 Journal of Biosciences and Medicines, 2014, 2, 36-43 Published Online June 2014 in SciRes. http://www.scirp.org/journal/jbm http://dx.doi.org/10.4236/jbm.2014.24007 How to cite this paper: Siddalinga Murthy, K.R. and Kantharaju, S. (2014) Studies on Xyloglucanase during the Germination of Seeds of Tamarindus indica. Journal of Biosciences and Medicines, 2, 36-43. http://dx.doi.org/10.4236/jbm.2014.24007 Studies on Xyloglucanase during the Germination of Seeds of Tamarindus indica K. R. Siddalinga Murthy, S. Kantharaju Department of Biochemistry, Central College Campus, Bangalore University, Bangalore, Karnataka, India Email: krsmurthy2001@yahoo.com, kantharaj2553@gmail.com Received March 2014 Abstract Germinating seeds of Tamarindus indica contain endo-β-1, 4-xyloglucanases which degrade tama- rind xyloglucan, but not carboxymethylcellulose (CMC). The xyloglucanases are isolated from the germinating tamarind seeds using 50 mM acetate buffer, pH 5.5 containing 0.5 M NaCl. The Km value is 0.667 g/liter and the enzyme is optimally active at pH 5.5 and stable between pH 4 - 6.5. The optimum temperature is 45˚C and is quite stable upto 50˚C. The activity declined by 50% at 60˚C and is completely inactivated at 70˚C. Highest xyloglucanase activity and specific activity are observed on the 23rd day of germination. The polyacrylamide gel electrophoresis (PAGE) indicated the presence of five isozymes of xyloglucanases which are visualized by activity staining separate- ly with congo red and grams iodine. Isozyme 2 is the major xyloglucanase present throughout the germination period. Keywords Tamarindus indic a, Xyloglucan, Xyloglucanase, Tamarind Seed 1. Introduction Xyloglucans were first found as amyloids in cell walls of seeds [1]. They are the major hemicellulosic structural polysaccharide of the primary cell walls of dicotyledons. Characteristic feature of xyloglucan is that they con- sists of a linear backbone chain of 1, 4-linked β-D-glucose residues and sidechains of α-D-xylose and β-D-galac- tose. The xyloglucans from Gramineae [2]-[4] and Solanaceae [5]-[10] have more unsubstituted glucose resi- dues compared to those from dicotyledonous plants. The major polysaccharide of tamarind seed is the soluble galactoxyloglucan (50% - 58%) consisting of a linear backbone chain of β-1, 4-linked D-glucose residues which are covalently linked by α-1, 6 linkage to xylose and some xylose residues are linked by β-1, 2 linkage to D-ga- lactose. The extent of xylose substitution by β-D-galactose varies considerably, but the glucose backbone struc- ture is typically substituted by 50% to 75% xylose. The high degree of substitution produces a stiff, extended conformation [11]. The backbone of xyloglucan can be cleaved by many endo-β-1, 4 glucanases whose substrates are mainly cellulose and different β-glucans and xyloglucanases (XG) which are highly specific towards