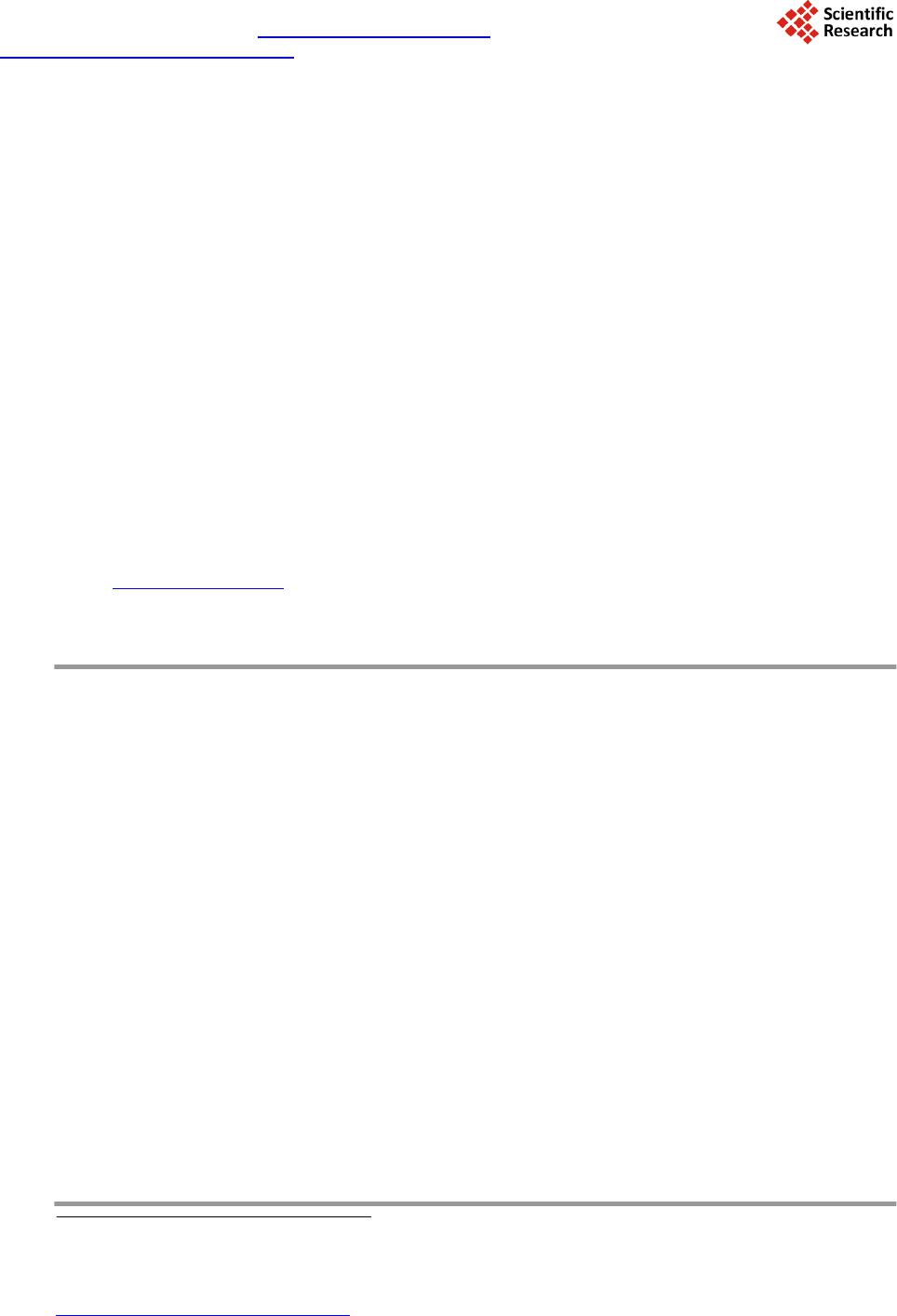
 Journal of Biosciences and Medicines, 2014, 2, 7-12 Published Online May 2014 in SciRes. http://www.scirp.org/journal/jbm http://dx.doi.org/10.4236/jbm.2014.23002 How to cite this paper: González-Olivares, L.G., et al. (2014) Viability and Proteolytic Capacity of Lactobacillus bulgaricus 2772 and Lactobacillus rhamnosus GG during Cheese Ripening. Journal of Biosciences and Medicines, 2, 7-12. http://dx.doi.org/10.4236/jbm.2014.23002 Viability and Proteolytic Capacity of Lactobacillus bulgaricus 2772 and Lactobacillus rhamnosus GG during Cheese Ripening L. G. González-Olivares1, Z. L. López-Cuellar1, J. Añorve-Morga1, M. J. Franco-Fernández2, A. Castañeda -Ovando1, E. Contreras-Ló pez1, J. Jaimez-Ordaz1, G. M. Rodriguez-Serrano3* 1Centro de InvestigacionesQuímicas, Universidad Autónoma del Estado de Hidalgo, Carr. Pachuca-Tulancingo km. 4.5, Pachuca, Hgo., C.P. 42067, México 2Instituto de CienciasAgropecuarias, Universidad Autónoma del Estado de Hidalgo, Rancho Universitario Av. Universidad Km. 1 Ex-Hda.deAquetzalpa AP 32, Tulancingo, Hgo, México 3Departamento de Biotecnología, Universidad AutónomaMetropolitana, UnidadIztapalapa, AP 55-355 México D.F., México Email: *gmrs @xa nu m.ua m. mx Received January 2014 Abstract Nowadays, probiotics have been utilized as starter cultures in the elaboration of fermented dairy products such as cheese. The survival of probiotic microorganisms in this type of products is very important in order to have a beneficial effect after their consumption. In addition to this, milk proteins are considered an important source of bioactive peptides. These peptides have been identified in hydrolyzed products of milk proteins and dairy products such as cheese. In this study, the protective effect on the survival of Lactobacillus rhamnosus GG was determined in a cheese which was inoculated only with this probiotic microorganism, and in another cheese additionally inoculated with Lactobacillus bulgaricus 2772 (exopolysaccharide producer bacteria). The ripen- ing of these cheeses took place for 28 days at two different temperatures (4˚C and 14˚C). The pro- teolytic capacity was analyzed by measuring the concentration of free amino groups, through the trinitrobenzenesulfonic acid method (TNBS). The separation of peptides was carried out by po- lyacrylamide gel electrophoresis (SDS-PAGE) with 15% T. At the end of the study it was found that the population