The Effect of Using Nano ZrO on the Properties of W-ZrC Composite Fabricated through Reaction Sintering5
2
asibility of fabricating W/ZrC com-
Table 4 Comparing to prior ZrC-W composites [2] these
values for mechanical properties are acceptable. Anyway
the variances might be because the reaction between
ZrO2 and WC did not go to completion and because of
the effect that the un-reacted components have on the
properties of the composite.
4. Conclusions
In this study the fe
posite through reaction sintering was studied and the
differences of composite made from nano and micron
ZrO2 were investigated. The W/ZrC composite was pro-
duced by mixing WC and ZrO2 powders, gel casting the
mixture to produce a green body and then sintering at
2100˚C. The XRD patterns and SEM images indicate that
W/ZrC composite has been fabricated successfully by
this method, although some extra compounds have been
formed. Moreover the XRD pattern of nano sample
(a)
(b)
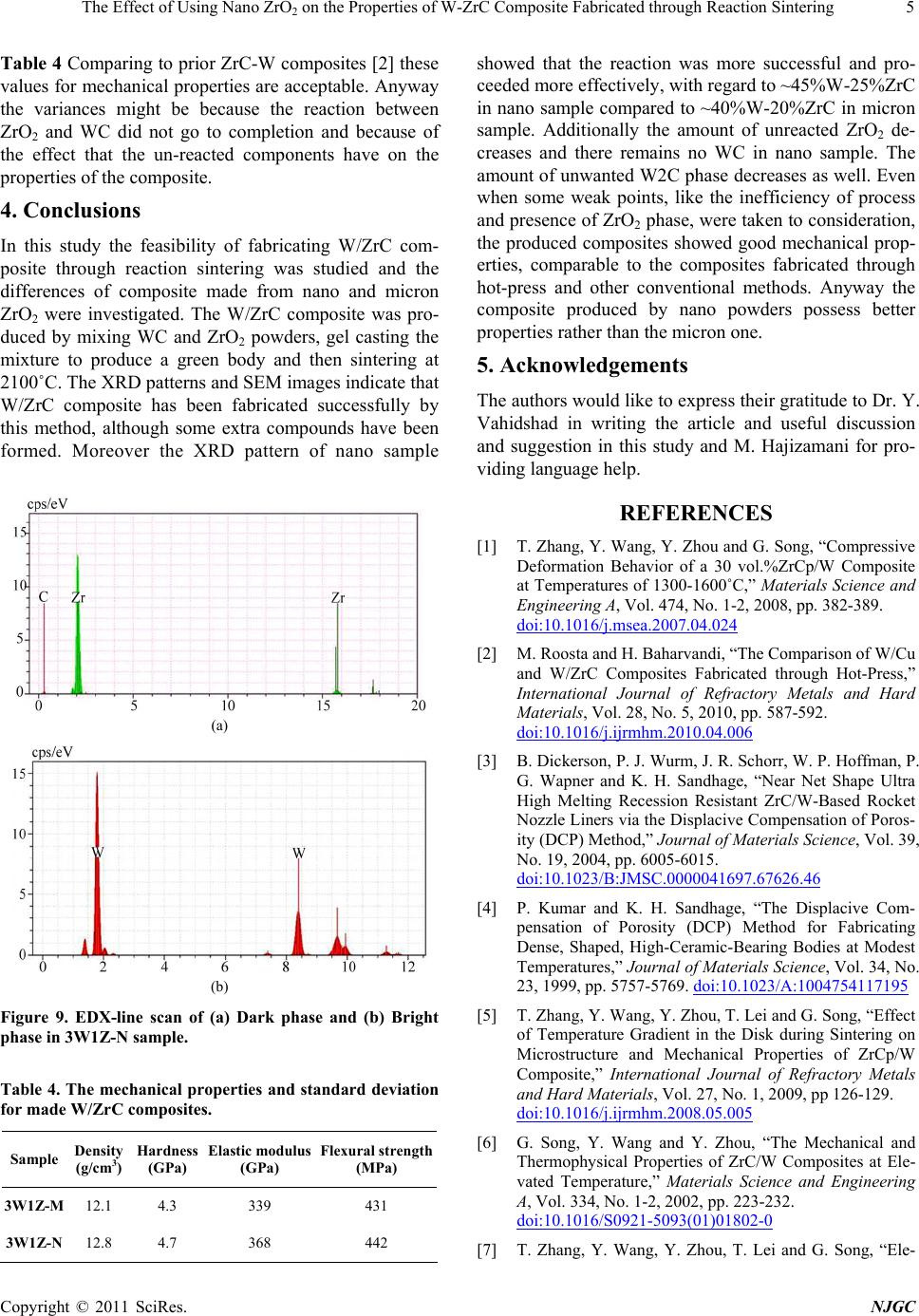
Figure 9. EDX-line scan of (a) Dark phase and (b) Bright
phase in 3W1Z-N sample.
