 Journal of Agricultural Chemistry and Environment, 2014, 3, 26-34 Published Online April 2014 in SciRes. http://www.scirp.org/journal/jacen http://dx.doi.org/10.4236/jacen.2014.32B005 How to cite this paper: Del Carmen Gutierrez, M., et al. (2014) Evaluation of the Antioxidant Activity of Ethanolic Extracts of Some Varieties of Onions. Journal of Agricultural Chemistry and Environment, 3, 26-34. http://dx.doi.org/10.4236/jacen.2014.32B005 Evaluation of the Antioxidant Activity of Ethanolic Extracts of Some Varieties of Onions María del Carmen Gutierrez, Patricia Della Rocca, Elizabeth De Seta, Fernando Reina Department of Chemical Engineering, National Technological University, Buenos Aires Regional Faculty (UTN-FRBA), Buenos Aires, Argentina Email: info@quimica.frba.utn.edu.ar Received January 2014 Abstract The content of polyphenolic substances in commercial onions has been determined. The antioxi- dant activity of their ethanolic extracts, as well as their effects on the oxidation of edible corn oil during accelerated ageing was studied. Maceration of taxonomically identified commercial vegeta- ble samples, previously peeled and thinly sliced, was carried out at ambient temperature, out of direct light, with occasional agitation and ultrasound, employing 95% ethyl alcohol as the extrac- tion solvent, allowing them to stand for 7 days. The total polyphenolic contents were determined on the filtrated extracts using the Folin-Ciocalteau method. The antioxidant activity was evaluated on emulsions of ethanolic extracts of onion prepared in edible commercial corn oil, using sorbitan monooleate as emulsifying agent. The peroxide values were analyzed using the iodometric me- thod; oxidation induction times were obtained from the peroxide evolution graphs, using the tan- gent method. Oil samples emulsified with ethanolic onion extracts showed an extension of the induction period. A 7-day ageing study at 45˚C was additionally performed to determine the conjugated dienes on pure commercial corn oil and its emulsions by visible spectrophotometry. The spectral analysis showed an increase of the measured absorbancies in oil samples without additives and no change for the oils emulsified with onion extract. An increasing of diene values was observed for corn oil without additives during ageing; no changes in the value were observed in oils emulsified with onion extracts. Keywords Onion ; Antioxidant Activity; Polyphenolic; Ethanolic Extracts; Emulsified Oil 1. Introduction Epidemiologic studies have determined that the consumption of vegetables and fruit is related to the reduction of the risks of contracting a cardiovascular disease or cancer Foods containing significant amounts of bioactive components may provide desirable health benefits and play important roles in the prevention of chronic diseases [1].  