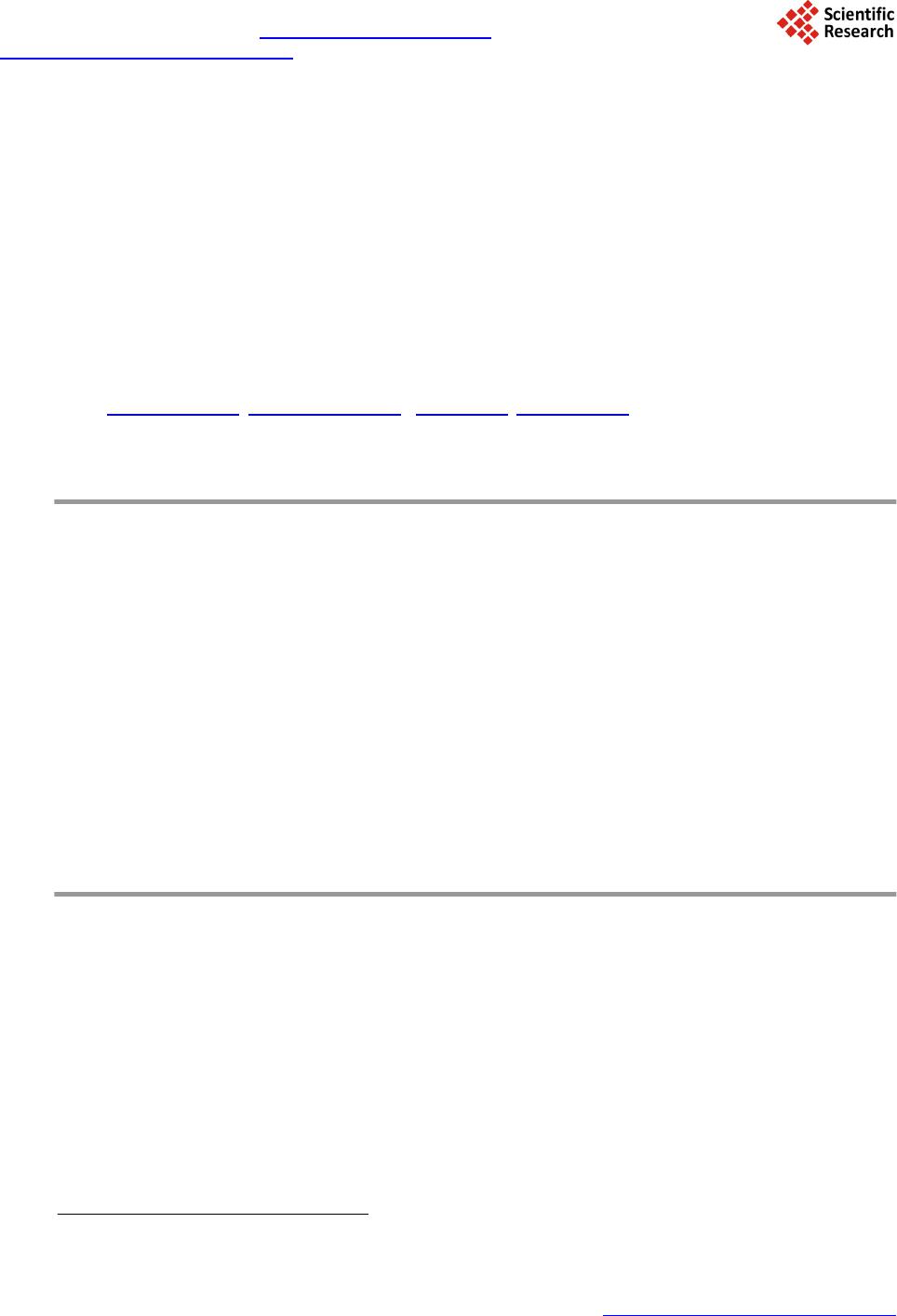
 Journal of Geoscience and Environment Protection, 2014, 2, 68-76 Published Online April 2014 in SciRes. http://www.scirp.org/journal/gep http://dx.doi.org/10.4236/gep.2014.22011 How to cite this paper: Zhang, T. et al. (2014). Preparation and Characterization of a New Desulfurizer and Its Performance on Removal of SO2. Journal of Geoscience and Environment Protection, 2, 68-76. http://dx.doi.org/10.4236/gep.2014.22011 Preparation and Characterization of a New Desulfurizer and Its Performance on Removal of SO2 Ting Zhang, Jun qing Li, Shurong Yu*, Yi Wang School of Petrol-Chemical Engineering, Lanzhou University of Technology, Lanzhou, China Email: zhangting@lut.cn, qing_5975@qq.com, *yusr@lut.cn, wangyi@lut.cn Received January 2014 Abstract Sulfur dioxide is one of the major pollutants resulting from fuel combustion. In this study, CaO and attapulgite were utilized as raw material for synthesizing CaO/attapulgite (CaO/ATP) desulfurizer. The physicochemical characteristics of CaO/ATP desulfurizer were evaluated by various tech- niques such as XRD, SEM, FT-IR. The performance of samples was studied in dynamic conditions. Major factors affecting on desulfurization such as weight ratio of CaO to total, types of modifiers, roasting time and temperature were investigated. The desulfurization agent synthesized under optimal synthesis conditions with CaO content of 30 wt% and NaOH modifier, and the desulfuriza- tion roasting time of 2 hours and roasting temperature of 600˚C, exhibit sulfur tolerance of 10.15 wt%. This desulfurizer with excellent absorbency and catalysis of desulfurization, economical and environment-friendly, could be especially useful in industrial applications. Keywords Attapulgite; CaO; Desul furi zation; SO2; Ad sor p tion 1. Introduction Sulfur dioxide (SO2) emission from coal-fired power plants and refinery operations is one of the most important pollution among other pollutants. Discharge of SO2 from various sources can easily create air pollution and cause harmful effects on living things and the environment. For instance, it causes acid rain affecting negatively on plant and animals (Aytar , 2011; Cao, 1996; Chen, 2003). It is noted that the dry processes are generally advantageous over the wet systems in terms of secondary treatment of wastes produced during wet methods. So because of their simplicity and relatively low cost, dry sorption technologies are applied more than the wet methods. Application of calcium-based sorbents, spray dry flue gas desulfurization, metal oxides sorption and activated carbon or coke processes are the main dry sorption processes used for SO2 and NOx removal (Che n, 2003). Lime/gyp method is a conventional dry desulfurizing method, which uses CaO or Ca(OH)2 as desulfurizers. *Corresponding author.  