Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 125-133 doi:10.4236/jbnb.2011.22016 Published Online April 2011 (http://www.SciRP.org/journal/jbnb) Copyright © 2011 SciRes. JBNB Preparation, Properties, and Cell Attachment/ Growth Behavior of Chitosan/Acellular Derm Matrix Composite Materials Tao Lu1, Ruixin Li2, Yan Zhang2, Yuxian Yan2, Yong Guo2, Jing Guan2, Jimin Wu2, Zhihong Li2, Bo Ning2, Shujie Huang2, Xizheng Zhang2,* 1Affiliated Hospital of Chinese People’s Armed Police Force Medical College, Tianjin, China; 2Institute of Medical Equipment, Tianjin, China. Email: z84656716@yahoo.com Received December 3rd, 2010; revised January 1st, 2011; accepted January 9th, 2011. ABSTRACT Composite membranes and sponge scaffolds consisting chitosan (CS) and acellular derm matrix (ADM) in six ratios were prepared by solvent evaporation technique and freeze-drying method, respectively. The composite materials were characterized by water contact angle measurement, scanning electron microscopy (SEM), water absorption and HaCat cells compatibility. The SEM result showed that CS/ADM three-dimensional (3D) micro-porous structures were suc- cessfully produced. The water absorption value of all scaffolds was over 18 times of its initial weight, which is high enough for skin regeneration scaffold, but there were no significant differences of water absorption ratio between de- ionized water and PBS solution for same scaffold (P > 0.05). HaCat cells were distributed uniformly on the surfaces of membrane 4 - 6, and an almost confluent monolayer was formed on membrane 6 on the fifth day, whereas cells main- tained round and spherical in shape on the surface of membrane 1. The results showed that the cell compatibility of pure CS membrane needed to be improved, and addition of ADM realized this purpose. The results of compatibility of HaCat cells on scaffolds showed that the cell proliferated well on the scaffolds 3 and 4. In our study, the cell’s attach- ment and growth on the composite membranes was mainly determined by the content of the membrane, whereas the cell’s attachment and growth in the scaffolds was determined by both the content and structure of the scaffolds. Keywords: Chitosan, Acellular Derm Matrix, Membrane, Scaffolds, HaCat Cell Compatibility 1. Introduction Skin being the largest and most highly complex organ in the human body is the most affected organ in injuries [1]. Every thing is done to reduce risks for health, especially from the growing number of synthetic compounds and new formulations [2]. Skin corrosivity testing in vivo may cause severe discomfort and pain to test animals. Therefore, many attempts have been made to replace the in vivo test in laboratory animals. Skin corrosion is de- fined as the production of irreversible tissue damage in the skin following the application of a test material (OECD, 2002) [3]. The new OECD Test Guideline 431 “In Vitro Skin Corrosion” (OECD, 2004) [4] defines the requirements for in vitro skin models to be validated for skin corrosivity testing and defines general and func- tional model conditions that need to be evaluated before the skin models will be routinely used. The most impor- tant general conditions are a multi-layered, functional stratum corneum with the necessary lipid profile, and absence of any contamination. The most important func- tional conditions specified in TG 431 are a stable and sufficiently high cell viability (expressed as metabolic conversion capacity), a sufficient resistance to a slowly penetrating cytotoxic marker chemical, reproducibility of data over time and between laboratories, and finally, ca- pability to correctly classify twelve reference chemicals specified in TG 431. But in China, only a few works have been done about it, so we try to reconstruct a new human epidermal model scaffold. Chitosan (CS) is an abundant, naturally occurring polysaccharide obtained by deacetylation of natural chi- tin [5]. It is biocompatible, biodegradable, easily formed into structures under mild processing conditions and can be chemically modified, so it is a natural choice as drug- delivery carrier [6,7], cartilage/skin tissue engineering  Preparation, Properties, and Cell Attachment/Growth Behavior of Chitosan/Acellular 126 Derm Matrix Composite Materials scaffolds [8-10], and