M. del Carmen Gutierrez et al. At present we pay the interest in the biological effects of phenolic compounds as it was found that diets rich in fruits and vegetables appear to protect against cardiovascular disease [2] [3] and some forms of cancer [4]. Currently there is an increasing preference for the use of natural antioxidants [5] although many of them have been used since antiquity. Many plant extracts have demonstrated considerable stabilizing effect against lipid oxidation reactions and, consequently, they can have significant commercial potential as source of nutraceutical or functional food ingredients [6]. Antioxidants with an important activity have been found in berries [7], cherries [8], citrics [9], k iw is [10], o live s [11], coc oa [12], potatoes [13], tomatoes [14], garlics [15], onions [16] and soybeans [17]. Most of the spices, e.g. red pepper [18], ginger [19] and rosemary [20] have also relevant antioxidant properties. Onion is proposed as a viable source of phenolic co mpounds and flavonoids. Studies conducted over different cultivars demonstrate that total oxidant activity is lower in white onion varieties; red varieties have a 100 mg/100 g average content of gallic acid equivalents [21]. Th e essential oil reveals interesting proper ties, such as antimicrobial agent and moderate reducing power feasible to implement in food [22]. Also, phenolic extracts obtained from wastes of onion were used to evaluate the capacity inhibiting of processes inflammatory and oxidation of low-density lipo protein (LDL) [23]. Likewise, different methods of extraction of the active components have been used for their assessment, resulting in variations of the reported activities [24] [25]. When extraction is carried out by the method of microwave-assisted greater efficiency is obtained with higher antioxidant activities [26]. Antioxidant capacity scores were reported [27] from the oxygen radical absorption capacity measurements (ORAC). Most investigations have been dedicated to quantifying polyphenols and its antioxidant capacity. Despite the results achieved, further studies should be performed in order to enhance the knowledge about extraction and identification of activ e components present in less studied vegetables . The efficiency of extraction of natural antioxidants (NAO) depends on the fraction, the type of vegetable used, and the ability of the active components to provide sufficient antioxidant capacity. Therefore a simple method of extraction of polyphenolic components from commercial onions has been developed by the authors, in order to evaluate their performance as an alternative source of natural antioxidants. 