T. Zhang et al. The lime/gyp method is considered to be more effective and cheaper than other desulfurizing methods, such as activate carbon adsorption and Claus recycle, etc. But the calcium-based desulfurizer has a big problem: It is easy to scaling during the process of desulfurization, because CaSO4, whose molar volume is three times as much as CaO, is generated, and the micro pore of desulfurizer would be soon jammed by CaSO4, which prevent the reaction and decreased SO2 removal efficiency (Chen, 2004; Fro st, 1998; Görkem, 2006; Her ná nd e z -Maldonado, 2005). In the field of dry and regenerable sorbents, considerable attention has been devoted to activated carbon or ac- tivated coke due to their large adsorption capacity of SO2 at near room temperatures (Li, 2007; Li, 2008). Much work has also been done on metal oxides such as CuO, Fe2O3, MnO, CaO, Co3O4, ZnO and CeO2 for H2S or SO2 capture at higher temperatures (Li n, 1998). Despite that the metal oxides alone exhibit high sulfur sorption capacity, their performance and life time are adversely affected by problems such as sintering, evaporation, and mechanical disintegrations. To overcome these problems, Al2O3 and natural zeolites were added to metal oxides or their mixtures as structure stabilizer (Liu, 2011; Ma, 20 03; P an, 2005). Jianrong Ma et al. (Cao, 1996) i nve s- tigated a novel regenerable Fe/activated coke (AC) desulfurizer prepared by impregnation of Fe(NO3)3 on an ac- tivated coke. Experiment results showed that at 200˚C the SO2 adsorption capacity of the Fe/AC was higher than that of AC or Fe2O3. Attapulgite (or palygorskite-as it often called) is a crystalline hydrated magnesium aluminum silicate with unique three-dimensional structure and has a fibrous morphology. Attapulgite has the structural formula Si8O20Mg5(Al)(OH)2(H2O)4∙4H2O. The distinguishing feature of its structure is that the Si-O tetrahedral form long strips, each an amphibole unit wide, on alternate sides of the oxygen sheet in a manner which confers a regular corrugated Si-O structure. The formulas are written as such to indicate the two types of water present; magnesium coordinated water and adsorbed water. The structure of the mineral results in zeolite-like channels, which are approximately 3.7×6.0 and 5.6×11.0Å wide, respectively. These