regenerative membrane [11]. The physical properties of a polymer can be altered by intro- ducing a second polymer that improves the properties of the original polymer in certain aspects, such as hydro- phobility, cell compatibility. CS and some of its com- plexes have been studied for a number of biomedical applications, including wound dressings, drug delivery systems and space-filling implants [12-18]. In a study comparing purified collagen, naturally occurring ex- tracellular matrix (ECM) scaffolds, and synthetic scaf- fold materials for in vitro endothelial cell attachment [19], it was found that ECM possessed the ability to recruit circulating marrow-derived progenitor cells and attract mature endothelial cells from selected organs such as the heart and liver to promote successful vascularization of engineered tissue structures. These studies reveal that extracellular components in a cell-free or acellular derm matrix (ADM) are critical for success in biomedical ap- plications as scaffolds. HaCat cells, a human keratinocyte line, are commonly utilized as an in vitro cell model for toxicity testing and the discernment of process of chemi- cally induced skin carcinogenesis. In this study, we prepared CS/ADM composite mem- branes and scaffolds by solvent evaporation technique and freeze-drying method, respectively, and investigated the characteristics of composite materials by water con- tact angle measurement, scanning electron microscopy (SEM) observation, water absorption ratio and HaCat cells compatibility test. 2. Methods 2.1. Materials CS (Mw ≈ 20 000, degree of deacetylation of 75% - 85%) was purchased from the Sigma Chemical Company (Sigma-Aldrich Co.Ltd., USA) and used without further purification. Dulbecco’s modified eagle’s medium (DMEM), fetal calf serum (FCS) and trypsin-EDTA (1×) were purchased from Gibco Laboratories (Invitrogen Corporation, CA, USA). All other chemicals were of analytical grade and used without further purification. 2.2. Preparation of CS/ADM Composite Membranes and Scaffolds ADM was prepared as follows: Porcine skin of 0.3 - 0.4 mm in thickness was obtained by removing the epider- mal. It was digested in 0.25% trypsin solution at 37℃ for 24 h to remove the epidermis and other cellular com- ponents. The remaining dermis was then immersed in 0.5% Triton X-100 solution for 36 h with continuous shak- ing to further remove cellular components, and subse- quently washed with deionized water to obtain ADM. ADM particles was prepared by milling the prepared ADM. Acetic acid was used as a solvent. To avoid the ADM particles aggregation, an alternative method was devel- oped. First, ADM particles were scattered in water by stirring, and then CS powder, not solution, was added with strong stirring to ensure that the powder was uni- formly mix with ADM particles. Finally, acetic acid was added to the solution. Since the CS powder was already uniformly dispersed, the addition of acetic acid caused the CS powder to immediately dissolve, thus avoiding the aggregation caused by ADM particles. Therefore, a homogeneous solution was obtained. The proportions of CS powder and ADM particles are listed in Tab.1. The resulting solution was allowed to stand at 4℃ until all air bubbles had disappeared. Composite membranes were prepared by solvent evaporation technique as follows: the mixture solution was cast into cell culture plates and allowed to dry at ambient temperature to form the composite membranes. The prepared composite membranes were neutralized with 1 wt.% aqueous NaOH solution for 30 min and subsequently washed with deionized water to remove the remaining NaOH. Composite porous scaffolds were pre- pared by using the same mix solutions: the mixture solu- tion was cast into cell culture plates and frozen at –20℃, and the composite scaffolds were prepared by freeze- drying method. The prepared composite scaffolds were neutralized with 1 wt.% aqueous NaOH solution for 2 h and subsequently washed with deionized water to remove the remaining NaOH. All the membranes and scaffolds were sterilized before seeding cells. 