2. Materials and Methods 2.1. Plant Material White onion taxonomically identified as Allium cepa, provided by the National Institute of Agricultural Tech- nology (I.N.T.A. Mendoza) and commercial white onion purchased in local grocery store. Bulbs from each va- riety were stored at 4˚C until sampling. 2.2. Sample Preparation Bulbs were randomly selected for extraction. Onions were peeled, eliminating the skin and the first and second layers, and chopped in fine pieces. 2.3. Extraction Maceration was performed at room temperature, out of direct light, with occasional agitation and ultrasound (to facilitate extraction) of an exactly weighed sample quantity, using 99.5% - absolute - pro analysis ACS ethyl alcohol as extraction solvent, in a 50% concentration (in masses), allowing them to stand for 7 days. Ethanolic extracts were filtered us ing glass woo l and diluted 10:1 with 80% ethanol to a total of 5 mL. 2.4. Spectrophotometric Analysis Absorbance (AU) readings were made in duplicate using a Shimadzu series UV1700 uv-visible spectrophoto- meter. 2.5. Determination of Total Polyphenols Spectrophotometric determination of Total Polyphenol in the extracts obtained was performed using the Folin-  M. del Carmen Gutierrez et al. Ciocalteu method, based on the Singleton and Rossi (1965) procedures and modified by Waterhouse (2001), Determinations were carried out, both for the classified and for the commercial onion samples. Concentrations of polyphenols in sample extract were calculated by linear regression onto the standard curve of monohydrated gallic acid, ACS analytic reagent, as standard. 2.6. Antioxidant Capacity Emulsions of ethanolic extract of onion were prepared in 10% concentration (in masses) in edible commercial corn oil with composition of 51 g/100g polyunsaturated fat and vitamin E (25 mg/100g of oil) using sorbitan monooleate (SPAN 80) in a concentration of 1% m/m as emulsifying agent. A sample of the same commercial corn oil without additives was used as blank; a corn oil sample with a synthetic antioxidant (butylhydroxytoluene) added, in 0.01% concentration (in masses) was also prepared for comparison. The samples were stored for 45 days on a heater at 45˚C temperature and protected from direct light, with occasional agitation. The peroxide values were analyzed by the iodometric method, employing p.a. ACS potassium iodide solution, analytic reagent ACS sodium thiosulfate pentahydrate for the