channels may be filled with water or organic molecules. The water is partly arranged in these channels and water molecules are also bound to the magnesium cations of the Mg(Al, Fe) brucite-like ribbon edges that border the channels running along the length of the crystals. Specific surface areas of about 200m2/g may, therefore, result form fine particle size rather than significant contributions from internal channel surface (Perderiset, 1988; S he n, 2012; W a ng, 2005). Because of its structural morphology, attapulgite has received considerable attention with regard to the adsorption of organ- ics on the clay surface and to their use as support for catalysts (Wa ng, 2011; Wie c kowska , 1995; Yan, 2002). In this study, CaO and attapulgite were utilized as raw material for synthesizing CaO/attapulgite (CaO/ATP) desulfurizer. Major factors affecting on desulfurization such as weight ratio of CaO to total, types of modifiers, roasting time and temperature were investigated. 2. Experimental 2.1. Material and Equipment Attapulgite clay came from Linze County, Gansu Province, China (60-mash sieve, specific surface area 110 ~ 150 m2/g), with ATP content of 31% ~ 57%, illite and kaolinite content of 18% ~ 23%, quartz and feldspar con- tent of 15% ~ 25%, dolomite and montmorillonite content of trace. Its chemical components (%) are as follows: SiO2, 57.61; Al2O3, 14.06; MgO, 1.10; CaO, 5.26; Fe2O3, 4.96; K2O, 2.57; Na2O, 1.41; Ti, 0.3873; Mn, 0.0603; P, 0.0585; Sr, 0.0451; Ba, 0.0424; Zr, 0.0197; V, 0.0093; Cr, 0.0071; Zn, 0.0067; La, 0.0041; Ni, 0.0032; Cu, 0.0030; Y, 0.0024; Pb, 0.0015; Th, 0.0014; Nb, 0.0013; Co, 0.0010. The other reagents supplied by their manu- facturers was all analytical pure. 2.2. Preparation Method of the Desulfurizer CaO, ATP and some water were mixed and well-distributed at a certain ratio. The mixture was aged for a day, shaped, and dried in the oven at the temperature of 105˚C. The samples were roasted in the high temperature oven at 600˚C for 2 hours, then cooled. The CaO/ATP desulfurizer samples were obtained. 2.3. Characterization and Analysis SEM was utilized to determine the crystal morphology and chemical element composition. Measurements were made on a JSM-5500 SEM instrument using a digital imaging process. FT-IR was used to confirm the chemical groups in the prepared CaO/ATP desulfurizer. Measurements were made on a Nicolet AVTAR 360 FT-IR spec- trometer after samples were mixed with 300 mg of spectroscopic grade KBr and ground in an agate mortar. The 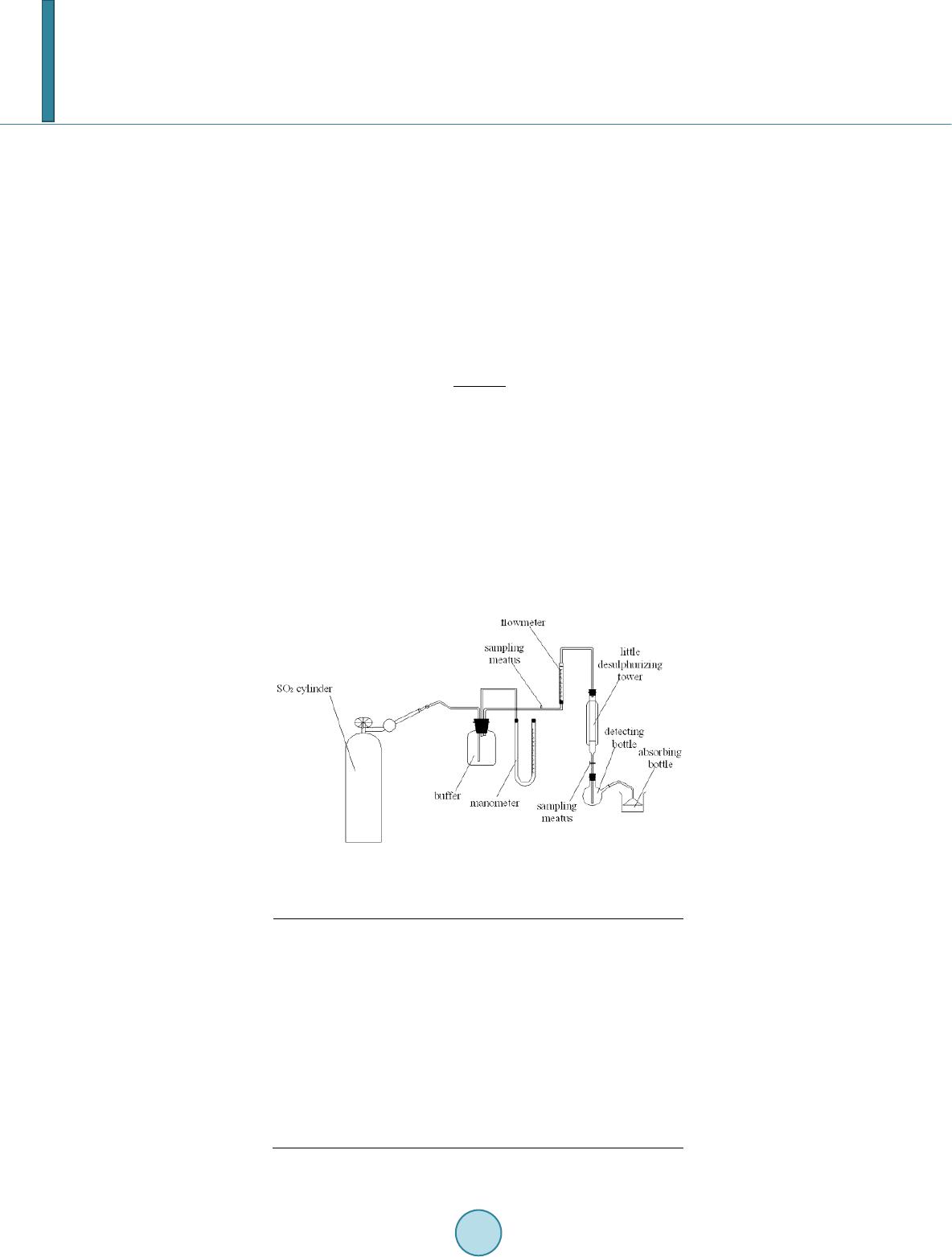 T. Zhang et al. crystalline form of the samples was identified by a Panalytical X’Pert PRO XRD instrument (Cu Ka radiation), operating at 40 kV and 30 mA. Surface area, pore volume, and pore size distribution were obtained on a ASAP2010 BET apparatus. 