Paper Menu >>
Journal Menu >>
 Advances in Bioscience and Biotechnology, 2011, 2, 103-107 ABB doi:10.4236/abb.2011.22016 Published Online April 2011 (http://www.SciRP.org/journal/abb/). Published Online April 2011 in SciRes. http://www.scirp.org/journal/ABB Anti-yeast activities of Origanum oil against human pathogenic yeasts Amber Adams, Satyanshu Kumar, Marck Clauson, Shivendra Sahi* Department of Biology, Western Kentucky University, Kentucky, USA. Email: shiv.sahi@wku.edu Received 8 October 2010; revised 21 February 2011; accepted 1 March 2011. ABSTRACT Outbreak of autoimmune diseases by pathogenic yeasts has led to a serious medical threat. As these organisms evolve resistance to existing antifungal drugs, the concern could be further compounded. The realm of plant derived products offers a wide spectrum of potentially valuable alternatives to the existing synthetic fungicides. Essential oils from sev- eral medicinal plants have been shown to exhibit pharmacological attributes. In the present study, anti-yeast properties of Oregano essential oil (OEO) were examined in vitro against four human patho- genic yeasts i.e., Candida albicans, Cryptococcus al- bidus, Cryptococcus neoformans and Rhodotorula ru- brum. OEO concentration of 200 μg/mL was found to be growth inhibitory against all four yeasts examined, thereby showing its potential to function as a natural anti-yeast agent. Keywords: Orig anum vulgare; Candida albicans; Cryptococcu s neo formans; Rhodotorula rubrum; Cryptococcus albidus 1. INTRODUCTION Aromatic plants and spices have been used in traditional medicine since ancient times due to different potent ac- tivities of their components. Essential oils produced by plants in defense against pathogens are derived from terpenes, sesquiterpenes and their oxygenated com- pounds. They are a rich source of bioactive compounds possessing antimicrobial, spasmolytic, carminative, he- patoprotective, anticancer and antiviral properties and have been pharmacologically evaluated for treatment of many infectious diseases [1]. However, there is only lim- ited information available on the antifungal activities of different essential oils toward human fungal pathogens. Candida albicans, Cryptococcus albidus, Cryptococcus neoformans and Rhodotorula rubrum are some of the most common pathogens infeeting immunocompromised patients. C. albicans, an opportunistic fungal pathogen, resides commensally in the mucocutaneous cavities of skin, vagina and intestine of humans [2]. It is one of the most frequent causes of fungal infections under altered physiological and pathological conditions like infancy, pregnancy, diabetes, prolonged broad-spectrum antibi- otic administration, steroidal chemotherapy and AIDS [3]. Whereas, C. neoformans is a basidiomycetous fun- gus infecting immunocompromised patients. Infection starts from the lungs and migrates to the central nervous system resulting in meningoencephalitis. Fluconazole, a fungistatic agent, has been used successfully to treat fungal infections caused by C. albicans and other related Candida species. However, emergence of intrinsically resistant species like Candida glabrata and Candida krusei to azoles is a worrisome development [4]. Human fungal infections mainly among immunocompromised patients have increased at an