 Journal of Biosciences and Medicines, 2014, 2, 69-73 Published Online June 2014 in SciRes. http://www.scirp.org/journal/jbm http://dx.doi.org/10.4236/jbm.2014.24011 How to cite this paper: de Souza, R.R. and Ferreira, R.D.M. (2014) Immobilization of Lipase from Candida rugosa on Meso- porous MCM 41. Journal of Biosciences and Medicines, 2, 69-73. http://dx.doi.org/10.4236/jbm.2014.24011 Immobilization of Lipase from Candida rugosa on Mesoporous MCM 41 Roberto Rodrigues de Souza, Renata D. M. Ferreira Department of Chemical Engineering, Universidade Federal de Sergipe (UFS) Campus São Cristóvão, Aracaju, Brasi l Email: rrsouza@ufs.br, ren ata .d mferr eira@ gma il. com Received March 2014 Abstract The use of enzymatic route for production of biofuels is growing up due the mild reaction condi- tions that this method provides, as well as reducing SOx emission. To reduce costs, it’s necessary to immobilize the enzyme, making possible to use it continuously as biocatalyst. The aim of this work was to measure the influence of the mass of support and pH used for immobilization of commer- cial lipase from Candida rugosa acquired by Sigma laboratory. The immobilization method chosen was adsorption on mesoporous and hydrophobic support MCM 41, this has been treated with ni- tric acid 10% v/v to remove any organic residue. Then, 20 ml of enzymatic solution in phosphate buffer (pH 6.0, 7.0 and 8.0; 50 mM) and 1 g/L was placed under constant stirring with 0.30 and 0.45 g of support. Aliquots were taken from the reaction medium and analyzed by spectropho- tometry at 10 minutes intervals. A volume of 0.2 ml of supernatant was put with 1.8 ml of sub- strate p-NFL at 0.18 g/L, and the absorbance at 410 nm was analyzed. In four cases there was a sharp reduction of supernatant’s activity at first 10 minutes, that ratifies the big affinity of the en- zyme for the support and the negative influence of pH about the activity. Using the calibration curve, it was possible to calculate the final activity of each immobilization batch. This work sug- gests the occurrence of diffusional effects, which means that the enzyme mobility was restricted due the excessive amount of support, and then, it lost a part of accessibility to substrate, reflecting in not expressive activity values, and changing the state of ionization of the components of the system. Keywords Immobiliz ation, Lip ase, Mesopo rous 1. Introduction Many compounds are currently produced through reactions that use chemical catalysts or expensives experimental conditions. However, this practice is aggressive to the environment, generating effluents with high cost of treatment, and consuming too energy. Enzymatic catalysis appears thus to reduce the energy demand contributing to a reduction in byproduct formation. However, the acquisition cost of enzymes is still high, making difficult the access of biocatalyst to industries. An alternative to this financial barrier is immobilize the enzyme [1].  