2.3. Water Contact Angles and Wettability of Composite Membranes The water contact angles of the surface of the composite membranes were measured with HARKE SPCA contact angle goniometer (Beijing Harke Instrument Company). Distilled water was deposited on the surface of the sam- ples with an automatic pipette. After being deposited, all contact angle measurements were taken within 20 s. With a digital camera a computer image of the drop was de- termined, and the water contact angle θ was calculated according this formula: tg (θ/2) = h/r, where h is the high of the water drop and r is the contact radius between wa- ter drop and the bottom. Figure 1 shows the schematic diagram of water contact angle. All measurements were r h θ/2 Figure 1. Schematic diagram of water contact angle. Copyright © 2011 SciRes. JBNB  Preparation, Properties, and Cell Attachment/Growth Behavior of Chitosan/Acellular 127 Derm Matrix Composite Materials taken at an ambient temperature (25℃) and they were repeated four times for each sample. 2.4. H.E staining of Sponge Scaffold Scaffold samples were first dehydrated with an increas- ing series of alcohol concentrations (30%, 50%, 70%, 90%, 100%) and then embedded in paraffin. Paraffin- embedded samples were sectioned at a thickness of 5 mm. After removing the paraffin, samples were stained with hematoxylin and eosin. After sealing, samples were ex- amined by light microscopy to inspect the degree of acelluar. 2.5. SEM Observation For SEM observation, the specimens were fixed with 1.5% glutaraldehyde in 0.14 m sodium cacodylate buffer (pH 7.3), then dehydrated in graded alcohols, critical- point dried, sputter-coated with gold and analyzed in a SEM equipped (HITACHI S-3400N) at an accelerating voltage of 30 KV and current of 119 µA. 2.6. Water Absorption Ratio of Sponge Scaffold The CS/ADM composite scaffolds were incubated in deionized water and PBS solution at 37℃ for 24 h, and swelling continued to reach constant weight of the sam- ple. Before weighing the sample, surface water was re- moved with filter paper. The water absorption ratio (R) was calculated by the following equation: R = (Ws − Wi) / Wi where Ws is the weights of swollen state and Wi is the weight of initial sample (before being immersed in water or PBS). Each value was averaged from three parallel meas- urements. 2.7. Cell Compatibility HaCat cells, an immortalized but non-tumorigenic human epidermal keratinocyte cell-line which retain their dif- ferentiation potential [20], were routinely cultured in high glucose DMEM medium supplemented with 10% (v/v) fetal calf serum, 10 µg/ml of streptomycin and 10 U/ml of penicillin, and incubated at 37℃ in a 5% CO2, 95% air humidified atmosphere. 2.7.1. Cell Compatibility of Membranes HaCat cells were detached from the culture plated with 0.25% Trypsin, centrifuged, and then suspended in me- dium. The cell concentration was determined by manual count with a hemocytometer. 350 µl cells suspension at density of 12 × 104/ml was seeded to each well (6-well culture plate) with composite membranes covered bottom and four wells for each sample. Cell proliferation was measured at 1, 3, 5, 7 days using the 3-(4, 5-dimethyl- thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay (Tianjin Runtai Reagent Company). Each sample was measured at 8 times. H.E staining also applied to the cultural plates with cells. 2.7.2. Cell Compatibility of Scaffolds Scaffolds were sliced into (0.0040 ± 0.0001)gram pieces, and sterilized before use. 100 µl cells suspension at density of 10 × 104/ml were seeded to each small pre- wetted scaffold, and four scaffolds for each kind of sam- ple. Cell proliferation was measured at 1, 3, 5, 7 days using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay in TECAN Elisa Reader. 2.8 Statistical Analysis Differences between groups were analyzed by the SARS (version 11.0, http://www.pinggu.org/bbs/a-385796.html). Differences were considered to be significant at P < 0.05. 