2.4. Determining the Properties of the CaO/ATP Desulfurizer The ability of desulphurization of CaO/ATP desulfurizer was determined by the experiment equipment showed in Figure 1. The experimental conditions can be seen in Table 1. SO2 gas went through the little desulfurizing tower continuously and was absorbed and transferred by CaO/ATP desulfurizer. SO2 concentration both at the inlet and at the outlet were determined ever few minutes until the desulfurizer was fully penetrated (SO2 con- centration at the inlet equals to that at the outlet). Then the desulfurization efficiency curves (penetrating curves) were obtained and the sulfur tolerance (S) was calculated by following formula: (1) Ga—the weight of the desulphurizer after desulphurization; Gb—the weight of the desulphurizer before desulphurization. 3. Results and Discussion 3.1. Characterization of the Synthesized Samples The phase structures of samples were investigated by XRD, and the obtained results are shown in Figure 2 and Figure 3. XRD patterns of the samples prepared with different CaO content are contrasted with those of ATP. Samples CaO/ATP with different CaO content showed the characteristic peaks (26.8˚, 29.9˚, 39.6˚, 43.3˚, 47.6˚, Figure 1. Experimental equipment. Table 1. Characteristics of experimental conditions. Gas SO2 Temperature 20˚C Pressure Normal Desu l f urizer C a O / ATP Column length (m) 0.20 Column diameter (m) 0.02 Particle diameter (m) 0.003 Total porosity 0.34 Apparent density (kg/m3) 398.089 BET surface area (m2/g) 206.9 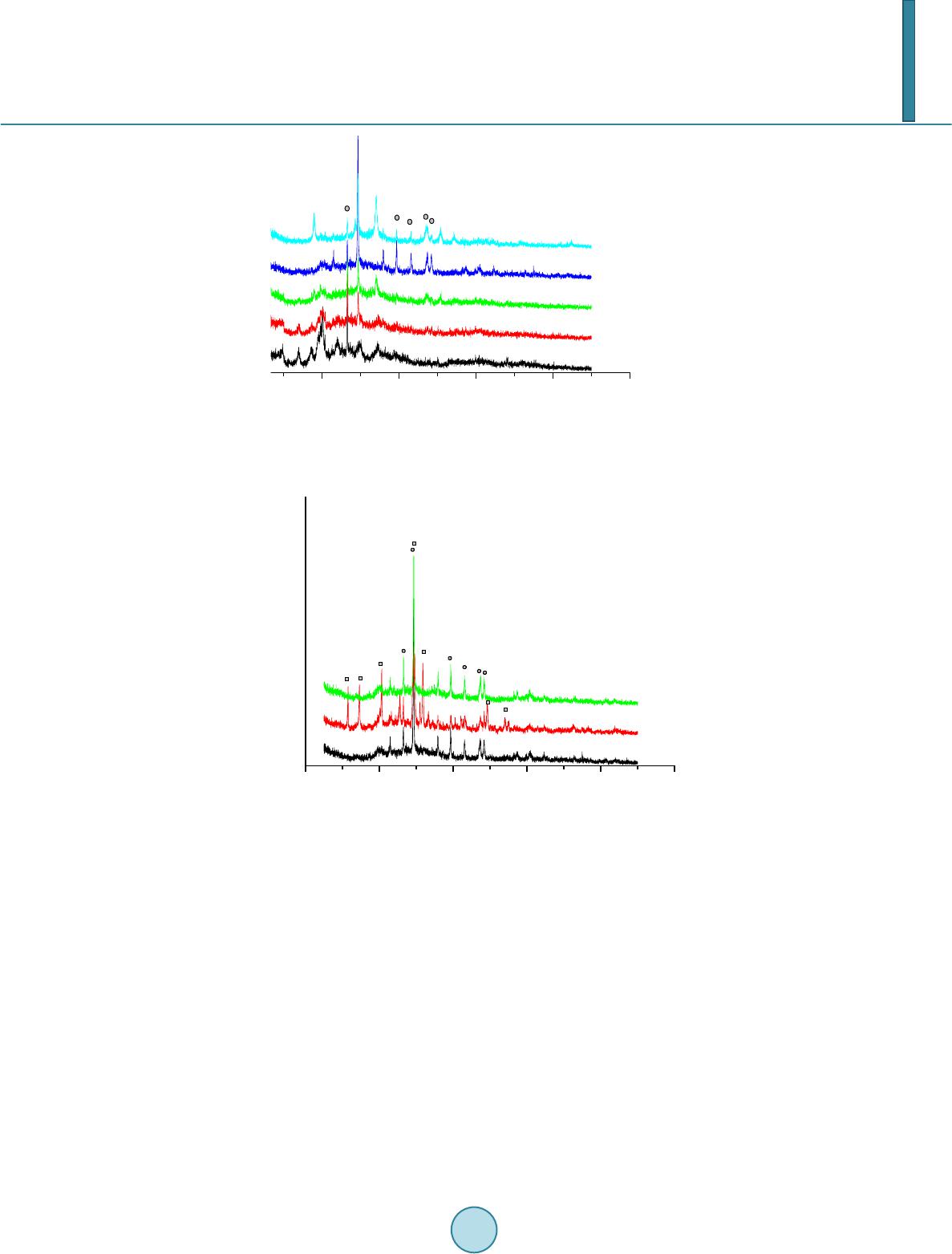 T. Zhang et al. Figure 2. XRD patterns of ATP and CaO/ATP samples with different CaO content (○—CaO). Figure 3. XRD patterns of CaO/ATP samples with different modifier (□— CaSO4, ○—CaO). 48.4˚) of cubic spinel structure known from bulk CaO phase. The intensity of some characteristic peaks of CaO of CaO/ATP sample became stronger along with CaO content increasing. Figure 3 showe d XRD patterns of CaO/ATP samples were disposed by H2SO4, NaOH respectively. From Figure 3 we can see that the samples which were disposed by NaOH did not change remarkably compared with the samples which were not disposed. The main reaction of that is ion exchange. But when H2SO4 was used as modifier, CaO reacted with H2SO4 and generated CaSO4. SEM micrographs were collected to illustrate