preparation of the titrating solution, pro analysis trichloromethane (chloroform) and soluble starch as indicator, according to the American Oil Chemists’ Society AOAC Official Meth o d 942.27 [28]. With the results ob taine d, the evolu tion of the mEq O2/kg generated in the oxidation process was recorded as function of the storage time. For each of the samples under study, oxidation induction times were determined from the charts by the tangents method. 2.7. Conjugated Dienes assay A 7 day ageing study, at a temperature of 45˚C, was additionally performed to determine pr imary lipid ox idation products: Conjugated Dienes were determined on both the pure commercial corn oil and the samples of the emulsions of ethanolic onion extract in edible corn oil, by UV-Visible spectrophotometry, measuring the absorbances at 233 nm, 268 nm and 278 nm and 2.2.4-trimethylpentane (Isooctane) for chromatographic use, as a sample preparation solvent, as described in Current Protocols in Food Analytical Chemistry (2001) D21.1-D2.1.15 [29 ]. From the results obtained the concentration of conjugated dienes [CD] was calculated as follows where: A = Absorbance at 233 nm. ε = Molar absorptivity of Linoleic A c id Hydroperoxide = 2.525 × 104 M−1cm−1 l = Optical path of the cuvette 1 cm And the con j ugated die ne value (CD value): where: 2.5 × 104 is the correction factor. m = sample mas s (g) 3. Results and Discussion 3.1. Total Polyphenols The calibration curve obtained, using gallic acid as external standard, corresponds to a first-order equation, and = 0.006x + 0. 027, with a correlation coefficient R2 = 0.9975. The taxonomically identified onion extract has a total polyphenol concentration of 322.8 mg /l (DE 17.8 mg/l ), being slightly higher than that corresponding to the commercial onion extract (309.9 mg/ l, DE 17.8). 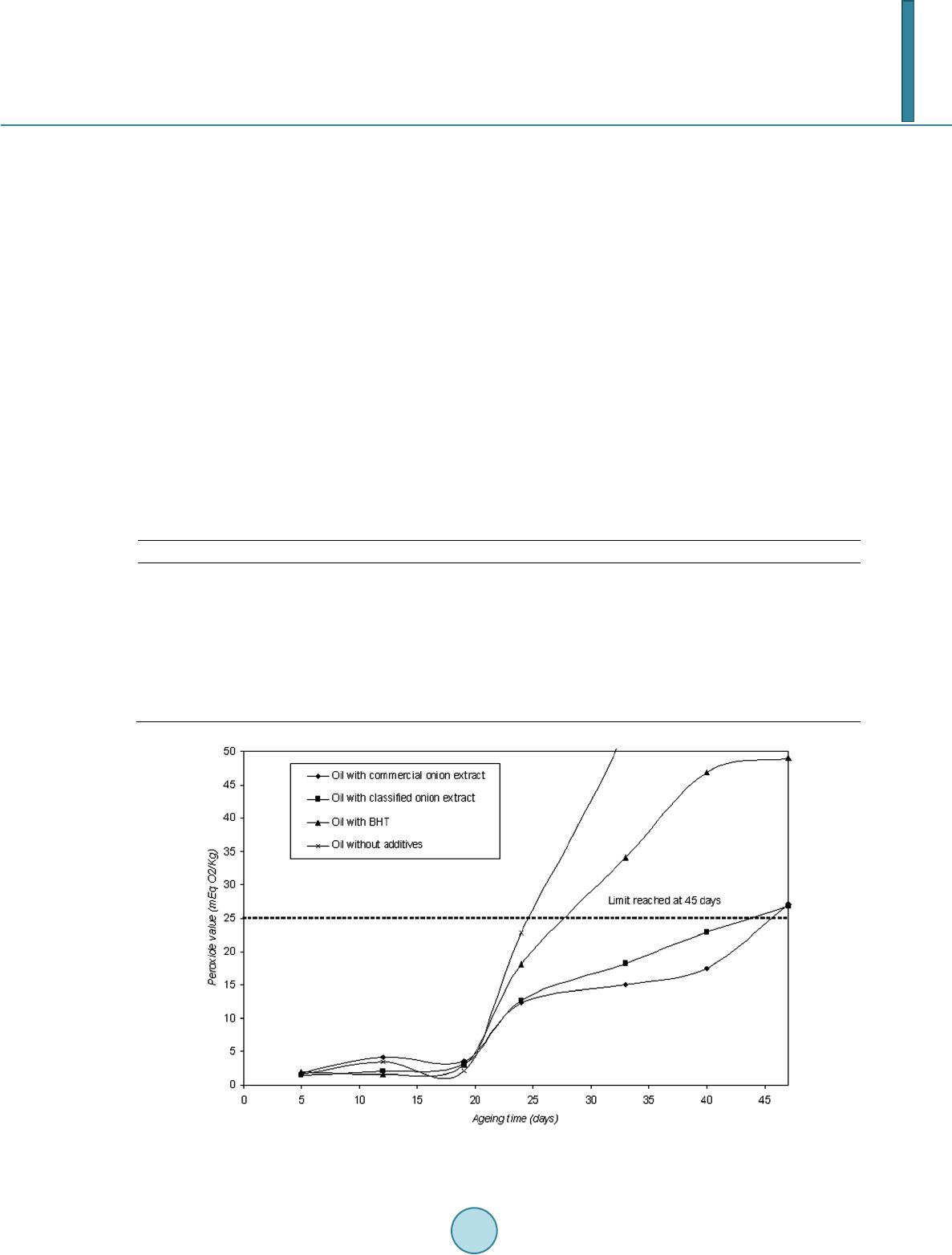 M. del Carmen Gutierrez et al. 3.2. Peroxide Values The values obtained for peroxide values (in mEq O2/kg) at different storage times for the samples analyzed are shown in Table 1. References: 1) Edible oil with aggregated ethanolic extract of commercial allium cepa. 2) Edible oil with aggregated ethanolic extract of allium cepa classified by INTA. 3) Edible oil with aggregated Butylhydroxytoluene. 