density was higher in the cheeses ripened at 14˚C while at 4˚C, it decreased. A higher proteolytic activity at 14˚C was also observed and it was determined by a higher concentra- tion of free amino groups. Likewise, during the analysis of electrophoresis gels, a higher concen- tration of peptides smaller than 10 kDa was found in the samples of cheeses ripened at 14˚C. These results increase the expectations to find peptides with a biological function. Keywords Probiotics, Lactic Bacteria, Ripeni ng Chees e, Bioactiv e Peptide s, Proteoly sis *Corresponding author.  L. G. González-Olivares et al. 1. Introduction The cheese variety in the market and the constant growth of its production provide the opportunity to adopt new food trends. In the past years, an important trend in developed countries has been the introduction of functional foods, especially in the dairy sector. The introduction of the probiotic concept has been carried out successfully, especially owing to the production of metabolites that are released during fermentation, primarily peptides of low molecular weight with biological activities potentially beneficial to health. Although most dairy products containing probiotics are based on fermented milks, new products have been developed; some examples are vis- ible in the cheese sector [1]. These developments include the use of probiotics as starter cultures. Meanwhile, peptides derived from the proteolytic breakdown of cheese caseins are produced during the ripening process. Many of these peptides may have biological activity [2]. After oral administration, bioactive peptides can exert a positive effect on the car- diovascular, digestive, immunologic and nervous systems, depending on their own sequence of amino acids. Bioactive peptides (peptides with a low molecular weight, 3 to 20 amino acids approximately) can influence cellular metabolism and work as pressure vessel regulators, growth factors, hormonal inductors and neurotrans- mitters [3]. It has been demonstrated that certain bacteria such as Lactobacillus helveticus, Lactobacillus rhamnosus GG and Lactobacillus delbrueckii subsp. bulgaricus have the capacity to generate peptides during dairy fermentation driven by their proteolytic system [3] [4]. The proteolytic system ensures the growth of lactic acid bacteria (LAB) in any medium. This system com- prises proteinases located in the wall cell that allows the degradation of caseins into oligopeptides [5] [6]. The second part of the proteolytic system is the peptide transport system which allows the transit of oligopeptides released within the cell. The intracellular peptidases that hydrolyze oligopeptides into peptides or amino acids constitute the last part of this system. Bioactive peptides are then peptides that were not transported to the inte- rior of the cell or peptides that were generated within the cell and were later excreted to the medium [5]. During the manufacture of cheese are formed a variety of peptides and some have been shown to have some biological activity. In the ripening, the casein phosphopeptides, which are naturally found in cheeses, generate peptides (secondary proteolysis) with anti-hypertensive activity. Such generation is highly dependent on the ri- pening stage the cheese is experiencing [2]. In order to provide a beneficial effect on the consumer’s health, probiotic microorganisms must survive in the extended period of storage required for cheese ripening which in some cases is over 6 months, plus the shelf life period of the product [7]. In the elaboration of cheese containing probiotics, the strains need to either grow in high quantities during ri- pening or, be incorporated in high concentrations in order to survive the process of ripening. During cheese ela- boration or ripening, probiotic microorganisms must not produce metabolites that may cause defects or interfere with the activity of other essential microorganisms that constitute the starter culture. With regards to the full use of the functional properties of probiotic bacteria, the processes of cheese produc- tion can be modified and adapted to the requirements of probiotics. In this research, the viability and proteolytic activity of Lactobacillus rhamnosus GG and Lactobacillus bulgaricus 2772 was studied during the ripening process of a cheese at 4˚C and 14˚C, for 4 weeks. 2. Materials and Methods 2.1. Cheese Making The cheese was produced from pasteurized and standardized cow milk with 3.6% of fat. Milk was heated at 36˚C, adding 0.20% (% p/v) of CaCl2 and 1% of inoculum of Lactobacillus rhamnosus GG and L. bulgaricus 2772. The pre-ripening was performed at 37˚C and 18.5˚C during a period of 30 and 40 minutes respectively. The amount of single-strength rennet added (Hansen’s standard, Chr. Hansen Lab., Copenhagen, Denmark) was 250 - 300 mL per 1000 L. After a coagulation time of 30 min, the curd was cut and agitated without heat for 40 minutes, proceeding to remove the whey. The cheese was pressed for 24 hours and was vacuum-packed and ri- pened for 4 weeks at 4˚C and 14˚C. Cheese was produced in duplicate. Samples were taken on days 0, 7, 14, 21 and 28 for the subsequent analyses.  