3. Results and Discussion 3.1. Water Contact Angles of CS/ADM Composite Membranes The material–cell interaction is affected by many factors, such as wettability (hydrophilicity/hydrophobility), sur- face free energy, chemistry, charge, roughness, and ri- gidity. The surface properties of scaffold are critical for its application because the surface of the scaffold is the place where the material interacts with the bioenviron- ment and where the cells attach and proliferate. Hydro- philicity is an important characteristic property for bio- materials. To determine the hydrophilicity of the mem- branes, their contact angles were investigated. The higher contact angle indicates lower hydrophilic- ity. The water contact angles on the membranes are shown in Figure 2. As a consequence, the contact angle 0 10 20 30 40 50 60 70 80 123456 w ater c ontact angle(degr ee) 0s 3s 6s 9s 12s 15s 18s *Significance is indicated: P < 0.05 vs. sample 1 Figure 2. Water contact angle of composite membranes (n = 4). Copyright © 2011 SciRes. JBNB  Preparation, Properties, and Cell Attachment/Growth Behavior of Chitosan/Acellular 128 Derm Matrix Composite Materials of all membranes decreased from 0s to 18s. In addition, the contact angle of membrane 1 to 5 exhibited no sig- nificant difference, and membrane 6 exhibited the high- est contact angle, which was primarily due to the surface become rougher with small ADM fibers scattered in the CS. Although the wettability of membrane was changed by blending with ADM, the contact angles for all the materials were less than 70°, indicating that all these ma- terials had good hydrophilicity. Additionally, by comparing the data at different meas- uring times, we found that the contact angles decreased with time, indicating that the hydrophilicity of the matri- ces increased with the absorption of water. The contact angle of membrane has been considered to be one of the physical parameters which related the af- finity between cells and the matrix membranes. However, no correlation between the initial cell anchoring rate and the wet contact angle was found for the HaCat cells in the present study. 3.2. H.E Staining of Scaffold H.E staining photograph of scaffold 4 is shown in Figure 3, in which whole cells are absent from the scaffolds. This indicated the preparation of acellular derm matrix was successful. 3.3. SEM Observation CS is a crystalline polysaccharide and is normally in- soluble in aqueous solutions above pH 7. However, in dilute acids (pH < 6), the free amino groups are proto- nated and the molecule becomes soluble. This pH-de- pendent solubility provides a convenient mechanism for processing under mild conditions [21]. Figure 4 shows the SEM image of scaffold 1, 4 and 5. This image re- vealed the freeze-drying process generated an open pore microstructure with a high degree of interconnectivity. But with the increasing of ADM content, the pore uni- formity decreased. Pure chitosan scaffold had the best pore structure. Figure 3. H.E staining of scaffold 4 (× 100). Figure 4. SEM image of scaffold 1, 4, 5. 3.4. Water Absorption Ratio of Composite Scaffolds The ability of a scaffold to preserve water is an important property for skin regeneration. The water absorption ra- tios of various scaffolds are shown in Table 2. The water absorption ability of the CS/ADM scaffold could be at- tributed to both of their hydrophilicity and the mainte- nance of their three-dimensional structure. Differences between sample 1 and other groups were analyzed by the SARS and the result was P < 0.05. So, there were sig- Copyright © 2011 SciRes. JBNB  Preparation, Properties, and Cell Attachment/Growth Behavior of Chitosan/Acellular 129 Derm Matrix Composite Materials nificant differences of water absorption ratio between pure CS scaffold and composite scaffolds. The main reason was the pore structure of pure CS was the best, which could be proved by SEM images in Figure 4. In addition, differences between deionized water and PBS solution groups were analyzed by the SARS and the re- sult was P > 0.05 for each sample. So, there were no sig- nificant differences of water absorption ratio between deionized water and PBS solution for same scaffold. The scaffold provides a necessary template and physi- cal support to guide the differentiation and proliferation of cells into targeted functional tissues or organs. The scaffold should contain its three-dimensional structures in liquid culture medium. It should absorb body fluid for transfer of cell nutrients and metabolites through the ma- terial [22]. Transport issues such as nutrient delivery, waste removal, protein transport, gaseous exchange, and general vascularization and guided tissue regeneration are governed by the pore structure of the scaffold [23]. In our study, the absolute water absorption value of all scaffolds was over 18 times of its initial weight, which is high enough for skin regeneration scaffold. This result is similar with other studies on CS scaffolds. Ma et al. [22] fabricated freeze-dried CS-collagen scaffolds that were crosslinked with glutaric dialdehyde (GTA). They found that crosslinking decreased the swelling ratio from 16 to 8. 