the morphologies of ATP, CaO/ATP, modified samples by MgCl 2, H2SO4 or NaOH, as depicted in Figure 4. Rod-shaped particles with lengths of 500 ~ 700 nm and widths of 100 ~ 150 nm are visible (Figur e 2(a)). After the introduction of CaO species into ATP, rod-shaped crystal still can be seen, but the surface area decreased (Figure 2(b)). Morphological observations of CaO/ATP mod- ified by NaOH is shown in Figure 2(c), which we cannot see the rod-shaped particles because NaOH destroyed three-dimensional space structure of ATP (Zhan g, 2005), the surface area increased instead. While the sample modified by H2SO4, which can be seen in F igur e 2(d), its surface presented a chaotic condition. The FT-IR results of CaO/ATP (different roasting temperature) can be seen in Figure 5. The strong wide ab- sorption bands appeared at 3440 cm−1 associated with the surface hydroxyl groups. The weak sharp absorption 020406080 100 Intensity 2θ/° CaO/AT P H 2 SO 4 disposal NaOH disposal 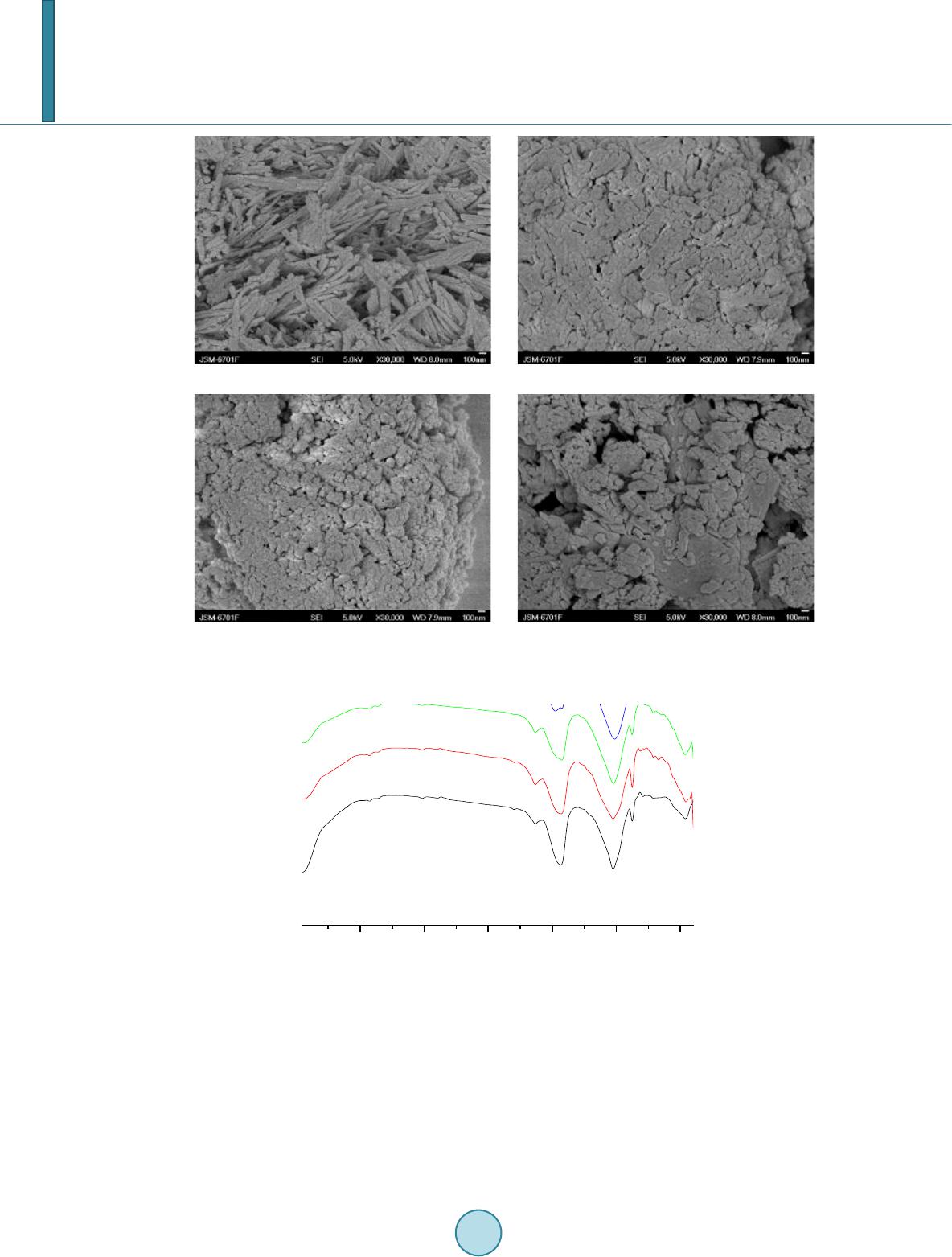 T. Zhang et al. (a) (b) (c) (d) Figure 4. SEM micrographs of ATP, CaO/ATP, CaO/ATP modified samples by H2SO4 or NaOH. (a) ATP ; (b) CaO/ATP; (c) CaO/ATP modified by NaOH; (d) CaO/ATP modified by H2SO4. 00 3000 2500 2000 1500 1000500 600 ℃ 500 ℃ 400 ℃ 440 1630 1430 1020 Figure 5. FT-IR results of CaO/ATP (different roasting temperature). bands at 1630 cm−1 is flexural vibrations of water H-O-H. The strong wide absorption bands at 1020 cm−1 is dis- symmetry stretching vibrations of Si-O-Si, and the absorption bands at 777 cm−1 and 476 cm−1 are symmetry stretching and flexural vibrations of Si-O. The strong wide absorption bands appeared at 3440 cm−1 became weak when increasing roasting temperature from 400˚C to 700˚C, for zeolitic water, crystal water and constitu- tion water of ATP crystal will decrease when temperature increase. Crystal structure changed at high tempera- ture. Over 600˚C, zeolitic duct was destroyed, and zeolitic water disappeared. 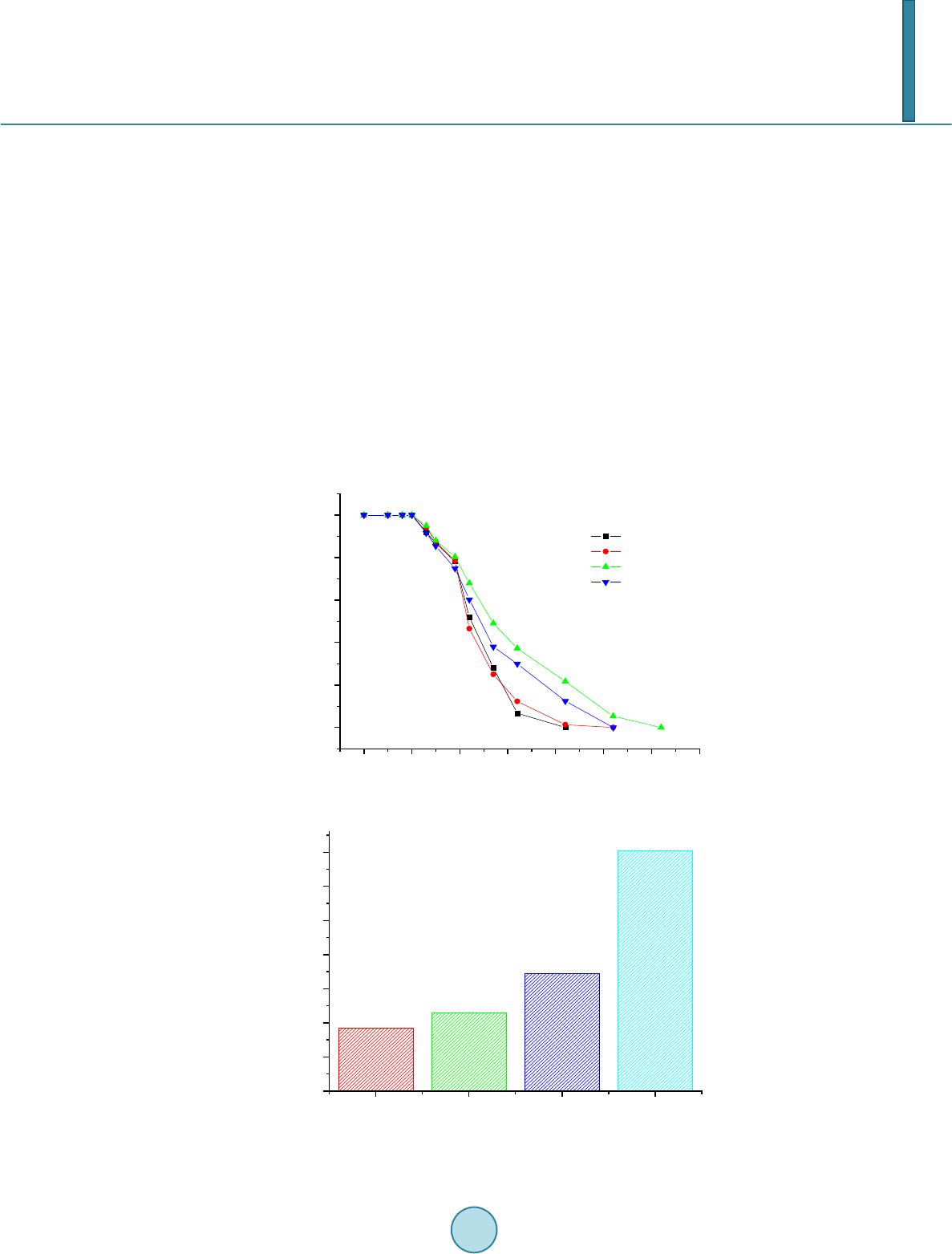 T. Zhang et al. 3.2. SO2 Desulfurizing Experiment by CaO/ATP Desulfurizer Except ATP, CaO was another main component of the desulfurizer. Its content would influent sulfate efficiency. The results were presented in Figure 6 and Figure 7. The SO2 removal efficiency represents the ratio of SO2 concentration at the inlet subtracting that at the outlet to that of inlet feed. Thus, the efficiency curves also indi- cate the penetrating curves. With increasing content of CaO, the penetrating time and sulfur tolerance were gradually increased. It was