L. G. González-Olivares et al. 2.2. Viability Determination The viability of microorganisms was determined through plate-count technique. Samples were collected in ste- rile bags. Ten grams of cheese were dissolved on 90 mL of sterile peptone water (1%) at room temperature and mixed in a Stomacher at 260 rpm for 3 minutes until obtaining a homogenous suspension. 1 mL of this suspen- sion was diluted in sterile peptone water 1%, considering a final dilution of 1 × 106. It was grown on a plate with an MRS (Man Rogossa and Sharpe) agar medium. The pH was adjusted to 5.20 with acetic acid 0.1N and incubated at 42˚C for 72 hours under anaerobic conditions. Later, a Gram staining was carried out in order to confirm strain purity. 2.3. Proteolytic Activit y The proteolytic activity was measured with 2,4,6-trinitrobenzenesulphonic acid (TNBS). 50 mL of deionized water was added to 20 g of cheese. This was mixed in a Stomacher at 260 rpm for 3 minutes. After that, 10 mL of the sample was centrifuged at 5000 rmp for 5 minutes at 4˚C and filtered through Whatman paper no. 4 under vacuum. The supernatant was centrifuged again at 5000 rpm during 10 minutes at 4˚C. 1 mL of phosphate buffer solution of 0.21 M, pH 8.2 was added in test tubes wrapped with aluminum foil and 125 µL of sample. The con- trol was prepared with deionized water. 1 mL of TNBS (0.10%) was added to phosphate buffer 0.21 M, pH 8.2, and each tube was agitated in vortex. The tubes were incubated for 1 hour at 50˚C in darkness. The reaction was interrupted after 60 minutes adding 2 mL of chloride acid 0.1 N. It was read in a spectrophotometer at 340 nm of wavelength against the control. 2.4. Polyacrylamide Gel Electrophoresis (SDS-PA GE ) Electrophoresis was carried out with polyacrylamide gel T = 15%, according to Laemmli [8] and Gonzalez- Olivares et al. [9].A standard of wide range molecular weights (Bio-Rad, EUA) was used. The gels were dyed with Coomassie Blue and analyzed through ImageJ software. 2.5. Statistical Analysis All the analyses were carried out in duplicate. The results were subjected to an analysis of variance (ANOVA) for mean comparison according to Tukey test with a level of significance of 0.05. 