xyloglucan and are virtually inactive towards the non-substituted β-glucans and carboxymethyl cellulose (CMC). The xyloglucanase of Aspergillus aculeatus was named as xyloglucan-specific endo-β-1, 4-glucanases [12]. Since then, number of  K. R. Siddalinga Murthy, S. Kantharaju works devoted to xyloglucanases has been increasing. A growing interest in xyloglucanases is due to the possi- bility of their application in a number of important biotechnological processes, such as conversion of the plant waste, modification of xyloglucans to endow them required rheological properties in food and feed industries, in pulp and paper industry, treatment of the fabrics to change its brightness and color and to remove the fuzz from the surface of textile materials in the textile industry. The object of the present work is to study xyloglucanases produced during the germination of tamarind seeds. 2. Materials and Method 2.1. Materials BSA, glycine, tris, phosphoric acid, hydrochloric acid, glycerol, methanol, acetic acid, bromophenol blue, acetic acid, sodium dihydrogen phosphate, disodium hydrogen phosphate, iodine, potassium iodide, congo red, are of analytical grade obtained from Sisco Research Laboratories Pvt. Ltd., India. The seeds of Tamarindus indica are collected using random sampling technique (RST) from local areas of Bangalore district, Karnataka State, India, during the February to May. After dehulling the seeds, immature and damaged seeds are removed and the mature seeds washed under tap water, dried and stored under refrigeration in plastic containers until further use. From this bulk population sample, subsamples are obtained. 2.2. Methods 2.2.1. Germination Studies Healthy Tamarind seeds (one hundred) are soaked in cold 50% H2SO4 for 60 mins and then washed thoroughly with distilled water and dispensed into the soil (1:1 ratio of acid washed sand and cocco peat). The seeds are germinated and the endosperms collected every alternate day, starting from 3rd day to 31st day (till the endos- perms are about to get detached from the seedlings). 2.2.2. Extraction of Xyl ogl u can ase A 20% extract of the fresh endosperm (15 day old germinated seed) is prepared in different buffer systems (0.05 M sodium phosphate buffer, pH 7.0 and 0.05 M acetate buffer pH 5.5 and also with the same buffers containing 0.5 M NaCl and 0.5% Triton X-100, respectively) by blending in a mixer for 5 mins and centrifuged at 10,000 rpm for 15 mins at 4˚C. The resulting supernatant is dialyzed overnight against 0.05 M acetate buffer pH 5.5, centrifuged at 10,000 rpm for 15 mins and used for the assay of xyloglucanase and total protein. 