because CaO was basic oxide, which could react with acidic gas SO2 quickly. In fact, the desulurizer has two effects on SO2, ATP adsorbed SO2 and CaO transferred it to sulfate. The higher CaO content was, the more SO2 was transferred. Meanwhile, CaO content could not be too high, that would cause mechanical intensity of the desulfurizer decreased. As a result, CaO content should be about 30 wt%, the effect of desulfurization of the samples was better. ATP is often modified by acid, such as sulfur acid and hydrochloric acid. When acid was dissociated in aqueous solution, H+ was produced and exchanged with K+, Na+, Ca2+ of ATP to enlarge pore volume of that. In this study, H2SO4 and NaOH were used for CaO/ATP modifying, and the result can be seen in Figure 8. Be- cause ATP was corporated with CaO, H2SO4 reacted with CaO and generated CaSO4, which blocked the micro- pore and reduced its activities. The samples modified by NaOH had higher surface area so that the higher sulfur tolerance can be obtained. Roasting temperature and time will influence the sulfur tolerance, as we saw from Figure 9 and Figure 10. At Figure 6. Influences of CaO content on SO2 removal efficiency. Figure 7. Influences of CaO content on sulfur tolerance. 010 20 30 40 50 60 70 0 20 40 60 80 100 SO2 removal efficiency/% Time/min CaO-0% CaO-10% CaO-20% CaO-30% CaO-0% CaO-10% CaO-20% CaO-30% 0 1 2 3 4 5 6 7 Sulfur tolerance/% CaO content/%  T. Zhang et al. Figure 8. Influences of modifier on sulfur tolerance. Figure 9. Influences of roasting temperature on sulfur tolerance. Figure 10. Influences of roasting time on sulfur tolerance. CaO/ATP CaO/ATP modified by H2SO4CaO/ATP modified by NaOH 0 2 4 6 8 10 12 Sulfur tolerance/% 300 400 500 600 700 0 2 4 6 8 10 12 Sulfur tolerance/% Roasting temperature/°C 1.0 1.5 2.0 2.5 3.0 0 2 4 6 8 10 12 Sulfur tolerance/% Roasting time/h  T. Zhang et al. 600˚C and roasting sample 2 hours, highest sulfur tolerance (10.15%) was achieved. It is known from Figure 5 that duct was destroyed when roasting over 600˚C, which will make the surface area of samples decrease. Roasting time will also influence the sulfur tolerance. The CaO/ATP samples were roasted to clot gradually. When roasted over 2 hours, the pore canal of samples would collapse because of losing water excessively. 