4) Edible oil without antioxidant aggr e gate. Figure 1 shows the evolution of the Peroxide Values. Figure 2 shows the time in which the maximum limit accepted by the Argentinean Food Code (CAA), 10 mEq O2/kg is reached. The kinetic curves of peroxide accumulation were used to determine the induction times, resulting in the fol- lowing: 17 d ays for the corn oil withou t aggregates (Figu re 3), 17.5 days for the oil with added BHT (Figure 4) and of 19.5 days for the emulsions of ethanolic onion extraction oil (Figure 5). An increase of the induction period is observed in the oil samples emulsified with ethanolic onion extract, in- cluding that classified as commercial, as well as a delay of the time in which peroxides reach the limit estab- lished by the Argentinean Food Code (CAA) of 10 mEq O2/kg in the kinetic curves of peroxide accumulation, thus demonstrating a net antioxidant effect produced by the polyphenolic contents present in the onion. Table 1. Peroxide values of the samples analyzed at different storage times. Time (days) PV 1 (MEqO/Kg) PV 2 (MEqO/Kg) PV 3 (MEqO/Kg) PV 4 (MEqO/Kg) 5 1.69 1.50 1.96 1.61 12 4.17 2.14 1.60 3.44 19 3.53 3.10 2.93 2.05 24 12.38 12.67 18.08 22.74 33 14.99 18.21 34.05 53.66 40 17.43 22.92 46.97 92.01 47 27.05 26.90 48.99 100.15 Figure 1. Evolution of the peroxide values. 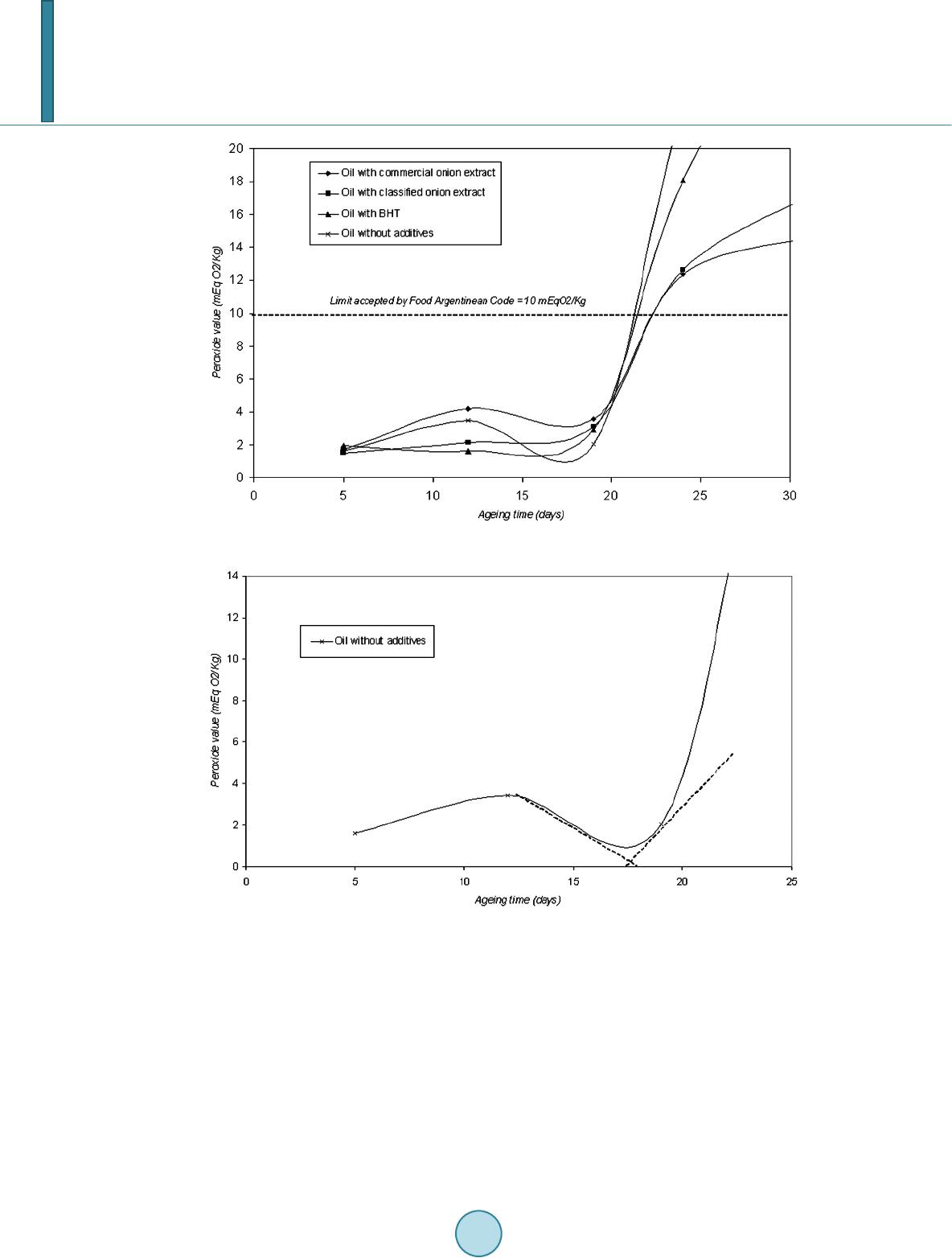 M. del Carmen Gutierrez et al. Figure 2. Evolution of the peroxide values. Figure 3. Evolution of the Peroxide Value for corn oil without aggregates. 3.3. Spectral Analysis by Means of UV-Visible Spectrophotometry and Determination of Conjugated Dienes Figures 6 and 7 show the spectral analysis, which reveals an increase of the absorbencies measured for the oil samples without additives and no modification for the oils emulsified with onion extracts. The information ob- tained is present e d o n Table 2 and Table 3. Results obtained for the concentration of conjugated dienes were 9.02 × 10−6 mmol/ml for the corn oil without additives at the beginning of the experiment, rising to 1.37 × 10−5 mmol/ml on the 7th day of ageing, corres- ponding to a dienes index of 12.5 mmol/g and 22.2 mmol/g respectively. On the other hand, the oils emulsified with onion extracts presented a concentration of conjugated dienes of 1.16 × 10−5 mmol/ml in th e initial level and 1.14 × 10−5 mmol / ml on the 7th day. The conjugated dienes indexes 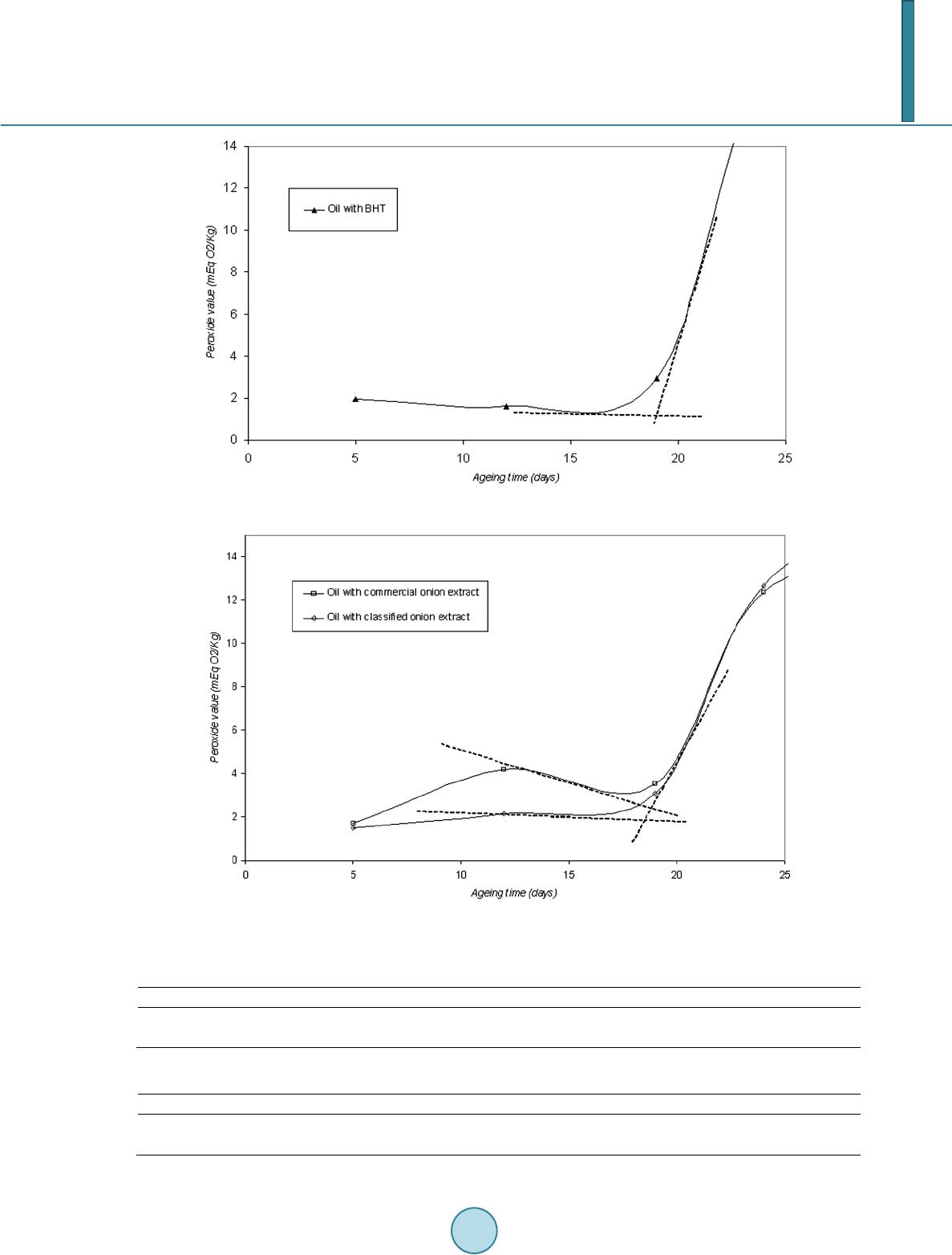 M. del Carmen Gutierrez et al. Figure 4. Evolution of the Peroxide Value for corn oil with BHT. Figure 5. Peroxide value evolution over time for emulsion obtained from the Allium cepa extracts in corn oil. Table 2. Sample absorbancies obtained at day 1. Samples Mass (g) Abs 233 nm Abs 268 nm Abs 278 nm Pure Oil 0.018 0.228 0.052 0.046 Oil + Extract Table 3. Sample absorbancies obtained at day 7. Samples Mass (g) Abs 233 nm Abs 268 nm Abs 278 nm Pure Oil 0.0155 0.347 0.066 0.059 Oil + Extract 0.0186 0.288 0.059 0.053 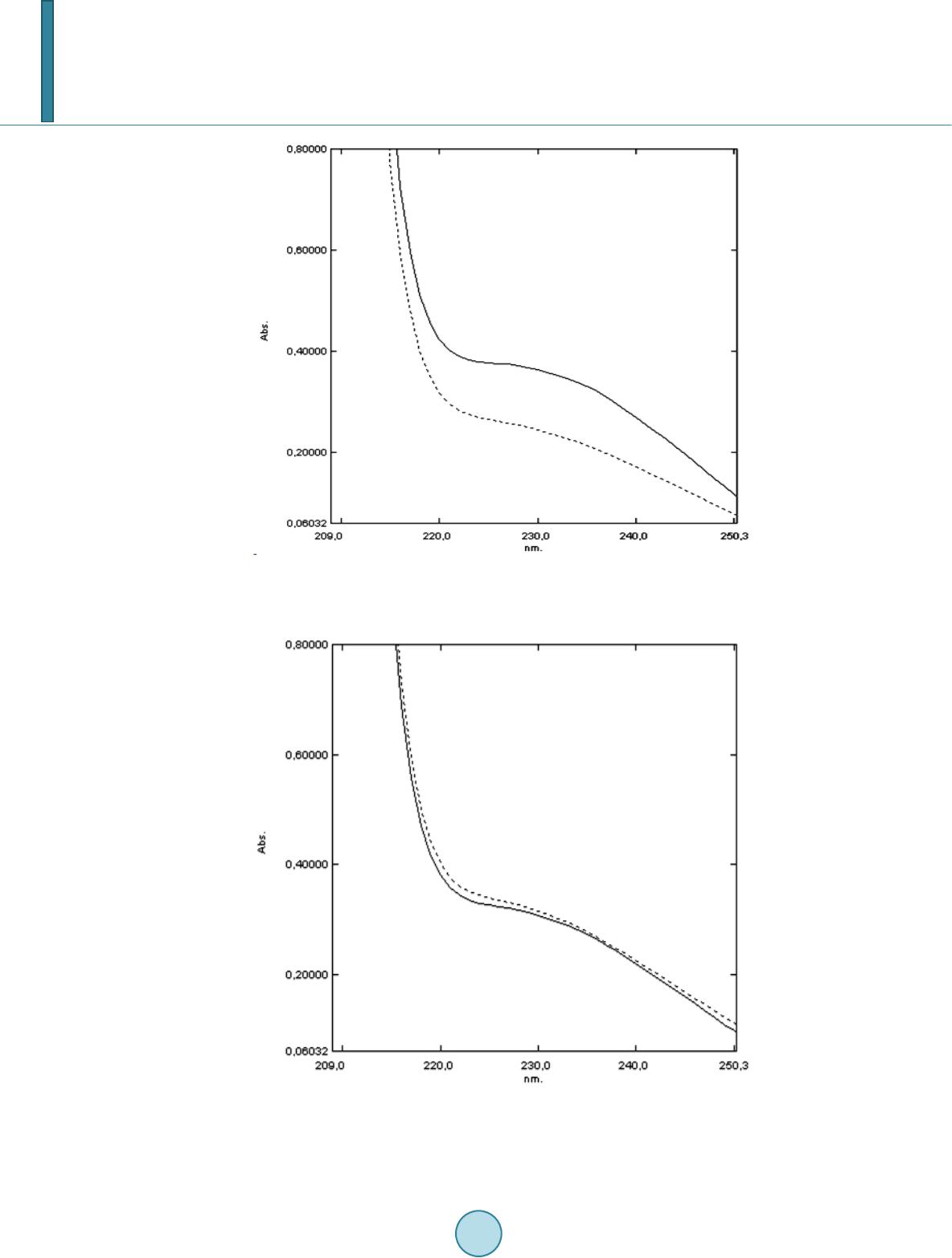 M. del Carmen Gutierrez et