2.2.3. Extraction of Xyloglucanase during Germination All procedures are carried out at 4˚C, unless otherwise specified. A 20% extract of the endosperm is prepared (at an interval of every 48 hours during germination) in 0.05 M acetate buffer pH 5.5 containing 0.5 M NaCl, cen- trifuged and the supernatant dialyzed overnight against 0.05 M acetate buffer pH 5.5. The dialyzate is centri- fuged at 10,000 rpm for 15 mins and used for the quantitative assay and electrophoretic analysis of xylogluca- nase and total protein estimation. 2.2.4. Xyloglucanase Assay Xyloglucanas e activity using tamarind seed xyloglucan as a substrate is determined by DNS method [13]. 1.0 ml of suitably diluted enzyme extract is added to 0.5 ml 0.05 M acetate buffer pH 5.5 and 1.0 ml 0.5% xyloglucan. The reaction mixture is incubated at 45˚C for 2 hours. The reaction is arrested by adding 0.25 ml DNS reagent. The activity is expressed as the number of µmoles of reducing sugar produced/hour. 2.2.5. Protein Estim ation Protein is quantitatively estimated using bovine serum albumin (BSA) as standard [14]. 2.3. Kinetic Parameters 2.3.1. Effect of Ti me Effect of time on xyloglucanase activity is determined by incubating the enzyme with 0.5% xyloglucan substrate  K. R. Siddalinga Murthy, S. Kantharaju for different time intervals at 45˚C and assaying the enzyme as described above. 2.3.2. Effect of Substrate Concentration Xyloglucanase is incubated with different concentration of the substrate for 2 hours at 45˚C and assayed as de- scribed above. Km and Vmax are determined by constructing LB-plot. 2.3.3. pH Op tim a pH optima for xyloglucanase is determined by assaying the enzyme using 50 mM buffers of different pH (ace- tate buffer pH 4.0, 4.5, 5.0 and 5.5, phosphate buffer pH 6.0, 6.5, 7.0 and 7.5, Tris-HCl buffer pH 8.0, 8.5 and 9.0, carbonate buffer pH 9.5, 10.0, 10.5 and 11.0) at 45˚C for 2 hours. 2.3.4. pH Stability The pH stability of xyloglucanase is determined by pre-incubating the enzyme with 50 mM buffers of different pH (acetate buffer pH 4.0, 4.5, 5.0 and 5.5, phosphate buffer pH 6.0, 6.5, 7.0 and 7.5, Tris-HCl buffer pH 8.0, 8.5 and 9.0, carbonate buffer pH 9.5, 10.0, 10.5 and 11.0) for 2 hrs at 4˚C. A known aliquot from the pre-incu- bated samples are removed and assayed at 45˚C for 2 hours as described above at an optimum pH of 5.5. 2.3.5. Temperature Optima The optimum temperature for xyloglucanase is determined by assaying the enzyme at different temperatures, such as, 5˚C, 10˚C, 17˚C, 22˚C, 27˚C, 32˚C, 37˚C, 45˚C, 52˚C, 60˚C, 70˚C and 80˚C using tamarind xyloglucan as the substrate, for 2 hours. 2.3.6. Temperature Stability Temperature stability of xyloglucanase is determined by pre–incubatng the enzyme at different temperatures, such as, 5˚C, 10˚C, 17˚C, 22˚C, 27˚C, 32˚C, 37˚C, 45˚C, 52˚C, 60˚C, 70˚C and 80˚C for 30 minutes, rapidly cooled to 0˚C and assayed using tamarind x yloglucan as the substrate at 45˚C for 2 hours. 