R. R. de Souza, R. D. M. Ferreira 1.1. Lipase “Carboxilestereases lipases are capable of catalyzing the hydrolysis of glycerides of long chain” [2]. Its applica- bility is great since in addition to the hydrolysis reaction, can catalyze esterification, transesterification and inte- resterification in non-aqueous or limited water resources. Therefore, lipases are excellent alternatives applicable to foods, oleo-chemical, pharmaceutical, biosensors industries, among others [3]. Lipase from Candida rugosa has been widely used in esterification reactions successfully [1]. 1.2. Adsorption Enzymes have been immobilized by three methods: physical adsorption, covalent attachment and involvement [4]. The adsorption consists of the union between the enzyme and the inert support through non-specific physico- chemical interactions, Van der Waals forces, hydrophobic and ionic interactions, and have shown good cost-effective with regard to efficiency and cost of the immobilization procedure, because it has simple method- ology [5]. [6] did a study comparing the efficiency of Rhisopusoryzae lipase immobilized by adsorption on silica air-gel front of the free enzyme, and concluded that the biocatalyst showed higher stability and higher activity than the free enzyme esterification (80% versus 35% of free enzyme). 1.3. Support [7] studied the effect of hydrophobicity of membranes used as support for the immobilization of lipase by cova- lent bond in the activity and stability of the enzyme, and obtained better results when used more hydrophobic membrane. Studies claim that the region surrounding the active site of lipases is hydrophobic, and because of that, they recognize hydrophobic surfaces as similar to their natural substrate and undergo interfacial activation [8]. [9] showed that, in general, substrates with high specificity had greater surface area for adsorption capacity, while the more hydrophobic again, the best results are attributed to the improved interfacial activity of the lipase. It is possible to predict, taking into account the objectives in view, the selected substrate should have high sur- face area, be thermally stable, chemically durable, resistant to contamination and reasonable cost [10]. Because of all these characteristics was chosen support material, the MCM 41, whose family is characterized by having a hexagonal arrangement of uniform pores and well-defined size, with linear channels constructed with a silica matrix [11]. 2. Experimental For conducting the experiments, 7.0 and 8.0, three buffer solutions of 50 mM potassium phosphate at pH 6.0 were made. Since the support was treated as follows: if weighed about 1 g of MCM 41 support. 