alarming rate during recent times [5], thereby warranting the need to identify and develop potent anti-yeast agents with broad spectrum activities. Origanum, comprising a wide range of species and subspecies, are valuable sources of spices and essential oils with the latter possessing broad spectrum antimicro- bial properties [6-8]. Gram negative and positive bacte- ria and their antibiotic resistant strains have shown sus- ceptibility to the Oregano essential oil (OEO) [9]. Fur- thermore, the potency of OEO in inhibiting mycelial growth and spore germination of Aspergillus niger and A. flavous have also been demonstrated. Several other studies also have reported the efficacy of OEO as an anti-yeast agent both under in vitro and in vivo condi- tions against a wide range of pathogenic yeasts [3,10]. In this study, anti yeast properties of OEO were examined in vitro against four human pathogenic yeasts namely Candida albicans, Cryptococcus albidus, Cryptococcus neoformans and Rhodotorula rubrum. Only a few stud- ies (including the present one) have demonstrated the time and concentration dependent efficacy of OEO as an anti-yeast agent on human pathogenic yeasts such as C.  A. Adams et al. / Advances in Bioscience and Biotechnology 2 (2011) 103-107 Copyright © 2011 SciRes. ABB 104 neoformans. To the best of our knowledge, this is the first report on the potency of OEO on the growth inhibi- tion of C. albidus. Since the effects of OEO an anti-yeast agent against Candida albicans have been demonstrated in earlier studies, we also included this strain in the pre- sent study to compare our results with those reported elsewhere. 2. MATERIALS AND METHODS 2.1. Plant Material and Extraction of Oil Fresh oregano plant material was purchased from Shen- andoah Growers in Harrisonburg, VA, USA. Stem and leaves were cut into small pieces, air-dried, powdered using a plant grinder and 100 g used for isolation of oil using hydro-distillation. 2.2. Yeast Culture Cultures of C. albicans and C. neoformans were ob- tained from Presque Island Cultures in Erie, PA, USA. C. albidus and R. rubrum were obtained from Department of Biology, Western Kentucky University, KY, USA. Initially culture were incubated at 37ºC for 3 days and subsequently transferred to room temperature (25ºC). All the yeast cultures were routinely sub-cultured for culture maintenance and inhibition test. 2.3. Culture Media Sabourad Dextrose Agar (DIFCO) and buffered yeast extract-peptone-dextrose (YPD) was used for yeast cul- tivation [11,12]. Initially all the yeast strains were main- tained on Sabourad Dextrose Agar (DIFCO) and later cultured in YPD (ATCC medium 1245) for solid me- dium growth inhibition test. The YPD broth (ATCC me- dium 1245) was also used for liquid medium yeast growth inhibition test experiments. 2.4. Liquid Medium Test Pre-cultures of C. albicans, C. neoformans, C. albidus and R. rubrum were maintained by inoculating 10 mL of YEPD broth with an appropriate yeast strain and incu- bating for 12 - 15 hr at 37ºC to ensure that yeast cells were actively dividing [3]. The pre-cultures were then used for inoculating the tubes in the test group. Test groups were prepared with 5 mL of medium containing 4.5 mL of YPD broth and 0.5 mL of yeast pre-cultures. Five and 10 μL of OEO corresponding to concentrations of 200 μg/mL and 400 μg/mL, respectively were added to the culture tubes containing medium in the test groups. Test tubes were incubated at 37ºC on a rotary shaker and growth measurements were taken over a period of one week by measuring the optical density using UV-Visible spectrophotometer at 600 nm (DU 530, Beckman Coul- ter). Blank and ethanol control groups with 200 μg/mL and 400 μg/mL of OEO in 5 mL YPD broth were also monitored. 