2.3.7. Polyacrylamide Gel Electrophoresis Polyacrylamide gel electrophoresis (PAGE) is performed [15] [16]. A discontinuous vertical slab gel system (8 cm X 8 cm) containing 10% separating gel and 4.5% spacer gel is used. The separating gel also contained 0.5 mg xyloglucan/ml. An aliquot of the enzyme extract suitably diluted with 40% glycerol solution containing bromophenol blue is carefully layered into the wells and subjected to electrophoresis for 2 hours at 10˚C ma in- taining a current of 20 mA. After electrophoresis, the gel is incubated for 5 hours in 0.05 M acetate buffer, pH 5.5. The gel is then stained separately using 0.1% solution of congo red [17] for 15 mins and destained with 1M NaCl and 1% grams iodine solution [18] for 5 mins. Xyloglucanase activity appeared as clear zone against red background (with congo red) and clear zone against green background (with grams iodine), respectively. 3. Result The enzyme xyloglucanase from 15 day old germinated tamarind seeds is extracted using various buffers. Ace- tate buffer, 50 mM, pH 5.5 containing 0.5 M NaCl is found to be ideal for the maximum extraction of xyloglu- canase. Hence, this buffer is used for extraction of xyloglucanases during the germination period. Experiments are carried out to determine the standard conditions for the assay of xyloglucanases from the ta- marind seed extract. The xyloglucanase activity of the enzyme extract is linear up to 1.0 mg protein (Figure 1) and up to 2 hours (Figure 2). Km and Vmax of the enzyme with tamarind seed xyloglucan as substrate is 0.667 g/ litre and 0.84 µmole/hour (Figure 3 and Figure 4). The enzyme extract did not show any activity towards car- boxymethylcellulose (CMC) or starch and indicated the absence of cellulolytic activity and α-glucanolytic activ- ity. The optimum pH is 5.5 (Figure 5) and was stable between pH 4.0 to 6.5 (Figure 6). The enzyme did not show any activity above pH 6.0. The optimum temperature is 45˚C (Figure 7) and the enzyme is quite stable from 10˚C to 50˚C. The activity declined by 50% at 60˚C and is completely inactivated at 70˚C (Figure 8). The tamarind seeds are germinated for 31 days and the changes in total xyloglucanase activity, specific activ- ity and protein are followed every alternate day (Figure 9). Analysis of the total xyloglucanase activity pattern during germination of the seed showed highest activity on 23rd day of germination. The total activity increased 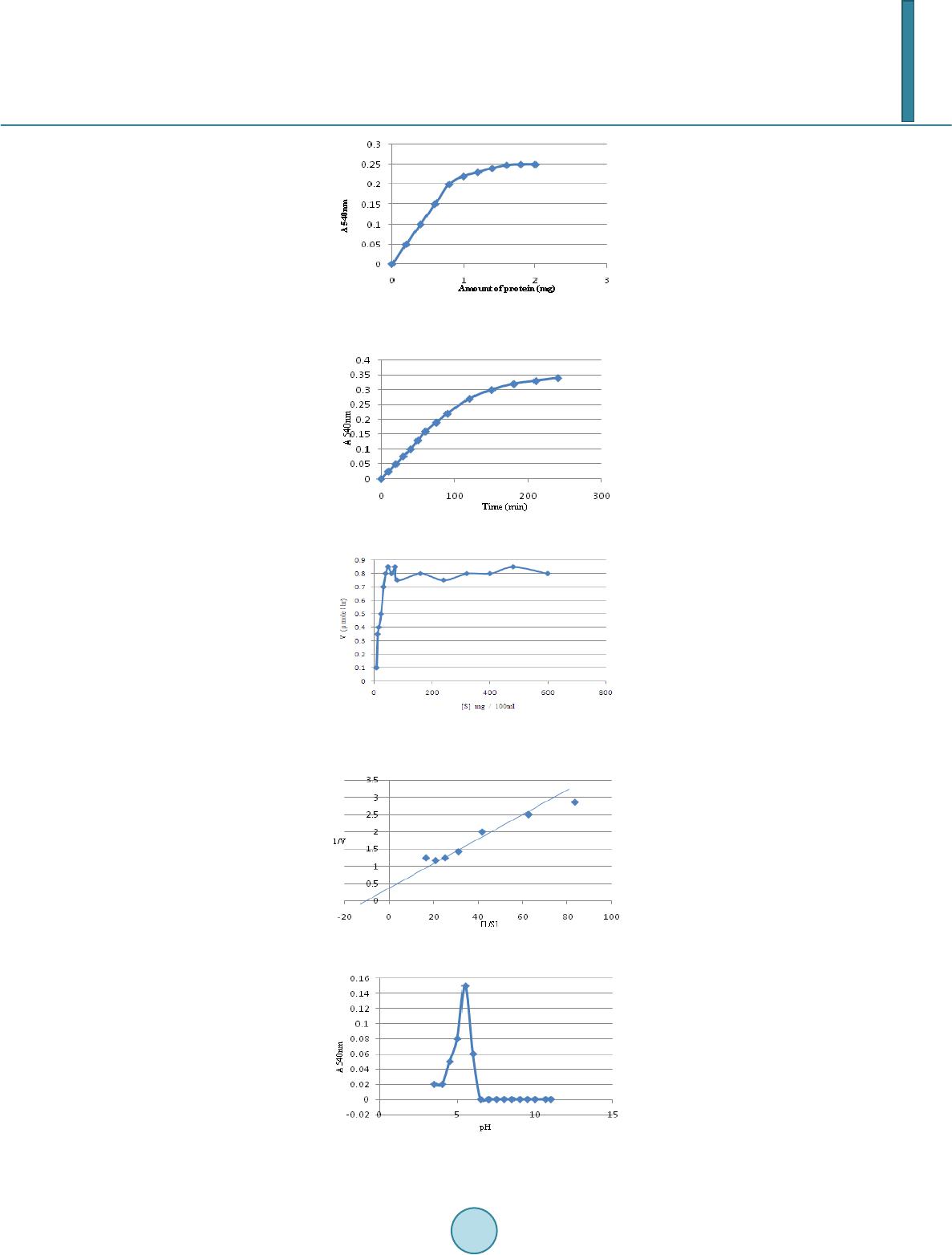 K. R. Siddalinga Murthy, S. Kantharaju Figure 1. Effect of protein concentration on xyloglucanase activity. Figure 2. Time curve for xyloglucanase. Figure 3. Effe ct of substrate concentration on xyloglucanase activity. Figure 4. Km & Vmax of xyloglucanase. Figure 5. Effect of pH on xyloglucanase activity. 