ties and standard deviation
r made W/ZrC composites.
(g/cm ) (GPa)
astic modulus
(GPa)
Flexural strength
(MPa)
Table 4. The mechanical proper
fo
Sample Density
3Hardness El
3W1Z-M 12.1 4.3 339 431
showed th rean was m successfu pro-
ceeded more effectively, with rega45%WrC
acomd to ~4-20%ZrC in micron
useful discussion
and M. Hajizamani for pro-
, Y. Zhou and G. Song, “Compressive
Deformation Behavior of a 30 vol.%ZrCp/W Composite
at Temperaturterials Science and
Engineering A, , pp. 382-389.
at thectioore
rd to ~
l and
-25%Z
in nano smple pare0%W
sample. Additionally the amount of unreacted ZrO2 de-
creases and there remains no WC in nano sample. The
amount of unwanted W2C phase decreases as well. Even
when some weak points, like the inefficiency of process
and presence of ZrO2 phase, were taken to consideration,
the produced composites showed good mechanical prop-
erties, comparable to the composites fabricated through
hot-press and other conventional methods. Anyway the
composite produced by nano powders possess better
properties rather than the micron one.
5. Acknowledgements
The authors would like to express their gratitude to Dr. Y.
d Vahidshad in writing the article an
and suggestion in this study
viding language help.
REFERENCES
[1] T. Zhang, Y. Wang
es of 1300-1600˚C,” Ma
Vol. 474, No. 1-2, 2008
doi:10.1016/j.msea.2007.04.024
[2] M. Roosta and H. Baharvandi, “The Comparison of W/Cu
and W/ZrC Composites Fabricated through Hot-Press,”
International Journal of Refractory Metals and H
Materials, Vol. 28, No. 5, 2010, pp. 587-592
ard
.
doi:10.1016/j.ijrmhm.2010.04.006
[3] B. Dickerson, P. J. Wurm, J. R. Schorr, W. P. Hoffman, P.
G. Wapner and K. H. Sandhage, “Near Net Shape Ultra
High Melting Recession Resistant ZrC/W-Ba
Nozzle Liners via the Displacive Co
sed Rocket
mpensation of Poros-
ity (DCP) Method,” Journal of Materials Science, Vol. 39,
No. 19, 2004, pp. 6005-6015.
doi:10.1023/B:JMSC.0000041697.67626.46
[4] P. Kumar and K. H. Sandhage, “The Displacive Com-
pensation of Porosity (DCP) Method for Fabricating
Dense, Shaped, High-Ceramic-Bearing Bodies
Temperatures,” Journal of Materials Science, V
at Modest
ol. 34, No.
23, 1999, pp. 5757-5769. doi:10.1023/A:1004754117195
[5] T. Zhang, Y. Wang, Y. Zhou, T. Lei and G. Song, “Effect
of Temperature Gradient in the Disk during Sintering on
Microstructure and Mechanical Properties of ZrCp/W
Composite,” International Journal of Refractory Metals
and Hard Materials, Vol. 27, No. 1, 2009, pp 126-129.
doi:10.1016/j.ijrmhm.2008.05.005
[6] G. Song, Y. Wang and Y. Zhou, “The Mechanical and
Thermophysical Properties of ZrC/W Composites at Ele-
vated Temperature,” Materials Science and Engineering
A, Vol. 334, No. 1-2, 2002, pp. 223-232.
doi:10.1016/S0921-5093(01)01802-0
[7] T. Zhang, Y. Wang, Y. Zhou, T. Lei and G. Song, “Ele-
3W1Z-N 12.8 4.7 368 442
Copyright © 2011 SciRes. NJGC