3.5. Morphology of HaCat Cell Figure 5 shows the morphology of HaCat cells on cul- ture plate photographed by a phase contrast microscope. Cells exhibited flagstone shape and form a confluent after being cultured for 3 days. 3.6. Morphology and Proliferation of HaCat Cell on the Surface of Composite Membranes 3.6.1. Morphology of HaCat Cell Adhered on the Surface of Composite Membranes Prepared pure CS membrane was transparent, whereas composite membranes were opaque with white small fibers scattered in the membranes. In our study, the pre- pared membranes stick to the culture plates tightly, so that the cells could adhere on the surface of the mem- brane instead of on the culture plates. The cytocompatibility of the matrices is very impor- tant for their applications. Since it is the biomaterial sur- face that first comes into contact with the living tissue when the biomaterial is planted in the body, the initial response of the body to the biomaterial depends on its surface properties. Surface properties that can influence biocompatibility include surface charge, surface topog- raphy, etc [24-29]. 1 d × 40 3 d × 40 3 d × 100 Figure 5. Phase contrast images of HaCat cell on culture plates. To examine the cell-matrix interactions, HaCat cells were cultivated on the surface of the composite mem- branes, their morphology and distribution were observed and photographed by a phase contrast microscope on day 1, 3, 5, 7 d. Figure 6 and Figure 7 show the morphology of HaCat cell on the composite membrane on day 1 and day 7, respectively. The micrographs of a 1-day culture reflect the status of HaCat cell attachment and spreading. Figure 6 shows that the attached cells on membrane 1 (pure CS membrane) were round and spherical in shape. Whereas the cells on the other membranes were flat, po- lygonal, and the difference of membrane 2 to 6 in spread- ing was not distinct. Chen et al. [30] concluded that cells Copyright © 2011 SciRes. JBNB 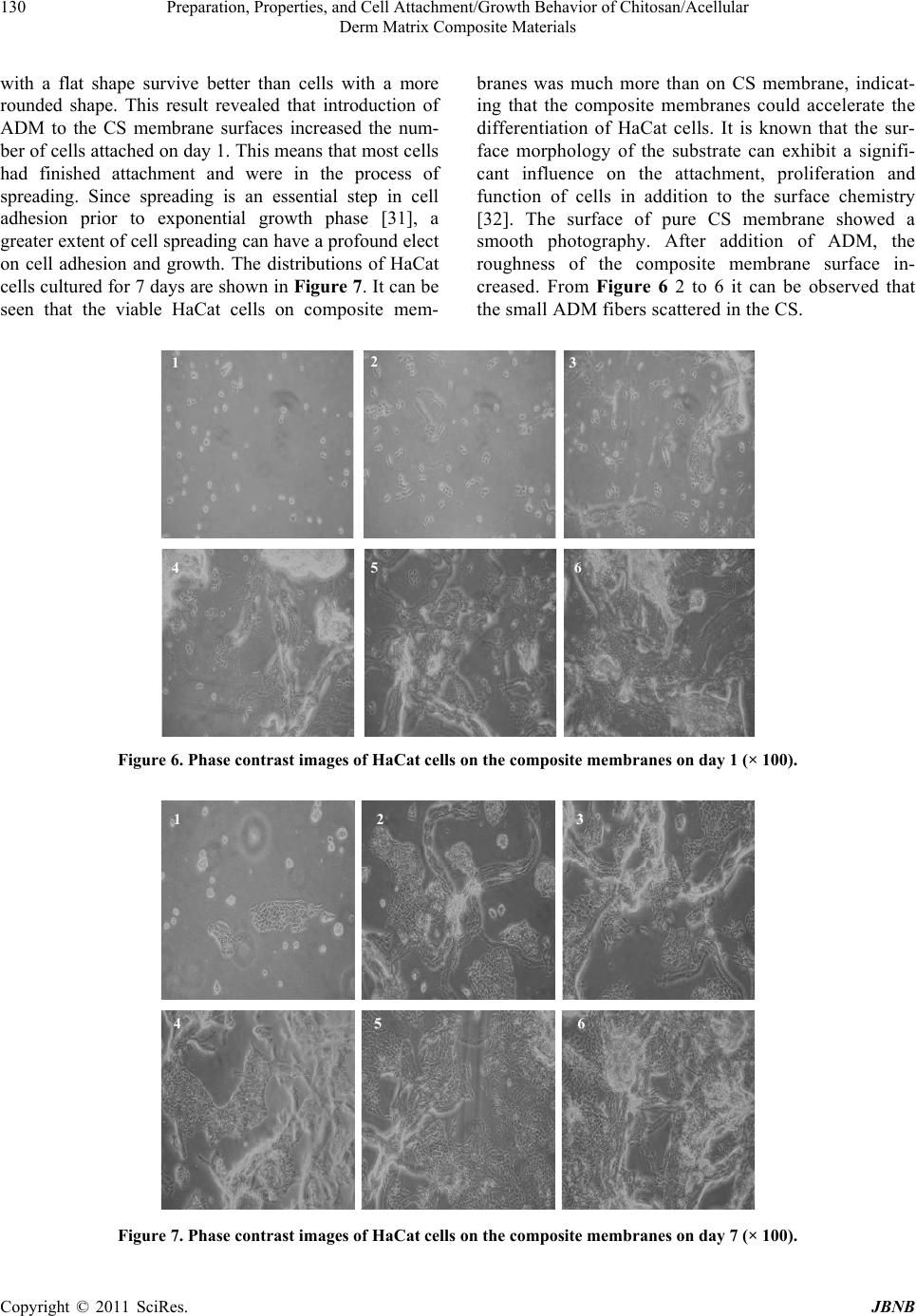 Preparation, Properties, and Cell Attachment/Growth Behavior of Chitosan/Acellular Derm Matrix Composite Materials Copyright © 2011 SciRes. JBNB 130 with a flat shape survive better than cells with a more rounded shape. This result revealed that introduction of ADM to the CS membrane surfaces increased the num- ber of cells attached on day 1. This means that most cells had finished attachment and were in the process of spreading. Since spreading is an essential step in cell adhesion prior to exponential growth phase [31], a greater extent of cell spreading can have a profound elect on cell adhesion and growth. The distributions of HaCat cells cultured for 7 days are shown in Figure 7. It can be seen that the viable HaCat cells on composite mem- branes was much more than on CS membrane, indicat- ing that the composite membranes could accelerate the differentiation of HaCat cells. It is known that the sur- face morphology of the substrate can exhibit a signifi- cant influence on the attachment, proliferation and function of cells in addition to the surface chemistry [32]. The surface of pure CS membrane showed a smooth photography. After addition of ADM, the roughness of the composite membrane surface in- creased. From Figure 6 2 to 6 it can be observed that the small ADM fibers scattered in the CS. 1 6 5 4 3 2 Figure 6. Phase contrast images of HaCat cells on the composite membranes on day 1 (× 100). 1 654 32 Figure 7. Phase contrast images of HaCat cells on the composite membranes on day 7 (× 100).  Preparation, Properties, and Cell Attachment/Growth Behavior of Chitosan/Acellular 131 Derm Matrix Composite Materials In order to visualize cell morphologies on different membrane surfaces, cells and membranes were stained using Hematoxylin and Eosin Staining Kit (H.E) and observed with a microscope (BX51, Olympus, Japan). Figure 8 shows a series of images of HaCat cells ad- hered onto the membranes which was stained red. HaCat cells were distributed uniformly on the surfaces of mem- brane 4-6, and an almost confluent monolayer was formed on membrane 6 on the fifth day, whereas cells maintained round and spherical in shape on the surface of membrane 1. The results showed that the cell compatibil- ity of pure CS membrane needed to be improved, and addition of ADM realized this purpose. 