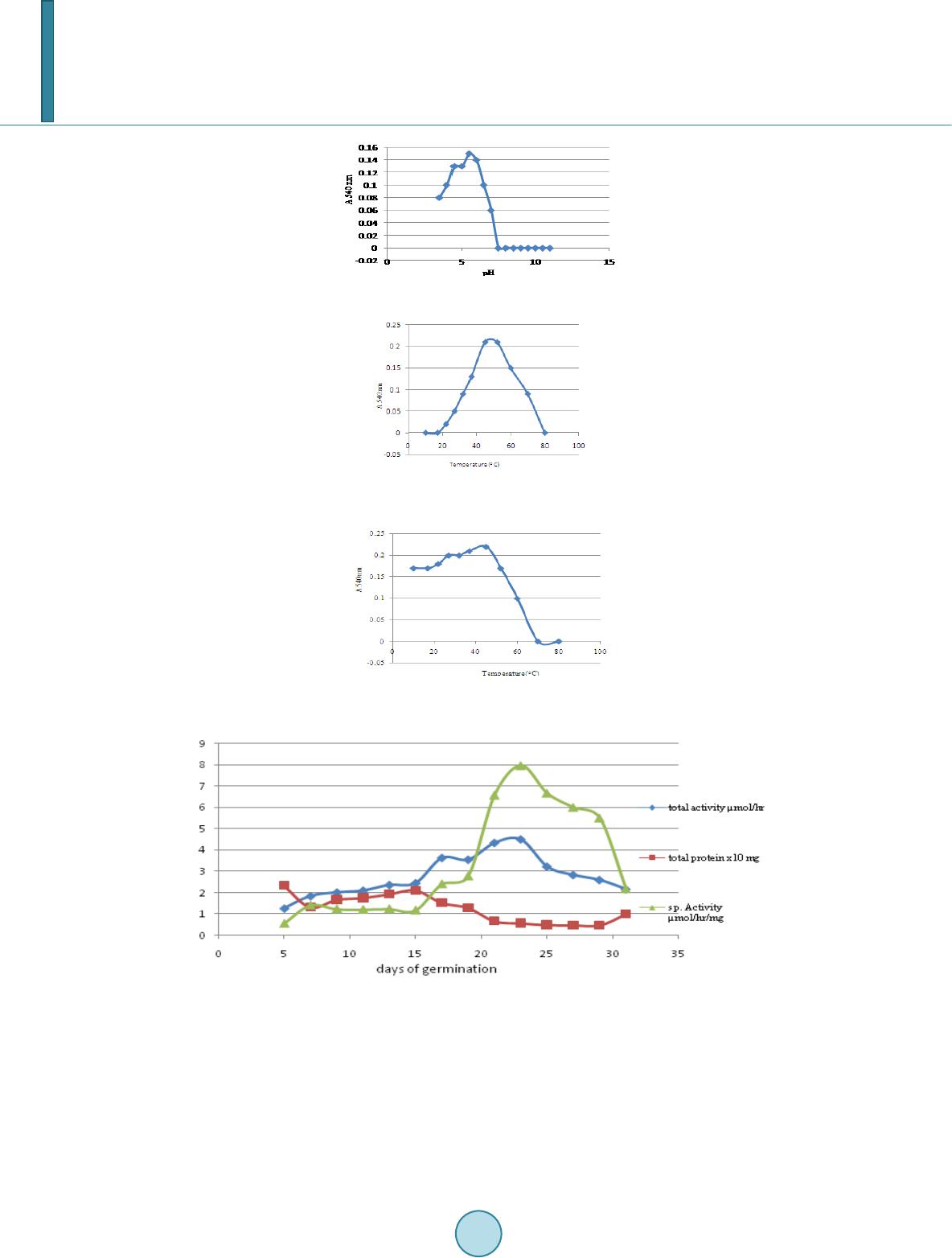 K. R. Siddalinga Murthy, S. Kantharaju Figure 6. pH stability ofxyloglucanase. Figure 7. Effe ct of temperature on xy- loglucanase activity. Figure 8. Temp erature stability of xyloglu- canase. Figure 9. Germination profile of xyloglucanas. gradually up to 23 days of germination (5-fold) and then decreased. The specific activity increased on 7th day and remained almost constant till 15th day and increased up to 23rd day of germination. The total protein in the endosperm decreased till 7th day of germination and then increased till 15th day and again decreased. Electrophoretic analysis of the xyloglucanase activity during the germination from 3rd to 31st day is shown in (Figure 10). Totally five isozymes were separated by PAGE. Band number 2 is the major xyloglucanolytic band, present on all the days while band numbers 3 & 4 appeared from 17th day onwards up to 31st day. Band number 5 appeared from 23rd day onwards. Band number 1 was present only from 3rd day onwards to 13th day.  K. R. Siddalinga Murthy, S. Kantharaju Figure 10. Electrophoretic pattern of xyloglucanase produced during germination of tamarind seeds. 4. Discussion Seed xyloglucanases have been investigated only in species that have stored xyloglucans in the cotyledons [19] [20]. In case of seeds, xyloglucan–specific endoglucanase has been isolated and purified from the cotyledons of nasturtium and lentils [20] [21]. They have also been detected in the cotyledons from tamarind, pea, bean and Hymeraea courbaril [19] [22]. The activity of xyloglucanases or CMC–degrading endoglucanases from the seeds of nasturtium [23], Hyme- raea courbaril [19], coffee-bean [24] [25] and cold-tolerant tomato lines [26] increased during the germination. Similarly, during tamarind seed germination, the activity of xyloglucanases increased continuously and is max- imum on 23rd day. In nasturtium cotyledons, the physiological role of xyloglucanase is correlated with the mobi- lization of storage xyloglucan from the cell walls following germination. Similarly, xyloglucanse is the major enzyme used for mobilizing tamarind seed xyloglycans which constitute about 55% of the endosperm. The activities of endoglucanases from seeds have been detected in cotyledons of germinating kidney beans [27], coffee bean [24] [25]. In these cases, the enzymes hydrolyzed CMC, but the activity on xyloglucans was not reported. However, in the seeds of Euphorbia heterophylla [28], it is shown that xyloglucan is depolyme- rized by CMCases which acts on xyloglucans and also by xyloglucan-specific endoglucanases which are unable to hydrolyze CMC. In contrast to the above, in nasturtium seeds, xyloglucan specific endo-1,4-β-glucanase hydrolyzes xyloglu- cans with no action on cellulose or other substrates with 1,4-β-D-glucosyl linkages [21]. In the present work we observed that tamarind seed xyloglucanases hydrolyzed only tamarind seed xyloglucans with no action against CMC or starch. Similarly xyloglucanases from Pencillium canescens, Penicillium verruculosum and Analipus japoniucs [29] exhibited activity towards xyloglucans, but not CMC. However, xyloglucanases from Chrysos- porium