3. Results and Discussion 3.1. Study of Viability For the cheeses ripened at 4˚C, it was observed that probiotic population diminished during the entire study. This could indicate that the microorganism had a higher stress time, not being able to reproduce. During storage at 14˚C, a higher survival was observed since the stress factor (temperature) allowed the survival of microor- ganisms and their adaptation to the medium (Figure 1). Various studies have shown the difficulty in survival of probiotic organisms during cheese ripening when they had been inoculated as the only starter strain, which has itself an adverse effect on cheese flavor [10] [11]. However, other authors have demonstrated that the addition of probiotics together with starter cultures allows the survival of the probiotics [12] [13]. Different approaches have been applied to improve the survival of pro- biotic microorganisms during ripening. In 1999, a patent for the production of probiotic cheese was granted, and in 2000, probiotic cheese containing Lactobacillus GG was introduced in the Finnish market. Lb. GG is one of the best probiotic bacterial strains with well-defined probiotic properties, however, there are no data regarding its probiotic properties when administered in a matrix of cheese [1]. 3.2. Determination of Free Amino Groups Using the Trinitrobenzenesulfonic Acid Method (TNBS) The concentration of free amino groups for each of the ripening stages analyzed in cheese carried out at different temperatures (4˚C and 14˚C) is shown in Figure 2. In this graph, it can be observed that from day 0, free amino groups were generated in amounts of 1.22 and 0.93 mg/L at 14˚C and 4˚C, respectively. At a temperature of 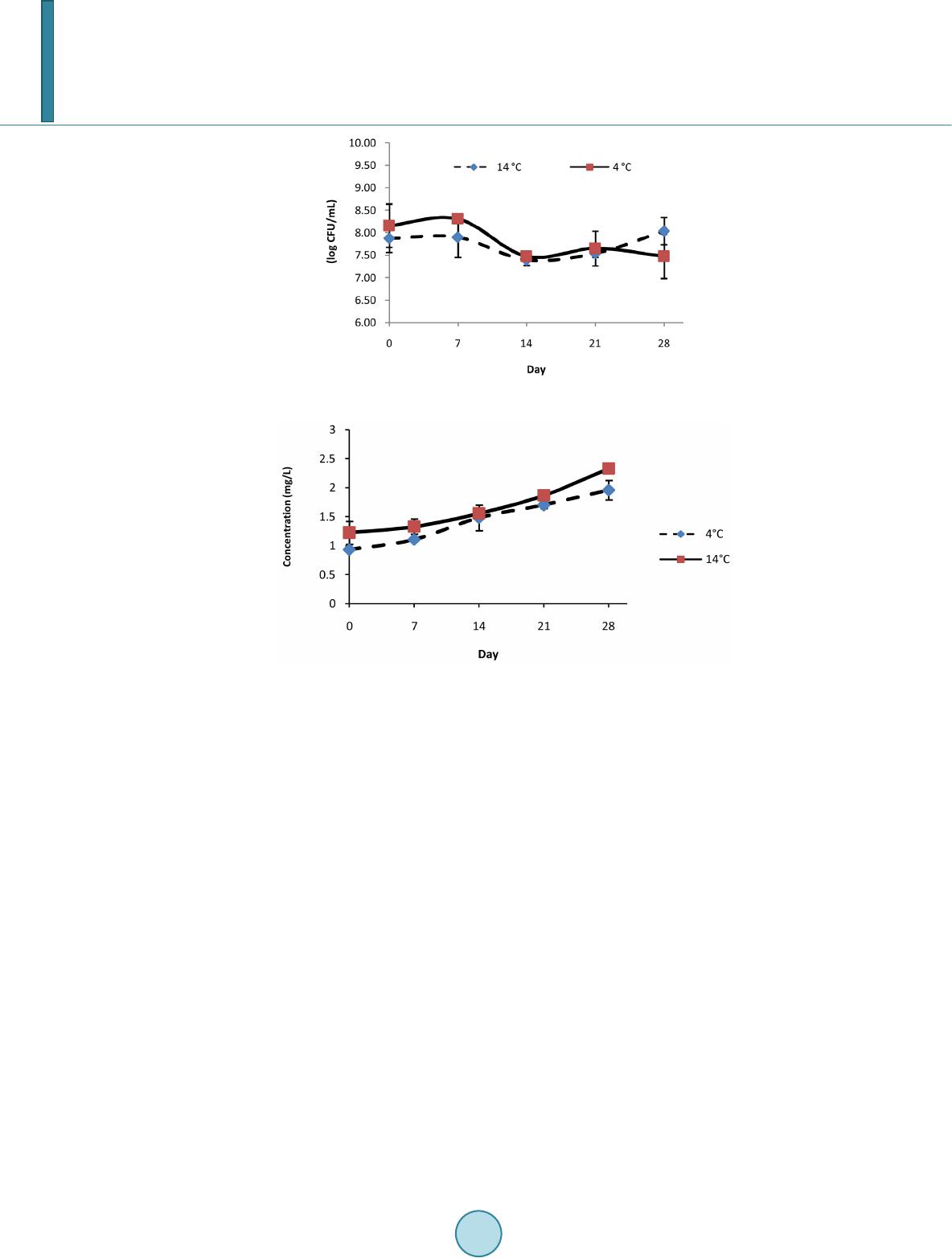 L. G. González-Olivares et al. Figure 1. Viabilityof L. rhamnosus GG during ripening at 4˚C and 14˚C. Figure 2. Concentration of free amino groupsduringcheeseripening at 4˚C and 14˚C 14˚C, the concentration of free amino groups did not have a significant difference from day 0 to day 14. Mean- while, there was a significant increase from day 14 to day 28, having a final concentration of 2.325 mg/L. This indicated an increase in the concentration of peptides generated. In the case of ripened cheeses at 4˚C, there was a significant difference only from day 0 to day 21, reaching a final concentration of 1.95 mg/L. During this pe- riod, a significant decrease in the