al. Figure 6. UV-Visible spectrophotometry for corn oil without antioxidant aggregates in its initial state (dotted line spectrophotometry) and after 7 days at a temperature of 45˚C (continuous line spectrophotometry). Figure 7. UV-Visible spectrophotometry for the emulsions of ethanolic extracts of onion (10% m/m) in commercial corn oil, in its initial state (dotted line spectropho- tometry) and after 7 days elapsed at a temperature of 45˚C (continuous line spectro- photometry).  M. del Carmen Gutierrez et al. were 14.9 µmol/g and 15.3 µmol/g respectively. Data shows the increase of concentration of conjugated dienes in the oil without antioxidants. Likewise no in- crease was found on oil samples with ethanolic onion extracts. Thus was demonstrated the antioxidant capacity of polyphenols extracted from some varieties of onion. 4. Conclusions The results obtained sugges t that commercial onions have a considerable content of polyphe n ols. In addition, the studies performed demonstrated the antioxidant power of their ethanolic extracts. Therefore, the use of commercial onions as sources to obtain polyphenolic substances and their applications as antioxidant additives can be con s idered entirely viable. Acknowledgements This resea rch was funded by National Tec h nological Universi t y, Buenos Ai re s Regional Facult y . Authors thank to Dr Isaac Marcos Cohen for his valuable contribution. References [1] Liu, R.H. (2003) Health Benefits of Fruit and Vegetables Are from Additive and Synergistic Combinations of Phyto- chemicals. American Journal of Clinical Nutrition, 78, 517S-520S. [2] Block, G. and Langseth, L. (1994) Antioxidant Vitamins and Disease Prevention. Food Technology, July, 80-84. [3] Hertog, M.G.L., Feskens, E.J.M., Hollman, P.C.H., Katan, M.B. and Kromhout, D. (1993) Dietary Antioxidant Flavo- noids and Risk of Coronary Heart Disease. The Zutphen Elderly Study. The Lancet, 342, 1007-1011. [4] Pokorny, J., Yanishlieva, N. and Gordon, M. (2001) Antioxidants in Food. Practical Applications. Woodhead Publish- ing Limited, Abington Hall, Abington, Cambridge, England. [5] Block, G. (1992) A Role for Antioxidants in Reducing Cancer Risk. Nutrition Review, 50, 207-213. http://dx.doi.org/10.1111/j.1753-4887.1992.tb01329.x [6] Shui, G. and Leong, L. (2006) Residue from Star Fruit as Valuable Source for Functional Food Ingredients and Anti- oxidant Nutraceuticals. Food Chemistry, 97, 277-284. http://dx.doi.org/10.1016/j.foodchem.2005.03.048 [7] Abuja, P., Murkovic, M. and Pfanhauser, W. (1998) Antioxidant and Prooxidant Activities of Eldeberry (Sambucus nigra) Extract in Low Density Lipoprotein Oxidation. Journal