10 ml of HNO3 10% v/v was mixed with support, stirring occasionally for 30-min. The solution was vacuum filtered and removed with suc- cessive washes of water and buffer solution. This procedure was required to remove all organic part that was still present on the support. The calibration curve p-NC (for nitro phenol) followed by the following method: A calibration curve p-NC (p-nitrophenol) in buffer solution, which weighed 0.0014 g of the reagent was made by dissolving the in buffer to complete 100 ml in a volumetric flask. Dilutions of this solution of p-NF were made in the same buffer and analyzed on a spectrophotometer the absorbance at 410 nm using as blank reaction buffer solution. Determination of enzymatic activity: Another calibration curve was made using the NFL lipase and p-(p-ni- trofenillaurato) as substrate. Was dissolved in 1 mL of DMSO 0.018 g of the substrate, and then added to the buffer until 100 mL was complete. This solution was reserved. The lipase solution was prepared by dissolving 0.1 g of the enzyme powder in 100 ml of buffer, subsequently performing various dilutions. The lipase solu- tion was placed with the substrate in a cuvette at a ratio of 2 mL 1.8 mL 0.2 mL of substrate to the enzyme solu- tion, and were patterned by a contact time 40 s. The pH variation was due to the buffer used at pH 6.0, 7.0 and 8.0. The immobilization was done in batch using a beaker on a magnetic stirrer, 20 ml contend p-NFL. Different masses of 41 MCM support were added in order to also assess the influence of the mass of support for immobi- lization. In an interval of 10 minutes, samples were collected and analyzed by spectrophotometer. 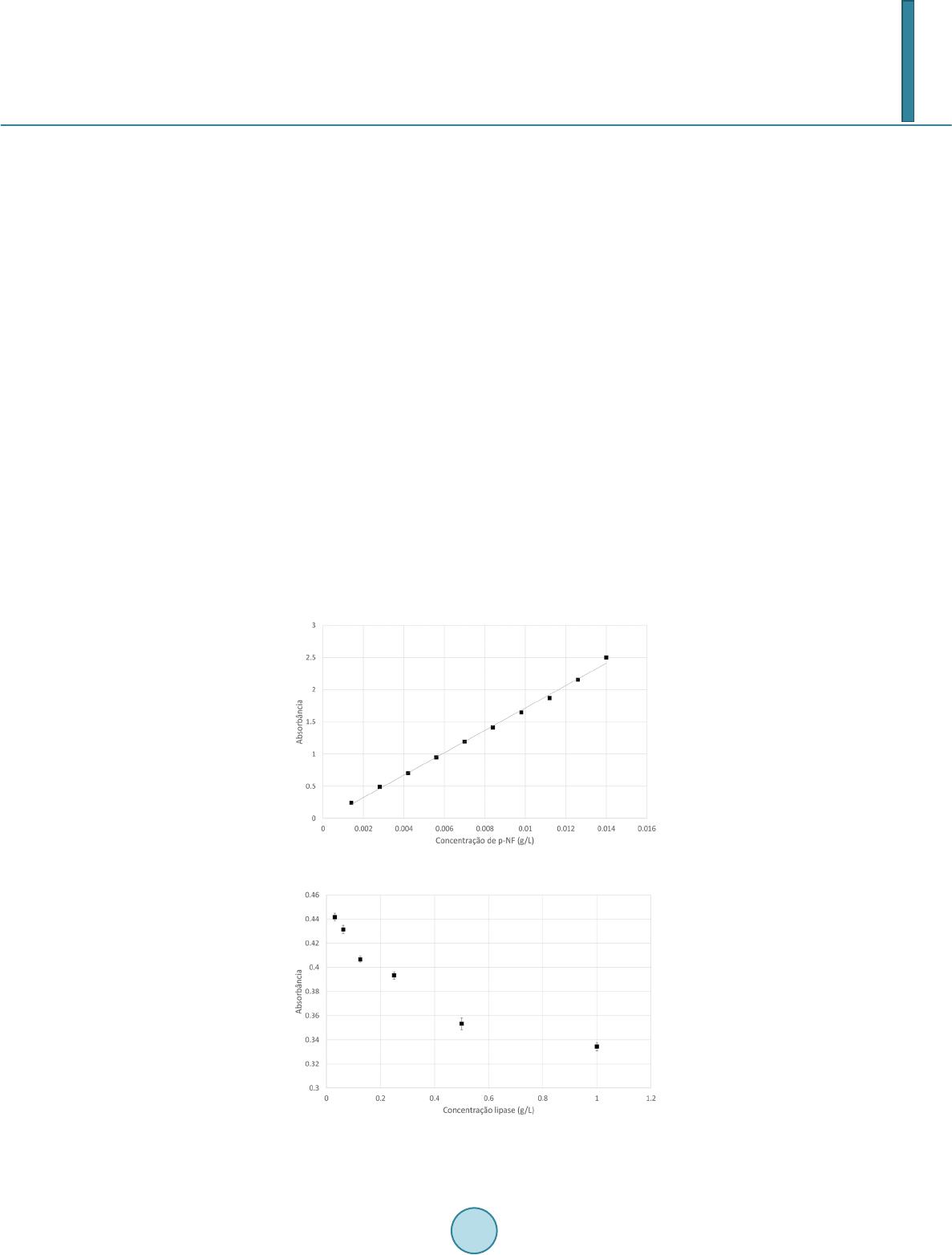 R. R. de Souza, R. D. M. Ferreira 3. Results and Discussion From Equation (1) obtained from the calibration curve of p-NF, shown in Figure 1, it was possible to know the concentration of p-NF was produced when using specific enzyme concentration, making direct connection with the enzymatic activity. (1) Figure 2 shows the relationship between the enzyme concentration and absorbance pH 6.0. It is known that the more concentrated the enzyme , the greater their activity because enzyme activity is a unit that corresponds to the amount of enzyme that catalyzes a reaction with rate of formation of 1 micro mol product per second , and p-NF formed intensifies its yellow color with concentration. However, the behavior obtained when using a medium at pH 6.0 was the opposite of the expected. The pH is one of the factors that can influence the activity of the enzyme, changing the state of ionization of the components of the system, and so it denatures. Therefore, the curve shows a drop in