concentration of free amino groups was observed compared with those ob- tained at 14˚C, which may be due to the fact that the proteinases and peptidases of their proteolytic system are more active at a temperature closer to their optimum. Even though thermophilic BAL have optimum growth temperatures of over 42˚C, enzymes present other optimum conditions of activity, close to 32˚C [2]. It has been observed that even in refrigerated conditions, LAB has proteolytic activity, generating low molecular weight peptides during the entire storage period. At both temperatures of ripening assayed, there was cheese protein degradation due to the action of the pro- teolytic system of lactic bacteria and, as a result, peptide generation takes place. This degradation of cheese pro- teins, especially caseins, is what supports the growth of the bacteria since the number of free amino acids found in milk is insufficient for the optimum growing of microorganisms [14]. 3.3. Electrophoresis in Polyacrylamide Gel (SDS-PAGE) Figure 3 and Figure 4 show the image of the electrophoresis gels with the samples obtained during the ripening of manchego cheese at 4˚C and 14˚C, respectively, inoculated with Lactobacillus bulgaricus 2772 and Lactobacil- lus rhamnosus GG. A decrease in milk proteins is clearly observed with time. Therefore, in the analysis peptidic fractions of 13.97 kDa to 1.32 kDa were found at both temperatures of ripening. Fractions smaller than 14.4 kDa are significantly important since peptides reported as bioactive have molecular weights smaller than this one [10]-[12]. A higher separation of peptides smaller than 6.5 kDa was observed at 14˚C, which is consistent with the production of free amino groups (Figure 4). The concentration of peptides continued to increase until the 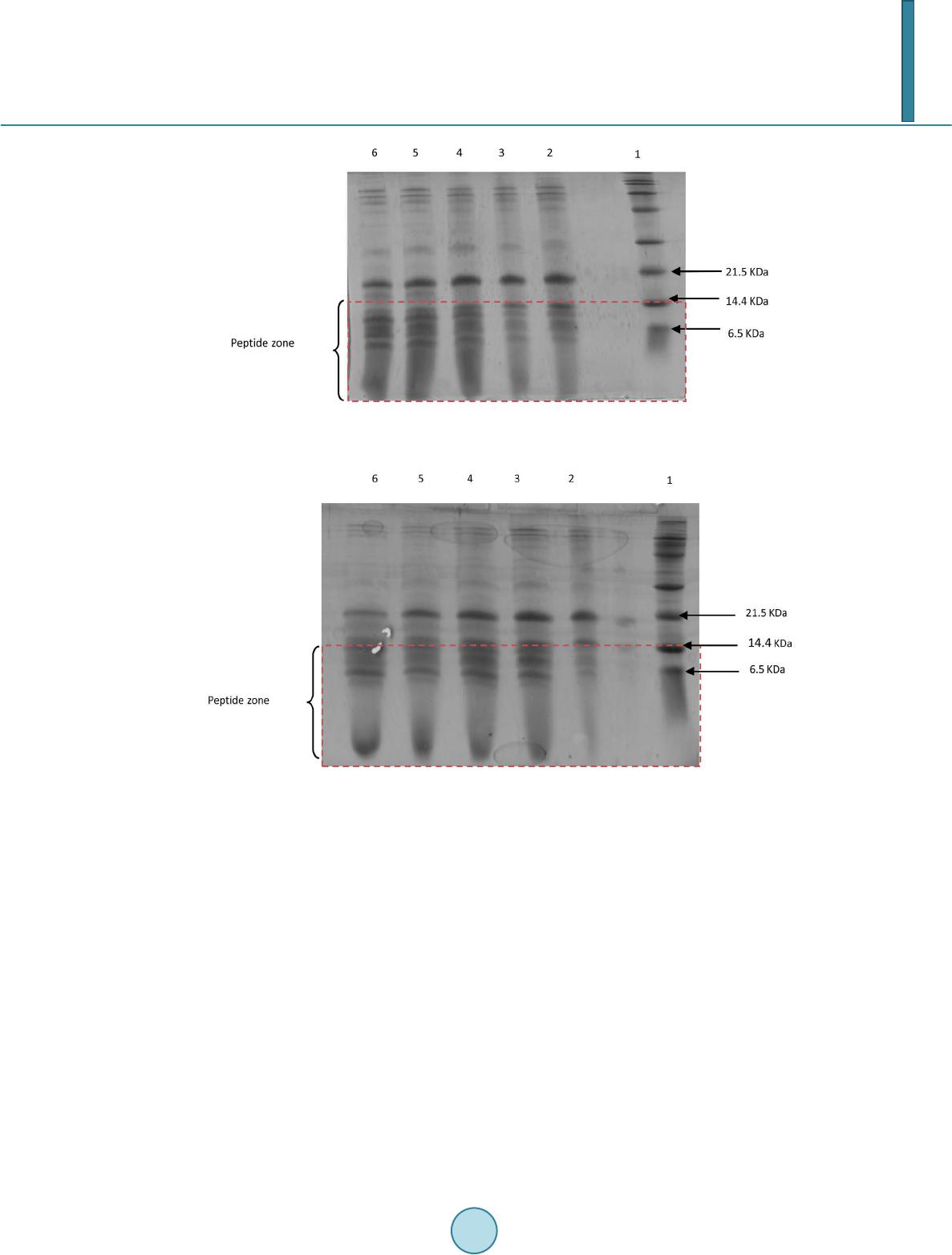 L. G. González-Olivares et al. Figure 3. SDS-PAGE. Peptides separation of cheese ripening at 4˚C, 1: polypeptide standard; 2: day 0; 3: day 7; 4: day 14; 5: day 21 and 6: day 28. Figure 4. SDS-PAGE. Peptides separation of cheese ripening at 14˚C, 1: polypeptide standard; 2: day 0; 3: day 7; 4: day 14; 5: day 21 and 6: day 28. last day of ripening at both temperatures. The generation of peptides is generated from αs1 and β caseins, since lactic bacteria hydrolyze them more frequently [15] [16]. According to the electrophoresis analyses, a complete separation of peptides could be observed, with both appearance and