of Agricultural and Food Chemistry, 46, 4091-4096. http://dx.doi.org/10.1021/jf980296g [8] Wang, H., Nair, M.G., Strasburg, G.M., Chang, Y., Booren, A.M., Gray, I.J. and DeWitt, D.L. (1999) Antioxidant and Antiinflammatory Activities of Anthocyanins and Their Aglycon, Cyanidin, from Tart Cherries. Journal of Natural Products, 62, 294-296. http://dx.doi.org/10.1021/np980501m [9] Saleh, M., Hashem, F.A. and Glombitza, K.W. (1998) Study of Citrus taitensis and Ra dical Scavenger Activity of the Flavonoids Isolated. Food Chemistry, 63, 397-400. http://dx.doi.org/10.1016/S0308-8146(97)00238-0 [10] Dawes, H.M. and Keene, J.B. (1999) Phenolic Composition of Kiwi Fruit Juice. Journal of Agricultural and Food Chemistry, 47, 2398-2403. http://dx.doi.org/10.1021/jf9810261 [11] Romani, A., Mulinacci, N., Pinelli, P., Vincieri, F. and Cimato, A. (1999) Polyphenolic Content in Five Tuscany Cul- tivars of Olea europaea L. Journal of Agricultural and Food Chemistry, 47, 964-967. http://dx.doi.org/10.1021/jf980264t [12] Sanbongi, C., Osakabe, N., Natsume, M., Takizawa, T., Gomi, S. and Osawa, T. (1998) Antioxidative Polyphenols Isolated from Theobroma cacao. Journal of Agricultural and Food Chemistry, 46, 454-457. http://dx.doi.org/10.1021/jf970575o [13] Friedman, M. (1997) Chemistry, Biochemistry, and Dietary Role of Potato Polyphenols. A Review. Journal of Agri- cultural and Food Chemistry, 45, 1523-1540. http://dx.doi.org/10.1021/jf960900s [14] Abushita, A., Hebshi, E. and Biacs, P. (1997) Determination of Antioxidant Vitamins in Tomatoes. Food Chemistry, 60, 207-212. http://dx.doi.org/10.1016/S0308-8146(96)00321-4 [15] Aruoma, O.I., Spencer, J., Warren, D., Jenner, P., Butler, J. and Halliwell, B. (1997) Characterization of Food Anti- oxidants, Illustrated Using Commercial Garlic and Ginger Preparations. Food Chemistry, 60, 149-156. http://dx.doi.org/10.1016/S0308-8146(95)00254-5 [16] Lachman, J., Pronek, A., Hejtmánkova, A., Dudjak, J., Pivec, V. and Faitová, K. (2003) Total Polyphenol and Main Flavonoid Antioxidants in Different Onion (Allium cepa L.) Varieties. Czech University of Agriculture, Faculty of  M. del Carmen Gutierrez et al. Agriculture, Prague, Czech Republic. [17] Ganthavorn, C. and Hughes, J.S. (1997) Inhibition of Soybean Oil Oxidation by Extracts of Dry Beans (Phaseolus vulgaris). Journal of the American Oil Chemists’ Society, 74, 1025-1030. http://dx.doi.org/10.1007/s11746-997-0020-5 [18] Markus, F., Daood, H., Kapitany, J. and Biacs, P.A. (1999) Change in the Carotenoid and Antioxidant Content of Spice Red Pepper (Paprika) as a Function of Ripening and Some Technological Factors. Journal of Agric ultural