absorbance with increasing enzyme concentration, which could mean that there denaturation of the lipase, preventing catalysis of the reaction and consequent formation of product. Having made such a negative influence on the enzyme, only pH 7.0 and 8.0 were studied during immobiliza- tion, together with the influence of the mass of support in the study of the influence of steric and conformational effects. Figure 3 shows the curve obtained with the immobilization of lipase at pH 7.0 for comparison between the masses of support used. We notice that there is a great affinity between the enzyme and support, for the first 10 minutes have de- creased enzyme activity measured in the supernatant. However, for a smaller mass support, the concentration achieved in the first 10 minutes and the adsorption was smaller, suggesting a larger amount of enzyme has mi- grated to the middle support, demonstrating that a large amount of support affect the adsorption of the enzyme support. Since Figure 4 shows a comparison between the pH’s studied using 0.30 g of MCM 41. The preference for Figure 1. Calibration curve of p-NF. Figure 2. Curve enzymatic activity represented by the rela- tionship between enzyme concentration and absorbance.  R. R. de Souza, R. D. M. Ferreira Figure 3. Curve of absorbance versus time of adsorption at pH 7.0 with mass equal to 0.30 and 0.45 g support. Figure 4. Curve of absorbance versus time of adsorption with mass equal to 0.30 g and pH 7.0 and 8.0 support. pH 7 is subtle, being perceived as the evaluation of the enzyme was adsorbed to the same time. It may be noted that the decrease of absorbance the first 10 minutes was 70% at pH 7, whereas at pH 8.0 was about 60%. Another observation that can be made through the figures shown above is that the optimum time for adsorp- tion was 10 minutes, as can be seen increased activity in supernatant posteriors in time, showing that the shaking may have caused desorption of lipase. 4. Conclusions The study of the influence of pH and mass media used in the immobilization of lipase showed the effect of these variables on the enzyme activity. The experiments carried out at pH 6.0 returned an effect of fall absorbance with increasing enzyme concen- tration, being an inappropriate means to maintain the lipase having their functions impaired catalysis by the en- zyme denaturation The pH was not as expression of enzyme activity, which means that the lipase allows a range between pH 7.0 and 8.0, with the results slightly better at pH 7.0. The optimal immobilization time was 10 minutes, demonstrat- ing high affinity of the enzyme for support. The mass of support best adsorbed enzyme was 0.30 g. In this case, note the influence of steric and conforma- tional effects of the excessive amount of the immobilization support. As a result the cost benefits with the use of support, which will be smaller. 