2.5. Solid Medium Inhibition Test for Anti Yeast Activity C. albicans and C. neoformans were assessed for OEO susceptibility using solid medium growth inhibition test by employing the disk diffusion method [5]. Aliquot (100 μL from 10 mg/mL OEO in ethanol (equivalent to 1000 µg OEO) was added drop wise to a 7 mm sterile paper disk under sterile condition. A control was main- tained by adding 90% ethanol to each sterile disk. All sterile disks were then allowed to air-dry in a sterile hood for a minimum of one hour or until they were ready for application on to the agar plate. Yeast strains were spread on the Sabourd’s dextrose agar plates to create a lawn pattern holding 0.5 McFarland standards liquid inoculants. Disks containing 1000 μg of OEO were placed to the freshly inoculated plates with each plate containing three disks. Ethanol control disks were also set up in a similar fashion. Plates were then incu- bated for 7 days at 37ºC and zones of inhibition were measured. Disk diffusion test for C. neoformans was conducted on YEPD agar plates using a slightly different method of inoculation. Plates were inoculated with 1 mL of C. neoformans cell suspension standardized to McFarland standards [13]. One mL inoculum was spread across the plate under sterile conditions. Plates were allowed to dry at room temperature in a sterile hood. OEO (1000 μg) containing disks were placed on the plates as described above. All the experiments were conducted in triplicate and liquid growth medium inhibition tests were duplicated for C. albicans, C. albidus, C. neoformans, and R. ru- brum. 3. RESULTS AND DISCUSSION To determine the efficacy of OEO in inhibiting the growth of the yeasts, Candida albicans was grown in liquid medium supplemented with 200 and 400 µg/mL of OEO for 21, 40, 60 and 85 hr (Figure 1(a)). There was an appreciable increase in the percent growth inhi- bition of this strain with an increase in the incubation period at both OEO concentrations. Although no signifi- cant difference was observed in percent growth inhibi- tion of C. albicans at both concentrations of OEO (200 and 400 µg/mL) at 40 hr incubation, at other time inter- vals the percent growth inhibition of the yeast strain at 400 µg/mL of OEO was relatively higher compared to those grown at 200 µg/mL of OEO. The inhibitory effect of OEO on the growth of this yeast strain was further corroborated by the disk diffusion method (Table 1, Fig- ure 2(a)). A zone of inhibition of 27 mm in diameter was 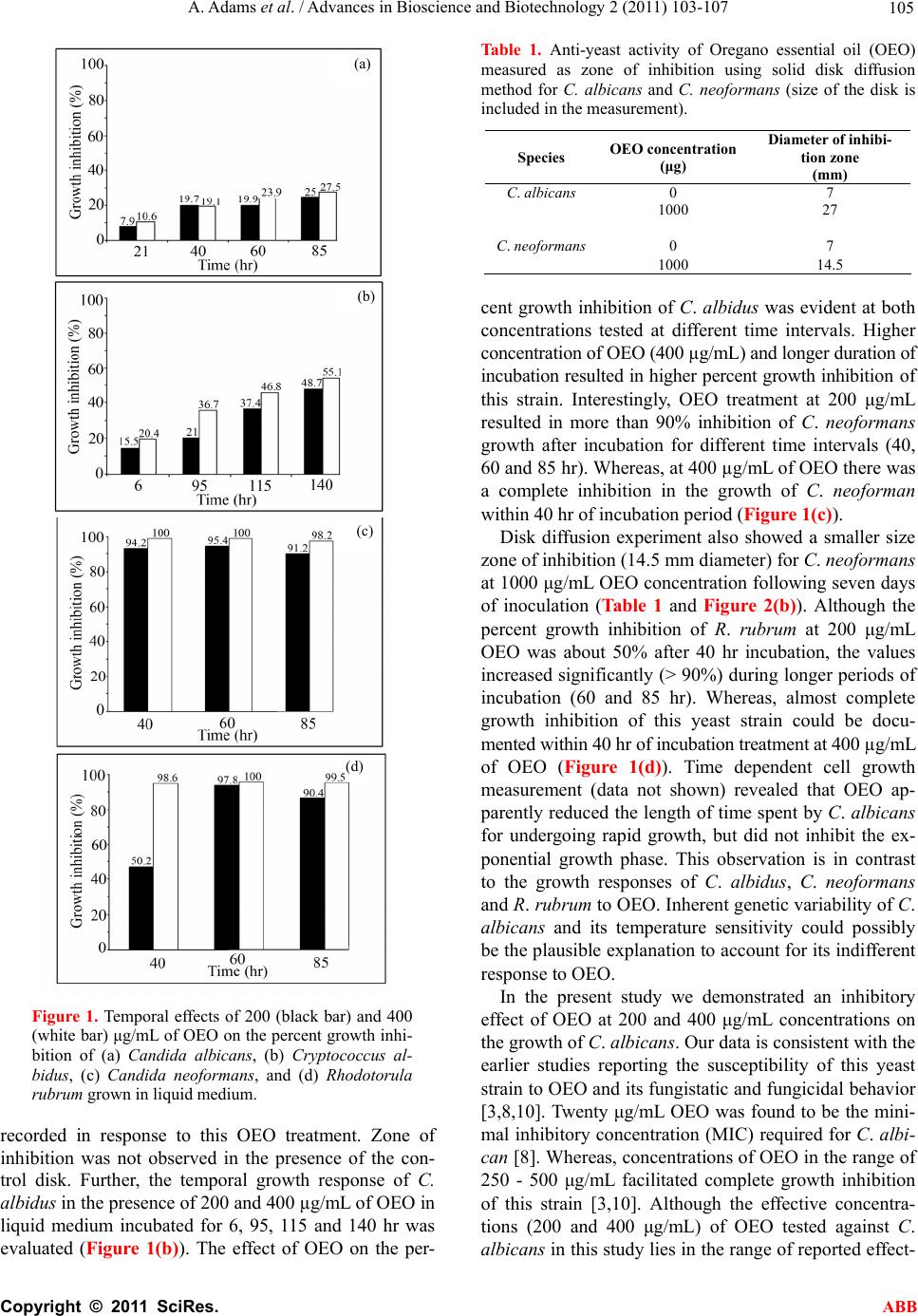 A. Adams et al. / Advances in Bioscience and Biotechnology 2 (2011) 103-107 Copyright © 2011 SciRes. ABB 105 Figure 1. Temporal effects of 200 (black bar) and 400 (white bar) μg/mL of OEO on the percent growth inhi- bition of (a) Candida albicans, (b) Cryptococcus al- bidus, (c) Candida neoformans, and (d) Rhodotorula rubrum grown