lucknowense and Trichoderma reesi [30] exhibited high activity towards xyloglucans and very low ac- tivity towards CMC. The Km value for tamarind xyloglucanase (0.667 g/litre) is in the range that is typical for Km values for fun- gal xyloglucanases (0.3 - 0.7 g/litre). The xyloglucanases of T reesi [30] and A japonicus exhibited maximal ac- tivities in weakly acidic region of pH 5 while tamarind seed xyloglucanase exhibited maximum activity at pH 5.5. The optimal temperature for the xyloglucanase activity for Penicillium species [29] ranged from 55˚C - 65˚C while tamarind seed xyloglucanase is maximally active at 45˚C. The xyloglucanase of Penicillium species [29] are stable upto 50˚C similar to tamarind seed xyloglucanase. Electrophoretic analysis of xyloglucanases during the germination period indicated the presence of five iso- zymes. Appearance of clear bands against colored background indicates that the xyloglucanases acts in the inte- rior of the xyloglucan substrate and hence are endoxyloglucanases. Specific xyloglucanase can be used for modification of the properties of tamarind seed xyloglucans and also produce oligosaccharide, which have high commercial value. There is great demand for food additives which can be used as functional substitutes for the calorie imparting contents of processed foods without adversely af- fecting the organoleptic quality. The tamarind seed hydrolyzate can be substituted upto 60% of the digestible carbohydrates in processed food products. Further it has been found that fat content can also be reduced upto 25% and enables a significant reduction in calorie content. Unlike other food additive gums, the tamarind seed poly- saccharide hydrolyzate can be used at high levels in processed foods without adversely affecting the processing thereof due to elevated viscosities.  K. R. Siddalinga Murthy, S. Kantharaju In view of the importance of xyloglucanases, further work is in progess to purify the xyloglucanases and study their properties. Acknowledgements We acknowledge University Grant Commission, New Delhi, for providing Fellowship and DOS in Biochemistry, Bangalore University, Bangalore for offering facilities to carry out the research work. References [1] Hayashi, T. (1989) Xyloglucans in the Primary Cell Wall. Annual Review of Plant Physiology and Plant Molecular Bi- ology, 40, 139-16 8. http://dx.doi.org/10.1146/annurev.pp.40.060189.001035 [2] Kato, Y., Iki, K. and Matsuda, K. (1981) Cell-Wall Polysaccharides of Immature Barely Plants. II Characterisation of a Xyloglu can. Agricultural and Biological Chemistry, 45, 2745-2753. http://dx.doi.org/10.1271/bbb1961.45.2745 [3] Kato, Y., Ito , S., Iki, K. and Matsuda, K. (1982) Xyloglucan and β-D-Glucan in Cell Walls of Rice Seedlings. Plant and Cell Physiology, 23, 351-364. [4] Kato, Y., Shiozawa, R., Takedu, S., Ito, S. and Matsuda, K. (1982) Structural Investigation