4. Conclusion A new CaO/attapulgite desulfurization agent was synthesized by mixing of CaO and attapulgite directly. The optimum synthesizing and operating conditions were as follows: weight ratio of CaO to attapulgite, 3:7; types of modifiers, NaOH (1 mol/L); roasting temperature and time, 600˚C and 2 hours; weight ratio of water to CaO/ATP desufurizer, 20 - 30 wt%. The desufurizer synthesized and operated under optimal synthesis condi- tions exhibited sulfur tolerance of 10.15 wt%. This new approach showed promising in utilizing natural resource of Gansu, China, such as attapulgite in the production of desulfurization agent, which could significantly reduce the production cost and make the tech- nique quite environmental friendly. Acknowledgem ents This work was financially supported by the National Natural Science Foundation of China (Grant No. 51302123). References Aytar, P., Gedikli, S., Şam, M., Ünal , A., Çabuk, A., Kolankaya, N., & Yürüm, A. (2011). Desulphurization of Some Low- Rank Turkish Lignites with Crude Laccase Produced from Trametes Versicolor ATCC 200801. Fuel Processing Tech- nology, 92, 71-76. http://dx.doi.org/10.1016/j.fuproc.2010.08.022 Cao, E., Bryant, R., & Williams, D. J. A. (1996). Electrochemical Properties of Na-Attapulgite. Journal of Colloid and In- terface Science, 179, 1 43-15 0. http://dx.doi.org/10.1006/jcis.1996.0196 Chen, B., & Zhang, X. X. (2003). The Research Development of the Numerical Model of Single-Particle Dry Method De- sulfurization. Industrial Boiler, 77, 16-19. Chen, B., Jin, H., & Zhang, X. X. (2003). Original Uneven Porosity Desulphurization Model Research of Small Particle. Journal of Lanzhou University, 39, 41-43. Chen, B., Jin, H., & Zhang, X. X. (2004). A Mathematic Model of Non-Homogenous Original Porosity for Dry method De- sulfurization. Environmental Pollution Prevention, 26, 46-47. Frost, R. L., Cash, G. A., Kloprogge, J. T. et al. (1998). Rocky Mountain Leather’, Sepiolite Andattapulgite—An Infrared Emission Spectroscopic Study. Vibrational Spectroscopy, 16, 173-184. http://dx.doi.org/10.1016/S0924-2031(98)00014-9 Görkem, B., & Oğuz, H. (2006). Development of an Active Sorbent from Fly Ash for Dry Desulphurization of Simulated Flue Gas in a Fluidized-Bed Reactor. Chemical Engineering Journal, 119, 147-15 2. http://dx.doi.org/10.1016/j.cej.2006.03.018 Hernánd ez-Mal d onado , A. J., Qi, G. S., & Yang, R. T. (2005). Desulfurization of Commercial Fuels by π-Complexation: Monolayer CuCl/γ-Al2O3. Applied Catalysis B: Environmental, 61, 236-24 2. http://dx.doi.org/10.1016/j.apcatb.2005.05.012 Li, A., Zhang, J. P., & Wan g, A. Q. (2007). Utilization of Starch and Clay for the Preparation of Superabsorbent Composite. J. Bior. Tech., 98, 327 -332. http://dx.doi.org/10.1016/j.biortech.2005.12.026 Li, J. J., Kobayashi, N., & Hu , Y. Q. (2008). The Activated