in liquid medium. recorded in response to this OEO treatment. Zone of inhibition was not observed in the presence of the con- trol disk. Further, the temporal growth response of C. albidus in the presence of 200 and 400 µg/mL of OEO in liquid medium incubated for 6, 95, 115 and 140 hr was evaluated (Figure 1(b)). The effect of OEO on the per- Table 1. Anti-yeast activity of Oregano essential oil (OEO) measured as zone of inhibition using solid disk diffusion method for C. albicans and C. neoformans (size of the disk is included in the measurement). Species OEO concentration (μg) Diameter of inhibi- tion zone (mm) C. albicans 0 7 1000 27 C. neoformans0 7 1000 14.5 cent growth inhibition of C. albidus was evident at both concentrations tested at different time intervals. Higher concentration of OEO (400 µg/mL) and longer duration of incubation resulted in higher percent growth inhibition of this strain. Interestingly, OEO treatment at 200 μg/mL resulted in more than 90% inhibition of C. neoformans growth after incubation for different time intervals (40, 60 and 85 hr). Whereas, at 400 µg/mL of OEO there was a complete inhibition in the growth of C. neoforman within 40 hr of incubation period (Figure 1(c)). Disk diffusion experiment also showed a smaller size zone of inhibition (14.5 mm diameter) for C. neoformans at 1000 μg/mL OEO concentration following seven days of inoculation (Table 1 and Figure 2(b)). Although the percent growth inhibition of R. rubrum at 200 μg/mL OEO was about 50% after 40 hr incubation, the values increased significantly (> 90%) during longer periods of incubation (60 and 85 hr). Whereas, almost complete growth inhibition of this yeast strain could be docu- mented within 40 hr of incubation treatment at 400 µg/mL of OEO (Figure 1(d)). Time dependent cell growth measurement (data not shown) revealed that OEO ap- parently reduced the length of time spent by C. albicans for undergoing rapid growth, but did not inhibit the ex- ponential growth phase. This observation is in contrast to the growth responses of C. albidus, C. neoformans and R. rubrum to OEO. Inherent genetic variability of C. albicans and its temperature sensitivity could possibly be the plausible explanation to account for its indifferent response to OEO. In the present study we demonstrated an inhibitory effect of OEO at 200 and 400 μg/mL concentrations on the growth of C. albicans. Our data is consistent with the earlier studies reporting the susceptibility of this yeast strain to OEO and its fungistatic and fungicidal behavior [3,8,10]. Twenty μg/mL OEO was found to be the mini- mal inhibitory concentration (MIC) required for C. albi- can [8]. Whereas, concentrations of OEO in the range of 250 - 500 μg/mL facilitated complete growth inhibition of this strain [3,10]. Although the effective concentra- tions (200 and 400 μg/mL) of OEO tested against C. albicans in this study lies in the range of reported effect- (a) (c) (d) (b)  A. Adams et al. / Advances in Bioscience and Biotechnology 2 (2011) 103-107 Copyright © 2011 SciRes. ABB 106 Figure 2. Typical zone of inhibition of OEO (1000 μg/mL) against (a) Candida albicans, and (b) Cryptocococcus neofor- mans. tive OEO concentrations, differences in the exact values of MIC of OEO required for C. albicans growth inhibi- tion may