of of a β-D-Glucan and Xyloglucan from Bamboo-Shoot Cell Walls. Carbohydrate Research, 109, 233-248. http://dx.doi.org/10.1016/0008-6215(82)84041-X [5] Eda, S. and Kato, K. (1978) An Arabinoxyloglucan Isolated from the Midrib of the Leaves of Nicotina tabacum. Agri- cultural and Biological Chemistry, 42, 351-357. http://dx.doi.org/10.1271/bbb1961.42.351 [6] Mori, M., Eda, S. and Kato, K. (1979) Two Xyloglucan Oligosaccharides Obtained by Bcellulose-Degradation of To - bacco Arabinoxyloglucan. Agricultural and Biological Chemistry, 43, 145-159. http://dx.doi.org/10.1271/bbb1961.43.145 [7] Ring, S.G. and Selvendran, R.R. (1981) An Arabinogalactoxyloglucan from the Cell Wall of Solanum tuberosum. Phy- tochemistry, 20 , 2411-2519. http://dx.doi.org/10.1016/0031-9422(81)83084-1 [8] Vincken, J.P., wijsman, A.J.M., Beldman, G., Niessen, W.W.A. and Voragen, A.G.J. (1996) Potato Xyloglucan Is Built from XXGG Type Subunits. Carbohydrate Research, 288, 219-232. http://dx.doi.org/10.1016/S0008-6215(96 )90 80 1-0 [9] Sims, I.M., Munro, S.L.A., Currie, G. and Bacic, A. (1996) Structural Characterisation of Xyloglucan Secreted by Sus- pension-Cultured Cells of Nicotiana plumbaginifolia. Carbohydrate Research, 293, 147-17 2. http://dx.doi.org/10.1016/0008-6215(96)00142-5 [10] York, W.S., Kolli, V.S.K., Orlando, R., Albershein, P. and Darvill, A.G. (1996) The Structures of Arabinoxyloglucans Produced by Solanaceous Plants. Carbohydrate Research, 285, 99-128. http://dx.doi.org/10.1016/S0008-6215(96)90176-7 [11] Powlowski, J., Mahajan, S., Schapria, M. and Master, E.R. (2009) Substrate Recognition and Hydrolysis by Fungal Xyloglu can—Specific Family 12 Hydr ase. Carbohydrate Research, 344, 1175-1179. http://dx.doi.org/10.1016/j.carres.2009.04.020 [12] Pauly, M., et al. (1999) A Xyloglucan -Specific Endo-β-1,4 -Glucanase from Aspergillus aculeatus: Expression Cloning in Yeast, Purification and Characterization of the Recombinant Enzyme. Glycobiology, 9, 93-10 0. http://dx.doi.org/10.1093/glycob/9.1.93 [13] Miller, G.L. (1959) Use Mof Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Biotechnology and Bioengineering Symposium, 5, 193-219. [14] Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein Measurements with the Folin Phenol Rea- gent. Journal of Biological Chemistry, 193, 265-275. [15] Davis, B.J. (1964) Disc Electrop horesis-II Method and Application to Human Serum Proteins. Annals of the New York Academy of Sciences, 121, 404-427. http://dx.doi.org/10.1111/j.1749-6632.1964.tb14213.x [16] Ornstein, L. (1964) Disc Electrophoresis. I. Background and Theory. Annals of the New York Academy of Sciences, 121, 321-349 . http://dx.doi.org/10.1111/j.1749-6632.1964.tb14207.x [17] Adeleke, A.J., Odunfa, S.A., Olambiwonninu, A. and Owoseni, M.C. (2012) Production of Cellulase and Pectinase from Orange Peels by Fungi. Nature Science, 10, 107-112. [18] Siddalinga