and Food Chemistry, 47, 100-107. http://dx.doi.org/10.1021/jf980485z [19] Kikuzaki, H. and Nakatani, N. (1993) Antioxidant Effects of Ginger Constituents. Journal of Food Science, 58, 1407- 1410. http://dx.doi.org/10.1111/j.1365-2621.1993.tb06194.x [20] Hall, C. and Cuppett, S. (1997) The Hydrogen Donating Mechanism of Rosmariquinone. Section 37 General Lipid Oxidation and Quality I, Presentado en: American Oil Chemist’s Society 88th Annual Meeting and Exposition, Seattle, WA, Mayo 11-14 . [21] Kaur, Ch., Joshi, S. and Kapoor, H.C. (2009) Antioxidants in Onion (Allium cepa L.) Cultivars Grown in India. J our- nal of Food Biochemistry, 33, 184-200. http://dx.doi.org/10.1111/j.1745-4514.2009.00212.x [22] Ye, C.-L., Dai, D.-H. and Hu, W.-L. (2013) Antimicrobial and Antioxidant Activities of the Essential Oil from Onion (Allium cepa L.). Food Control, 30, 48-53. http://dx.doi.org/10.1016/j.foodcont.2012.07.033 [23] Albishi, T., John, J. A., Al-Khalifa, A.S. and Shahidi, F. (2013) Antioxidant, An ti-Inflammatory and DNA Scission In- hibitory Activities of Phenolic Compounds in Selected Onion and Potato Varieties. Journal of Functional Foods, 5, 930-939. http://dx.doi.org/10.1016/j.jff.2013.02.005 [24] Budic-Leto, I., Lovri, T., Pezo, I. and Gajdo Kljusuri, J. (2005) Study of Dynamics of Polyphenol Extraction During Traditional and Advanced Maceration Processes of the Babic Grape Variety. Food Technology and Biotechnology, 43, 47-53. [25] Nawaz, H., Shi, J., Mittal, G.S. and Kakudac, Y. (2006) Extraction of Polyphenols from Grape Seeds and Concentra- tion by Ultrafiltration. Separation and Purification Technology, 48, 176-181. http://dx.doi.org/10.1016/j.seppur.2005.07.006 [26] Zill-e-Huma, M.A.V., Fabiano-Tixier, A.-S., Elmaataoui, M., Dangles, O. and Chemat, F. (2011) A Remarka ble Influ- ence of Microwave Extraction: Enhancement of Antioxidant Activity of Extracted Onion Varieties. Food Chemistry, 127, 1472-1480. http://dx.doi.org/10.1016/j.foodchem.2011.01.112 [27] Cao, G., Sofic, E. and Prior, R.L. (1996) Antioxidant Capacity of Tea and Common Vegetables. USDA-ARS, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington Street, Boston, Mas- sachusetts 02111, and Nutritional Science Department, University of Connecticut, Storrs, Connecticut 06269J. Agric. Food Chem., 44, 3426-3431. [28] AOCS (American Oil Chemists’ Society) (1998) Official method Cd 8-53. Peroxide Value. In Firesto ne, D., Ed., Offi- cial Methods and Recommended Practices of the American Oil Chemists’ Society, 5th Edition, AOCS, Champaign, III. [29] Current Protocols in Food Analytical Chemistry, D2.1.1-D2.1.15 (2001) John Wiley & Sons, Inc.
|