possibly be due to the presence of different chemotypes, variable growth conditions, harvesting and extraction methods of Origanum. A few studies have also demonstrated the time-and concentration-dependent efficacy of OEO as an anti-yeast agent on other human pathogenic yeasts such as C. neoformans, R. rubrum and Trichophyton beigelii [14,15]. However, to the best of our knowledge, there is no information available on the effect of OEO on the growth performance of C. albidus. Thus, our study provides the first report on the potency of OEO on the growth inhibition of this yeast strain. Thymol, a major constituent of OEO, has been shown to stimulate deformities in the envelope of the yeast (Saccharomyces cerevisiae) cells, and was presumed to influence the exponential growth phase of the yeast by inducing cracking of non-dividing cells, thereby, affect- ing the budding process [16]. This could possibly ex- plain the differential effects of OEO on four different yeast strains in the present study. In the faster growing strains (C. albicans and C. albidus) OEO was compara- tively less effective to halt cell division completely. Low concentrations of growth inhibiting molecules in OEO or rapid cell division of these two yeast strains in the liquid medium could be attributed as possible reasons. On the contrary, R. rubrum and C. neoformans took longer time to begin their exponential growth phases, thereby allow- ing OEO sufficient time to affect their dividing cells, resulting either in the complete inhibition or lengthy delay of the exponential growth phase. Origanum vulgare showed great potential as a natural anti-yeast agent, in addition to its already reported bac- tericidal and fungicidal activities. Different yeast strains responded differentially to OEO. C. albidus, C. neofor- mans and R. rubrum showed higher susceptibility to Oregano oils as compared to C. albicans. Present and earlier studies indicate that OEO hold great potential for combating many fungal and yeast pathogens. Further investigations are warranted to decipher as how the in- oculums size and length of lag phase affect the action of OEO on yeast growth. It is also imperative that the ef- fects of different growing/harvesting methods upon oil content and antifungal and anti-yeast action be deter- mined. With further experimentation and in vivo testing, essential oil from Origanum vulgare could serve as a highly effective anti-yeast agent in pharmacology. 5. ACKNOWLEDGEMENTS Financial support from WKU Honors College, Biology Department and Applied Research and Technology Program of Ogden College of Science and Engineering are gratefully acknowledged. We also thank Drs. Mohd Israr and Ajay Jain for their technical help and critical review of this manuscript, respectively. REFERENCES [1] Bruneton, J. (1999) Pharmacognosy, phytochemistry, medicinal plants. Intercept Ltd, London, Paris, New York . [2] Kaufman, H.K. (1997) Opportunistic fungal infections: Superficial and systemic candidiasis. Geriatrics, 52, 50-54. [3] Manohar, V., Ingram, C., Gray, J., Talpur, N.A., Echard, B.W., Bagchi, D. and Preuss, H.G. (2001) Antifungal ac- tivities of origanum oil against Candida albicans. Mo- lecular and Cellular Biochemistry, 228, 111-117. doi:10.1023/A:1013311632207 [4] Mandala, S.M., Thornton, R.A., Milligan, J., Rosenbach, M., Garcia-Calvo, M., Bull, H.G., Harris, G., Flattery, A.M., Gill, C.G., Bartizal, K., Dreikon, S. and Kurtz, M.B. (1998) Rustmicin, a potent antifungal agent inhibits sphingolipid synthesis at inositol phosphoceramide syn- thase. Journal of Biological Chemistry, 273, 14942-14949. doi:10.1074/jbc.273.24.14942 [5] Scorzoni, L., Benaduccil,T., Almeida, A., Sliva, D., Bol- zani, V. and Gianinni, M. (2007) The use of standard methodology for determination of antifungal activity of natural products against medical yeasts Candida sp. Bra- zilian Journal of Microbiology, 38, 391-397. doi:10.1590/S1517-83822007000300001 [6] Lawrence, B.M. (1984) The botanical and chemical as- pects of Oregano. Perfume and Flavor, 9, 41-52. [7] Lukas,B., Schmiderer, C., Mitteregger, U., Franz, C. and Novak, J. (2008) Essential oil compounds of Origanum vulgare (Lamiaceae) from Corsica. Natural Product Communications, 12, 1127-1131. [8] Bozin, B., Mimica-Dukic, N., Simin, N. and Anackov, G. (2006) Characterization of the volatile composition of essential oils of some Lamiceae spices and antimicrobial and antioxidant activities of the entire oil. Journal of Ag- ricultural and Food Chemistry, 54, 1822-1828. doi:10.1021/jf051922u [9] Paster, N., Juven, B.J., Shaaya, E., Menasherov, M., Nit- zan, R., Weisslowicz, H. and Ravid U. (1990) Inhibitory effect of oregano and thyme essential oils on moulds and food borne bacteria. Letters in Applied Microbiology, 11, (a) (b)  A. Adams et al. / Advances in Bioscience and Biotechnology 2 (2011) 103-107 Copyright © 2011 SciRes. ABB 107 33-37. doi:10.1111/j.1472-765X.1990.tb00130.x [10] Tampieri, M.P., Galuppi, R., Macchioni, F., Carelle, M.S., Falcioni, L., Cioni, P.L. and Morelli, I. (2005) The inhi- bition of Candida albicans by selected essential oils and their major components. Mycopathologia, 159, 339-345. doi:10.1007/s11046-003-4790-5 [11] Adam, K., Sivropoulou, A., Kokkini, S., Lanaras, T. and Arsenakis, M. (1998) Antifungal activities of Origanum vulgare susp Hirtum, Mentha spicata, Lavandula angus- tifolia and Salvia fruticosa essential oils against human pathogenic fungi. Journal of Agricultural and Food Chemistry, 46, 1739-1745. doi:10.1021/jf9708296 [12] Fromtling, R. and Bulmer, G. (1978) In vitro effect of aqueous extract of garlic (Allium sativum) on the growth and viability of Cryptococcus neoformans. Mycologia, 70, 390-397. doi:10.2307/3759038 [13] Bartner, A., Pfeiffer, K.P. and Batner, H. (1994) Applica- bility of disc diffusion methods required by the pharma- copoeias for testing antibacterial activity of natural compound. Pharmazie, 49, 512-516. [14] Voda, K., Boh, B., Vrtaznik, M. and Pohleven, F. (2003) Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versi- color and the brown rot Coniophora puteana. Interna- tional Journal of Biodeterioration and Biodegradation, 51, 51-59. doi:10.1016/S0964-8305(02)00075-6 [15] Radudiene, J., Judpintiene, A. Peeiulyte, D. and Janulis, V. (2005) Chemical composition and antimicrobial activ- ity of Origanum vulgare. Biologia, 4, 53-58. [16] Bennis, S., Chami, F., Chami, N., Bouchikhi, T. and Remmal, A. (2004) Surface alteration of Saccaromyces cervisiae induced by thymol and eugenol. Letters in Ap- plied Microbiology, 38, 454-458. d oi:10.1111/j.1472-765X.2004.01511.x |