Coke Preparation for SO2 Adsorption by Using Flue Gas from Coal Power Plant. Chemical Engineering and Processing, 47, 118-127. http://dx.doi.org/10.1016/j.cep.2007.08.001 Lin, Y. S., & Deng, S. G. (1998). Removal of Trace Sulfur Dioxide from Gas Stream by Regenerative Sorption Processes. Separation and Purification Technology, 13, 65-77. http://dx.doi.org/10.1016/S1383-5866(97)00062-2 Liu, B. S., Wei, X. N., Zhan, Y. P., Chang, R. Z., Subhan, F., & Au, C. T. (2011). Preparation and Desulfurization Perfor- mance of LaMeOx/SBA-15 for Hot Coal Gas. Applied Catalysis B: Environmental, 102, 27-36. http://dx.doi.org/10.1016/j.apcatb.2010.11.020 Ma, J. R., Liu, Z. Y., Liu, S. J., & Zhu, Z. P. (2003) . A Regenerable Fe/AC Desulfurizer for SO2 Adsorption at Low Tem- peratu res. Applied Catalysis B: Environmental, 45, 301-30 9 . http://dx.doi.org/10.1016/S0926-3373(03 )00 176 -0 Pan, Y. G., P erales, J. F., V elo , E., &Pui gjaner, L. (2005). Kinetic Behaviour of Iron Oxide Sorbent in Hot Gas Desulfuriza- tion. Fuel, 84, 1105-1109. http://dx.doi.org/10.1016/j.fuel.2004.11.025  T. Zhang et al. Perderi set, M., Baillif, P., & Jaurand, M. C. (1988). Chemical Analysis and Photoelectron Spectroscopy of the Adsorption of Macromolecules on the Surface of Attapulgite. Journal of Colloid and Interface Science, 12 1 , 381-391. http://dx.doi.org/10.1016/0021-9797(88)90441-9 Shen, Y. F., Su n, T. H., & Jia, J. P. ( 2012 ). A Novel Desulphurization Process of Coal Water Slurry via Sodium Metaborate Electroreduction in the Alkaline System. Fuel, 96, 250 -25 6. http://dx.doi.org/10.1016/j.fuel.2012.01.003 Wang, L., & Sheng, J. (2005). Preparation and Properties of Polypropylene/Org-Attapulgite Nanocomposites. Polymer Journal, 46, 6243-6249 . http://dx.doi.org/10.1016/j.polymer.2005.05.067 Wang, Y. P., Liu, Y., Don g, Y. H., & Ma, Y. J. ( 2011 ). Adsorption of Ammonia Nitrogen on Modified Palygorskites an d Zeolite in Waste Water. Applied Chemical Industry, 40, 985-992 . Wiecko wska , J. (1995). Catalytic and Adsorptive Desulphurization of Gases. Catalysis Today, 24, 405-465 . http://dx.doi.org/10.1016/0920-5861(95)00021-7 Yan, Y., Peng, X. F., Jia, L. et al. (2002 ) Study on the Distributed Pore characteristic of Dry Flue Gas Desulfurization. Energy environment, 20, 38-41 . Zhang, J., Chen, H., & Wan g, A. (2005). Study on Superabsorbent Composite. III. Swelling Behaviors of Polyacrylamide/ Attapulgite Composite Based on Acidified Attapulgite and Organo-Attapulgite. European Polymer Journal, 41, 2434- 2442. http://dx.doi.org/10.1016/j.eurpolymj.2005.03.022
|