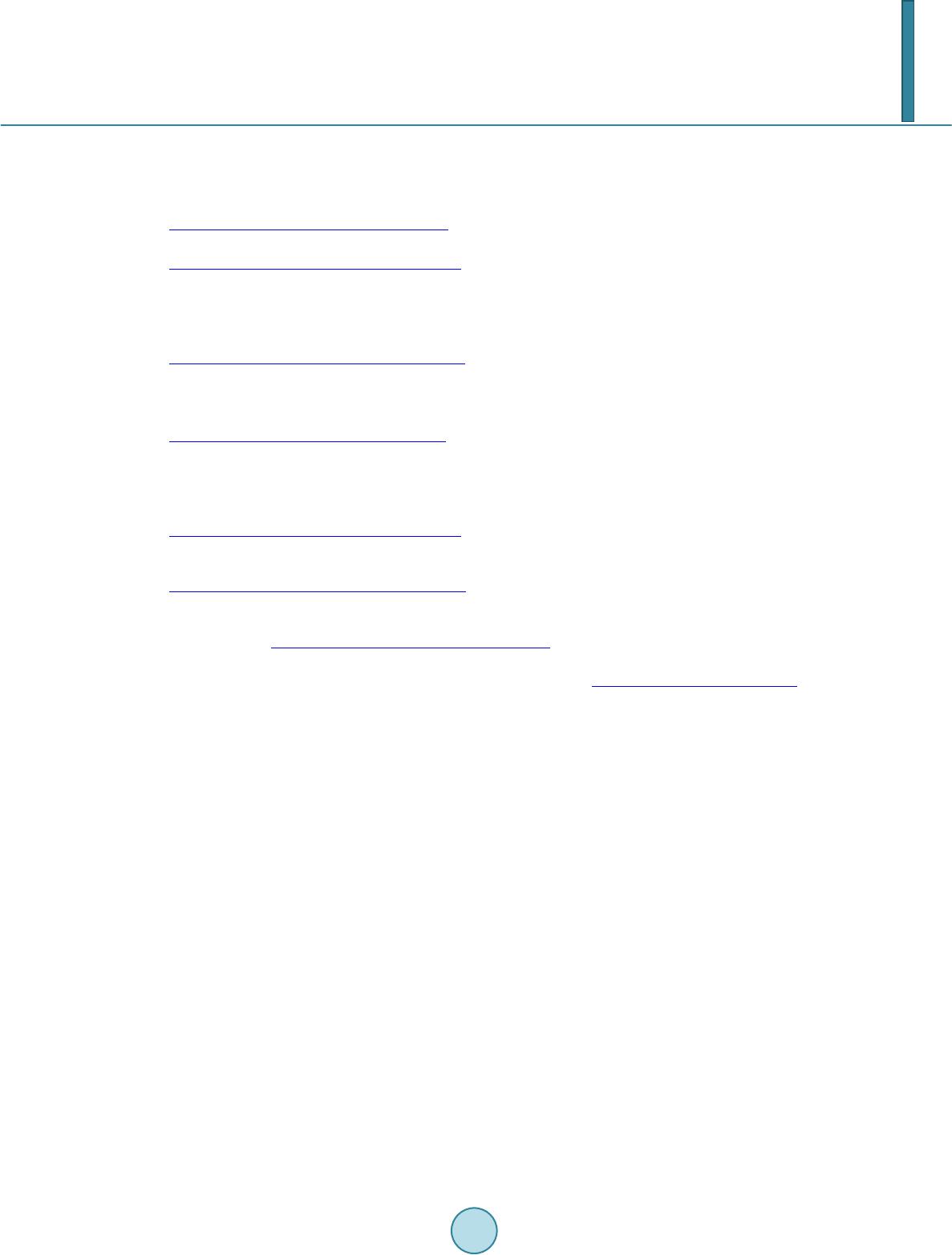 R. R. de Souza, R. D. M. Ferreira References [1] Zheng, M., Dong, L., Lu, Y., Guo, P., Deng, Q., Li, W., Feng, Y. and Huang, F. (2012) Immobilization of Candida Rugosa Lipase on Magnetic Poly(Allylglycidyl Ether-co-Ethylene Glycol Dimethacrylate)Polymer Microsphere for Synthesis of Phytosterol Esters of Unsaturated Fatty Acids. Journal of MolecularCatalysis B: Enzymatic, 74, 16-23. http://dx.doi.org/10.1016/j.molcatb.2011.08.008 [2] V erger, R. (1997 ) Interfacial Activation of Lip ases: Facts and Artifacts. Trends in B iote chno log y, 15 , 32-38. http://dx.doi.org/10.1016/S0167-7799(96)10064-0 [3] Bon, E.P.S., Ferrara, M.A. and Cor vo, M. L. (20 08 ) Enzimas em Biotecnologia: Produção, Aplicações e Mercado. In- terciênciaLtda, Rio de Janeiro. [4] Yang, J.J., Ma, X.O., Zhang, Z.S., Cheng, B., Li, S. and Wan g, G. (2010) Lipase Immobilized by Mo d ification- Coupled and Adsorption-cross-Linking Methods: A Comparative Study. Biotechnology Advances, 28, 644-650. http://dx.doi.org/10.1016/j.biotechadv.2010.05.014 [5] Regu l y, J.C. (2000) Biotecnologia dos Processos Fermentativos. Vol. 3, Editora Universitário/ UFP E. [6] Khar rat, N., Ali, Y.B., M arzou k, S., et al. (2011 ) Immobilization of Rhizopusoryzae Lipase on Silica Aerogels by Ad- sorption: Comparison with the Free Enzyme. Process Biochemistry, 46, 1083 -10 89. http://dx.doi.org/10.1016/j.procbio.2011.01.029 [7] Ch en, G., Kuo, C., Chen , C., et al. (2011) Effect of Membranes with Various Hydrophobic/Hydrophilic Properties on Lipase Immobilized Activity and Stability. Journal of Bioscience and Bioengineering. [8] Fern and ez-Lafuente, R., Armi sèn, P., Sabuquillo, P., Fernández-Lorente, G. and Guisán, J.M. (1998 ) Immobilization of Lipases by Selective Adsorption on Hydrophobic Supports. Chemistry and Physics of Lipids, 93, 185-197. http://dx.doi.org/10.1016/S0009-3084(98)00042-5 [9] Zh ou , Z., Inayat, A., Sch wieger, W. an d Hart mann, M. (2012) Improved Activity and Stability of Lipase Immobilized in Cage-Like Large Pore Mesoporous Organosilicas, Microporous and Mesoporous Materials. http://dx.doi.org/10.1016/j.micromeso.2012.01.003 [10] Kand asamy, R., Kennerdy, L.J. , Vidya , C., et al. (2010) Immobilization of Acidic Lipase Derived from Pseudomonas Gessardii onto Mesoporous Activated Carbon for the Hydrolysis of Olive Oil. Journal of Molecular Catalysis B: Enzy - matic, 62, 59-66. http://dx.doi.org/10.1016/j.molcatb.2009.09.004 [11] Kresge, C.T., Leonowics, M.E., Roth, W.J., Vartulli, J.C. and Beck, J.S. (1992) Ordered Mesoporous Molecular Sieves Synthesized by a Liq uid-Crytal Template Mechanism. Nature, 359, 710. http://dx.doi.org/10.1038/359710a0
|