Murthy, K.R., et al. (2008) Partial Purification and Biochemical Characterisation of Extracellular Pectinase from Aspargillus niger Isolated from Groundnut Seeds. Journal of Applied Biosciences, 96, 1223-122 8 . [19] Tine, M.A.S., Cortelazzo, A.L. and Buckeridge, M.S. (2000) Xyloglucan Mobilisation in Cotyledons of Developing Plantlets of Hymenaea courbaril L. (Leguminosae-Caesalpinoideae). Plant Science, 154, 117-126. http://dx.doi.org/10.1016/S0168-9452(99)00245-9  K. R. Siddalinga Murthy, S. Kantharaju [20] Steele, N.M., Sulova, Z., Campbell, P., Braam, J., Frakas, V. and Fry, S.C. (2001) Ten Isozymes of Xyloglucan Endo- transglycosylase from Plant Cell Walls and Cleave the Donor Substrate Stochastically. Biochemical Journal, 35 5, 671- 679. [21] Edwards, M., Dea, I.C.M., Bulpin, P.V. and Reid, J.S.G. (1986) Purification and Properties of a Novel Xyloglucan Specific Endo-(1-4)-β-D-Glucanase from Germinated Nasturtium Seeds (Tropaeolum majus L). Journal of Biological Chemistry, 2 61, 9489-9494. [22] Sulova, Z., Lednicka, M. and Farkas, V. (1995) A Colorimetric Assay for X yloglucan -Endo Endotransglycosylase from the Germinating Seeds. Analytical Biochemistry, 229, 80-85 . http://dx.doi.org/10.1006/abio.1995.1381 [23] Edwards, M., Dea, I.C.M., Bulpin, P.V. and Reid, J.S.G. (1985) Xyloglucan (Amyloid) Mobilisation in the Cotyledons of Tropaeolum majus L. Seeds Following Germinatiomn. Planta, 163, 133-140. http://dx.doi.org/10.1007/BF00395907 [24] Takaki, M. and Dietrich, S.M.C. (1980) Effect of GA3. and Light on Polysaccharide Levels and Metabolism in Ger- minating Coffee Seeds. Journal of Experimental Botany, 31, 1643-1649. http://dx.doi.org/10.1093/jxb/31.6.1643 [25] Giorgini, J.F. (1992) Purification and Partial Characterisation Two Isozymes of Cellulase from GA3-Tr eated Coffee Endosperm. Brazilian Journal of Plant Physiology, 4, 75-80 . [26] Leviatov, S., Shoseyov, O. and Wolf, S. (1995) Involvement of Endomannase in the Control of Tomato Seed Germina- trion under Low Temperature Conditions. Annals of Botany, 76, 1-6. http://dx.doi.org/10.1006/anbo.1995.1071 [27] Lew, F.T. and Lewis, L.N. (1974) Purification and Properties of Cellulase from Phaseolus vulgaris. Phyto chemist ry, 13, 1359-1366. http://dx.doi.org/10.1016/0031-9422 (74) 8029 2 -X [28] Suda, C.N.K. and Giorgini, J.F. (2003) Multiple Forms of Endo-1,4-β-Glucanases in the Endosperm of Euphorbia he- terophylla L. Journal of Experimental Botany, 54, 2045-20 52. ht tp:/ /d x.do i.o rg/1 0.10 93 /jxb /e rg2 29 [29] Sinitsyna, O.A., et al. (2009) Isolation and Properties of Xyloglucanases of Penicillium sp. Bi ochemistry (Moscow), 75, 41-49. http://dx.doi.org/10.1134/S0006297910010062 [30] Grishutin, S.G., et al. (2004) Specific Xyloglucanases as a New Class of Po lysaccharid e-Degrading Enzymes. Biochi- mica et Biophysica Acta, 16 74, 268-281. http://dx.doi.org/10.1016/j.bbagen.2004.07.001
|