3.6.2. Proliferation of HaCat Cells on the Surface of Composite Membrane MTT assay was used as a measure of relative cell viabil- ity. HaCat cells were cultured on the membranes for 1, 3, 5, 7 d, and cell proliferation was determined by the MTT method. Figure 9 shows the viability of HaCat cell on different surfaces with different cultural time. There was significant enhancement of cell proliferation and viability on the composite membranes compared with that on pure CS membrane (p < 0.05). Also, cells proliferation rate on membrane 6 was the highest, the reason perhaps was that the content of ADM was similar to the ECM environ- ment in vivo. In addition, cell adhesion on the surfaces increased and then decreased with a prolonged time pe- riod of culture. The cells formed a confluent monolayer on day 5, and their proliferation were inhibited in the following day resulted in a decrease of viability. From the results of cell culture, we can conclude that compos- ite film is bioactive to HaCat cells by increasing the at- tachment, spreading and proliferation of the cells. This trend was not consistent with their surface hydrophilicity indicated by the water contact angle. The present works revealed the attachment and growth behavior of HaCat mainly depend on the content of the membrane, not the contact angle of the membrane. 3.7. Proliferation of HaCat Cells in the Composite Scaffolds HaCat cells were cultured in the sponge scaffolds for 1, 3, 5, 7 d, and cell proliferation was determined by MTT method. The result of MTT test is shown in Figure 10. It was clear that HaCat cells proliferated well on the scaf- folds 3 and 4. On the other hand, the increasing ADM of Figure 8. H.E Staining images of HaCat on composite membranes on day 5. 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 1d 3 d 5 d 7d 1 2 3 4 5 6 Figure 9. Proliferation of HaCat cells on the surface of composite membrane. 0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 1 d 3 d 5 d 7 d 1 2 3 4 5 6 Figure 10. Proliferation of HaCat cells in the composite scaffolds. Copyright © 2011 SciRes. JBNB  Preparation, Properties, and Cell Attachment/Growth Behavior of Chitosan/Acellular 132 Derm Matrix Composite Materials sample 5 and 6 scaffolds resulted in lower numbers of HaCat cells. This trend was not consistent with the statis- tical results of cell proliferation on membranes. The rea- son was mainly that the cell growth depended on both material content and structure of the sponge scaffolds. Control of scaffold pore morphology is critical for con- trolling cellular colonization rates within cell scaffold co-culture in vitro. 4. Conclusions In this work, six composite membranes were prepared by solvent evaporation technique and six composite scaf- folds were prepared by freeze-drying method. The effect of ADM on the characteristics of composite membranes and scaffolds were investigated by static water contact angle measurements and water absorption. The water absorption value of all scaffolds was over 18 times of its initial weight, which is high enough for skin regeneration scaffold, but there were no significant differences of wa- ter absorption ratio between deionized water and PBS solution for same scaffold (P > 0.05). The scaffolds morphology were observed by SEM, and the results showed that CS/ADM three-dimensional (3D) micro- porous structures were successfully produced. Cell com- patibility of the composite membranes and scaffolds were analyzed by HaCat cells co-culture in vitro. The cell compatibility results revealed that composite mem- branes with higher levels of ADM content provided much better substrates in terms of cell attachment, growth and morphology. But in the scaffolds, the cell proliferation was determined not only by the hydrophil- city and content, but also by the structure of the scaffolds. As a consequence, a composite material includes mem- brane 6 and scaffolds 4 will be the best materilas for HaCat prolifferation. This study was the basic work for further investigation, a new human epidermal model composite material includes membrane and sponge scaf- fold will be reconstructed and the effect of 12 reference chemical substances in OECD TG 43 on the HaCat vi- ability in composite material will be investigated in our future research. 