disappearance of peptides, especially those larger than 6.5 kDa [9]. Peptides smaller than 6.5 kDa appeared from day 1 and their accumulation was constant though the formation of lower molecular weight peptides is observed in higher concentrations in cheese ripened at 14˚C. The appearance of low molecular weight peptides follows a waterfall pattern where caseins are the substrate of proteinases to produce interme- diate molecular weight peptides, which in turn become the substrate of peptidases that break down oligopeptides into low molecular weight peptides. 4. Conclusion Ripening at a temperature of 14˚C proved to be appropriate to allow the survival of Lactobacillus rhamnosus GG. In addition, at this temperature a higher degradation of proteins into low molecular weight peptides was ob- served. This confirms that proteolytic activity is favored at temperatures closer to the optimal for the proteolytic system of LAB. A temperature of 14˚C allows microorganisms to adapt to the medium and with time cell mul- tiplication takes place, which is intended to occur in a ripened cheese with probiotics in order to provide benefits  L. G. González-Olivares et al. to the host together with the production of low molecular weight peptides which could have a positive biological effect. References [1] Farnworth, E. (2008) Handbook of Fermented Functional Foods. Segunda Edición, CRC Press, Quebec, Canada, 209- 260. [2] Hartmann, R. and Meisel, H. (2007) Food-Derived with Biological Activity: From Research to Food Aplication. Current Opinion of Biotechnology, 18, 163-169 . http://dx.doi.org/10.1016/j.copbio.2007.01.013 [3] Figueroa-González, I., Hérnandez-Sánchez, H., Rod rí guez -Serr ano , G., Gómez-Ruiz, L., García-Garib ay, M. and Cruz- Guerrero, A. (2010) Antimicrobial Effect of Lactobacillus casei Strain Shirota Co-Cultivated with Escherichia coli UAM0403. Revista Mexicana de Ingeniería Química, 9, 11-16. [4] Figueroa, C. (2007) Péptidos bioactivos de la leche. Utilización del sistema proteolítico de Lactococcus lactis para la generación de péptidos potencialmente bioactivos. Universidad Autónoma Metropolitana, México. 