.A. and Hirst, L.W. (1997) Retrospective study of ocular surface squamous neoplasia. Aust NZJ Ophthal- mol, 25, 269-276. doi:10.1111/j.1442-9071.1997.tb01514.x [2] Chikama, T., Liu, C.Y., Meij, J.T.A., Hayashi, Y., Wang, I.J., Yang, L., Nishida, T. and Kao, W.W.Y. (2008) Excess FGF-7 in corneal epithelium causes corneal intraepithe- lial neoplasia in young mice and epithelium hyperplasia in adult mice. Am J Pathol, 172, 638-649. doi:10.2353/ajpath.2008.070897 [3] Toth, J., Karcioglu, Z.A., Moshfeghi, A.A., Issa, T.M., Al-Ma’ani, J.R. and Patel, K.V. (2000) Correlation be- tween HPV positivity and p53 gene mutation in conjunc-  I. Pecorella et al. / Health 3 (2011) 245-248 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 248 tival squamous cell carcinoma. Cornea, 19, 159-162. doi:10.1097/00003226-200003000-00007 [4] McKelvie, P.A., Daniell, M., McNab, A., Loughnan, M. and Santamaria, J.D. (2002) Squamous cell carcinoma of the conjunctiva: a series of 26 cases. Br J Ophthalmol, 86, 168-173. doi:10.1136/bjo.86.2.168 [5] Auw-Haedrich, C., Sundmacher, R., Freudenberg, N., Spelsberg, H., Feltgen, N., Maier, P. and Reinhard, T. (2006) Expression of p63 in conjunctival intraepithelial neoplasia and squamous cell carcinoma. Graefe’s Arch Clin Exp Ophthalmol, 244, 96-103. doi:10.1007/s00417-005-0025-4 [6] Pe’er, J. (2005) Ocular surface squamous neoplasia. Oph- thalmol Clin N Am, 18, 1-13. [7] Martins-Green, M., Boudreau, N. and Bissell, M.J. (1994) Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res, 54, 4334-4341. [8] van den Hooff, A. (1988) Stromal involvement in malig- nant growth. Adv Cancer Res, 50, 159-196. doi:10.1016/S0065-230X(08)60437-6 [9] Usui, Y., Waring, G.O., See, R.F. and Rao, N.A. (2004) Bilateral ocular surface squamous cell neoplasia: a clini- copathological case report. Br J Ophthalmol, 88, 595-596. doi:10.1136/bjo.2003.029710 |