5. Acknowledgements The research was supported by Key Project of AMMS Innovation Fund (2008ZD09) and General Program of Natural Science Foundation of Tianjin (07JCYBJC05100). REFERENCES [1] K. Helena, L. Manfred and S. Horst, “Assessment of the Human Epidermis Model Skin Ethic RHE for in Vitro Skin Corrosion Testing of Chemicals According to New OECD TG 431,” Toxicology in Vitro, Vol. 20, 2006, pp. 547-559. doi:10.1016/j.tiv.2005.11.008 [2] J. Hoffmann, E. Heisler and S. Karpinski, “Epidermal- skin-test 1000 (EST-1000)—A new Reconstructed Epi- dermis for in Vitro Skin Corrosivity Testing,” Toxicology in Vitro, Vol. 19, 2005, pp. 925-929. doi:10.1016/j.tiv.2005.06.010 [3] Organisation for Economic Co-operation and Develop- ment, “Acute Dermal Irritation/Corrosion,” OECD Guideline for the testing of Chemicals, No. 404, Organi- sation for Economic Cooperation and Development, Paris, France, 2002. [4] Organisation for Economic Co-operation and Develop- ment, “In Vitro Skin Corrosion: Human Skin Model Test,” OECD guideline for testing of chemicals, No. 431, Organisation for Economic Co-operation and Develop- ment, Paris, France, 2004. [5] J. K. F. Suh and H. W. T. Matthew, “Application of Chi- tosan-Based Polysaccharide Biomaterials in Cartilage Tissue Engineering: A Review,” Biomaterials, Vol. 21, 2000, pp. 2589-2598. doi:10.1016/S0142-9612(00)00126-5 [6] E.B. Denkbaş and M. Odabaşi, “Chitosan Microspheres and Sponges: Preparation and Characterization,” Journal of Applied Polymer Science, Vol. 76, 2000, pp. 1637- 1643.doi:10.1002/(SICI)1097-4628(20000613)76:11<163 7::AID-APP4>3.0.CO;2-Q [7] K. G. H. Desai and H. J. Park, “Preparation and Charac- terization of Drug-Loaded Chitosan–Tripolyphosphate Microspheres by Spray Drying,” Drug Development Re- search, Vol. 64, 2005, pp. 114-128. doi:10.1002/ddr.10416 [8] Iyabo Adekogbe and Amyl Ghanem, “Fabrication and Characterization of DTBP-Crosslinked Chitosan Scaf- folds for Skin Tissue Engineering,” Biomaterials, Vol.26, 2005, pp. 7241- 7250. doi:10.1016/j.biomaterials.2005.05.043 [9] P. J. Vandevord, H. W. T. Matthew, S. P. Desilva, l. Mayton, B. Wu and P. H. Wooley, “Evaluation of the Biocompatibility of a Chitosan Scaffold in Mice,” Jour- nal of Biomedical Materials Research, 2002, Vol. 59, pp. 585-90. doi:10.1002/jbm.1270 [10] L. Ma, C.Y. Gao, Z. Mao, J. Zhou, J. Shen and X. Hu, “Han C: Collagen/Chitosan Porous Scaffolds with Improved Bio- stability for Skin Tissue Engineering,” Biomaterials, Vol. 24, 2003, pp. 4833-4841. doi:10.1016/S0142-9612(03)00374-0 [11] A. N. L. Rocha, T. N. C. Dantas, J. L. C. Fonseca and M. R. Pereira, “Permeation of Drugs in Chitosan Mem- branes,” Journal of Applied Polymer Science, Vol. 84, 2002, pp. 44-49. doi:10.1002/app.10185 [12] H. Ueno, H. Yamada, I. Tanaka and N. Kaba, Matsuura M, Okumura M, Kadosawa T and T. Fujinaga, “Accelerating Effects of Chitosan for Healing at Early Phase of Experi- mental Open Wound in Dogs,” Biomaterials, Vol. 20, 1999, pp. 1407-1414. doi:10.1016/S0142-9612(99)00046-0 [13] C. Xiao, S. Gao, H. Wang and L. Zhang, “Blend Films from Chitosan and Konjac Glucomannan Solutions,” Journal of Applied Polymer Science, Vol. 76, 2000, pp. 509-515. Copyright © 2011 SciRes. JBNB  Preparation, Properties, and Cell Attachment/Growth Behavior of Chitosan/Acellular Derm Matrix Composite Materials Copyright © 2011 SciRes. JBNB 133 doi:10.1002/(SICI)1097-4628(20000425)76:4<509::AID- APP8>3.0.CO;2-2 [14] T. W. Chung, Y. F. Lu, S. S. Wang, Y. S. Lin and S. H. Chu, “Growth of Human Endothelial Cells on Photo- chemically Grafted Gly-Arg-Gly-Asp (GRGD) Chito- sans,” Biomaterials, Vol. 23, 2002, pp. 4803-4809. doi:10.1016/S0142-9612(02)00231-4 [15] Park Y. J., Lee M. Y., Lee J. Y., Seol Y. J., Chung C. P. and Lee S. J., “Controlled Release of Platelet-Derived Growthfactor-BB from Chondroitin Sulfate-Chitosan Sponge for Guided Bone Regeneration,” Journal of Con- trolled Release, Vol. 67, 2000, pp. 385-394. doi:10.1016/S0168-3659(00)00232-7 [16] T. Suzuki, Y. Mizushima, T. Umeda