29-46. [5] Juille, O., Le Bars, D. and Juillard, V. (2005) The Specificity of Oligopeptide Transport by Streptococcus thermophilus Resembles that of Lactococcus lactis and Not That of Pathogenic Streptococi. Microbiology, 151, 1987 -1994. http://dx.doi.org/10.1099/mic.0.27730-0 [6] Poolman, B., Kunji, E.R.S., Hagting, A., Juillard, V. and Konings, W.N. (1995) The Proteolytic Pathway of Lactococcus lactis. Society of Applied Bacteriology, 24, 65S-75S. [7] Ryhanen, E.L., Pihlanto-Lep pala, A. and Pahkala, E. (2001) A New Type of Ripened, Low-Fat Cheese with Bioactive Prop erti es. International DairyJournal, 11, 441 -44 7. http://dx.doi.org/10.1016/S0958-6946(01)00079-6 [8] Laemmli, U.K. (1970) Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature, 227, 680-6 85 . http://dx.doi.org/10.1038/227680a0 [9] Gonzál ez-Olivares, L.G., Jiménez-Guzmán, J., Cruz-Guerrero, A., Rodríguez-Serrano, G., Gómez-Ruiz, L. and Gar cía- Garibay, M. (2011) Liberación de Péptidos Bioactivos por Bacterias Lácticas en Leches Fermentadas Comerciales. Revista Mexicana de Ingeniería Química, 10, 179-188. [10] Steele, J., Broadbent, J. and Kok, J. (2013) Perspectives on the Contribution of Lactic Acid Bacteria to Cheese Flavor Developmen t. Current Opinion in Biotechnology, 24, 135-141 . http://dx.doi.org/10.1016/j.copbio.2012.12.001 [11] Tan, W.S., Budinich, M.F., Ward, R., Broadbent, J.R. and Steele, J.L. (2012) Optimal Growth of Lactobacillus casei in a Cheddar Cheese Ripening Model System Requires Exogenous Fatty Acids. Journal of Dairy Science, 95, 1680-16 89 . http://dx.doi.org/10.3168/jds.2011-4847 [12] Jyothsna, D., Michael, P. and Kasipathy, K. (2013) Effect of Encapsulation on the Survival of Probiotic Bacteria in the Presence of Starter and Non -Starter Lactic Acid Bacteria in Cheddar Cheese over a 6-Month Ripening Period. International Journal of Fermented Foods, 2, 63-76. [13] Karimi, R., Mortazavian, A.M. and Gomes Da Cruz, A. (2011) Viability of Probiotic Microorganisms in Cheese during Production and Sto ra ge: A Review. Dairy Science & Technology, 91, 283-308. http://dx.doi.org/10.1007/s13594-011-0005-x [14] Juillard, V., Furlan, S., Foucaud, C. and Richard, J. (1996) Mixed Cultures of Pro teinase-Positive and Protein ase- Negative Strains of Lactococcus lactis in Milk. Journal of Dairy Science, 79, 964-970. http://dx.doi.org/10.3168/jds.S0022-0302(96)76447-0 [15] Visser, S. (1992) Proteolytic Enzymes and Cheese Ripening. Journal of Dairy Science, 76, 329-350. http://dx.doi.org/10.3168/jds.S0022-0302(93)77354-3 [16] Gasson, M.J. and De Vos, W.M. (1994) Cap. 4 : The Proteolytic System of Lactic acid Bacteria. In: Genetics and Bio- technology of Lactic Acid Bacteria, Blackie Academic y Professional an Imprint of Chapman y Hall, 169-210.
|