and R. Ohashi, “Further Biocompatibility Testing of Silica-Chitosan Complex Membrane in the Production of Tissue Plasmi- nogen Activator by Epithelial and Fibroblast Cells,” Jour- nal of Bioscience and Bioengineering, 1999, Vol. 88: pp. 194-199. doi:10.1016/S1389-1723(99)80201-1 [17] I. Yamaguchi, S. Itoh, M. Suzuki, M. Sakane, A. Osaka and J. Tanaka, “The Chitosan Prepared from Crab Tendon I: The Characterization and the Mechanical Properties,” Biomaterials, Vol. 24, 2003, pp. 2031-2036. doi:10.1016/S0142-9612(02)00633-6 [18] J. Y. Lee, S. H. Nam, S. Y. Im, Y. J. Park, Y. M. Lee, Y. J. Seol, C. P. Chung and S. J. Lee, “Enhanced Bone Forma- tion by Controlled Growth Factor Delivery from Chitosan- Based Biomaterials,” Journal of Controlled Release, Vol. 78, 2002, pp. 187-197. doi:10.1016/S0168-3659(01)00498-9 [19] S. F. Badylak, F. Ling and R. Record and J. Hodde, “Vas- cularization of 3-Dimensional Scaffolds. Symposium on Tissue Engineering Science,” Aegean Conferences Series, Vol. 4, 2002, p. 63. [20] P. Boukamp, R. T. Petrussevska, J. Hornung, A. Mark- ham and N.E. Fusenig, “Normal Keratinization in a Spon- taneously Immortalised Aneuploid Human Keratinocyte cell Line,” The Journal of Cell Biology, Vol. 106, 1988, pp. 761-771. doi:10.1083/jcb.106.3.761 [21] V. Sundararajan, Madihally and Howard W.T. Matthew. “Porous Chitosan Scaffolds for Tissue Engineering,” Bio- materials, Vol. 20, 1999, pp. 1133-1142. [22] J. Ma, H. Wang, B. He and J. Chen, “A Preliminary in Vitro Study on the Fabrication and Tissue Engineering Ap- plications of a Novel Chitosan Bilayer Material as a Scaf- fold of Human Neofetal Dermal Fibroblasts,” Biomaterials, Vol. 22, 2001, pp. 331-336. doi:10.1016/S0142-9612(00)00188-5 [23] E. Behravesh, M. D. Timmer, J. J. Lemoine, M. A. K. Liebschner and A.G. Mikos, “Evaluation of the in Vitro Degradation of Macroporous Hydrogels Using Gravimeter, Confined Compression Testing and Microcomputed To- mography,” Biomacromolecules, Vol. 3, 2002, pp. 1263-1270. doi:10.1021/bm020067+ [24] J. R. Karel Smetana, Jaromir Lukas, Vera Paleckova and et al., “Effect of Ehemical Structure of Hydrogels on the Adhesion and Phenotypic Characteristics of Human Monocytes Such as Expression of Galectins and Other Carbohydrate-Binding Sites,” Biomaterials, Vol. 18, 1997, pp. 1009-1014. doi:10.1016/S0142-9612(97)00037-9 [25] E. T. den Braber, J. E. de Ruijter, L. A. Ginsel and et al., “Quantitative Analysis of Fibroblast Morphology on Mi- crogrooved Surfaces with Various Groove and Ridge Dimensions,” Biomaterials, Vol. 17, 1996, pp. 2037-2044. doi:10.1016/0142-9612(96)00032-4 [26] R. Singhvi, G. Stephanopoulos and I. C. Daniel, “Effect of Substrate Morphology on Cell Physiology,” Biotech- nology and Bioengineering, Vol. 43, 1994, pp. 764-771. doi:10.1002/bit.260430811 [27] Takahiro Suzuki and Yasuyuki mizushima, “Characteris- tics of Silica-Chitosan Complex Membrane and Their Relationships to the Characteristics of Growth and Adhe- siveness of L-929 Cells Cultured on the Biomembrane,” Journal of Fermentation and Bioengineering, Vol. 84, 1997, pp. 128-132. doi:10.1016/S0922-338X(97)82541-X [28] N. J. Hallab, K. J. Bundy, K. O’Connor and et al., “Cell Adhesion to Biomaterials: Correlations between Surface Charge, Surface Roughness, Adsorbed Protein and Cell Morphology,” Journal of Long-Term Effects of Medical Implants, Vol. 5, 1995, pp. 209-231. [29] T.G. Ruardy, H.E. Moorlag, J.M. Schakenraad and et al., “Growth of Fibroblasts and Endothelial Cells on Wet- tability Gradient Surfaces,” Journal of Colloid and Inter- face Science, Vol. 188, 1997, pp. 209-217. doi:10.1006/jcis.1997.4769 [30] C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, “Geometric Control of Cell Life and Death,” Science, Vol. 276, 1997, pp. 1425-1428. doi:10.1126/science.276.5317.1425 [31] E. A. Vogler and R. W. Bussian, “Short-Term Cell-At- tachment Rates: A Surface-Sensitive Test of Cell-Sub- strate Compatibility.” Journal of Biomedical Materials Research, Vol. 21, 1987, pp. 1197-1211. doi:10.1002/jbm.820211004 [32] Y. Zhu, C. Gao, T. He, X. Liu and J. Shen, “Layer-by- Layer Assembly to Modify Poly(L-Lactic Acid) Surface toward Improving Its Cytocompatibility to Human Endo- thelial Cells.” Biomacromolecule s, Vol. 4, 2003, pp. 446- 452. doi:10.1021/bm025723k |

