 Vol.3, No.3, 199-207 (2011) Natural Science http://dx.doi.org/10.4236/ns.2011.33026 Copyright © 2011 SciRes. OPEN ACCESS Reaction of thiocarboxanilide derivatives of 2-phenylimino-3-phenyl-4-thiazolidinone and 1,3-diphenyl-2-thioxo-4-imidazolone with hydrazonoyl halides and active chloromethylene compounds Hamdi M. Hassaneen1*, Omar A. Miqdad2, Nada M. Abunada2, Ahmad A. Fares1 1Chemistry, Faculty of Science, Cairo University, Cairo, Egypt; *Corresponding Author: hamdihass@gmail.com 2Department of Chemistry, Faculty of Applied Sciences, Al-Aqsa University, Gaza, Palestine; nadanadannrs@yahoo.com; miqdadomar@hotmail.com Received 7 January 2011; revised 10 February 2011; accepted 13 February 2011. ABSTRACT The potassium salts of thiocarboxanilide of 2- phenylimino-3-phenyl-4-thiazolidinone and 1,3- diphenyl-2-thioxo-4-imidazolone react with hy- drazonoyl halides in N,N-dimethylformamide to afford the corresponding 1,3,4-thiadiazoline de- rivatives. 2-Phenylimino-3-phenyl-4-thiazolidinone reacts with active chloromethylene compounds in N,N-dimethylformamide to give the corre- sponding thiazolylidenethiazolidin-4-one deriva- tives. The new compounds were characterized using IR, 1H NMR, 13C NMR and mass spectra. Keywords: Hydrazonoyl Halides; 1,3,4-Thiadiazoline; Thiazolidinone 1. INTRODUCTION 1,3,4-Thiadiazole and its derivatives possess an inter- esting biological activity probably conferred to them by the strong aromaticity of this ring system [1]. It is known that many of its derivatives have antibacterial [2], an- timicrobial [3], antimycobacterial [4,5], antifungal [6,7], antidepressant [8], anti-inflammatory [9], analgesic[10] activities and cardiotonic [11] action being notable. Be- sides, the thiazoline ring is associated with a variety of pharmacological actions, including antimicrobial [12], anti-inflammatory [13], anti-tumor [14], and antioxidant [15] actions. Moreover, imidazolinone derivatives con- stitute an important class of therapeutic activities such as anticonvulsant [16], potent central nervous system (CNS) depressant [17], and acting on α-adrenergic and/or imi- dazoline receptors [18]. Recently, some new imidazoli- none derivatives have been reported as antimicrobial [19,20], histamine H3-receptor antagonist [21] and L-DOPA prodrugs in the treatment of Parkinson’s dis- ease [22]. Some workers have recognized 5-imidazolone as having anticancer activity [20]. Prompted by these findings and due to our interest in the synthesis of new heterocyclic compounds with potential biological activi- ties [23-26] and in continuation of our work on the syn- thesis of hetaryl-ylidene derivatives [27-29], we report herein the synthesis of some new 1,3,4-thiadiazoline- (thiazoline)-2-hetarylylidene derivatives that might be of pharmacological importance. 2. EXPERIMENTAL The melting points were determined on an electro- thermal melting point apparatus and are uncorrected. IR spectra were recorded in KBr discs on a Pye Unicam SP 3300 and a Shimadzu FT-IR 8101 PC infrared spectro- photometers. The NMR spectra were recorded on a Var- ian Mercury VX-300 MHz NMR spectrometer in DMSO-d6 solutions using TMS as an internal reference. 1H NMR spectra were run at 300 MHz and 13C NMR spectra were run at 75.46 MHz in dimethylsulfoxide (DMSO-d6). Electron impact mass spectra were recorded on a Shimadzu GCMS-QP 1000 EX spectrometer at 70 eV. Elemental analyses were carried out at the Micro Analytical Center at Cairo University, Giza, Egypt. 2-Phenylimino-3-phenyl-4-thiazolidinone 2 [30], 1,3- diphenyl-2-thioxo- 4-imidazolone 13 [31] and hydrazo- noyl halides 1a [32], 1b [33], 1c [34], 1d [35], 1e [36], 1f [37], 1g [38], 1h [39], 1i [40], 1j [41] and 1k [42] were prepared according to the reported literature methods. Synthesis of 3,5-disubstituted 2-[2’-phenylimino-3’- phenyl-4’-oxothiazo lidin-5'-ylidene]-2,3-dihydro-1,3,4- thiadiazole derivatives 7a-k, 3,5-disubstituted 2-[1’,3’- diphenyl-2’-thioxo-4’-oxoimidazolidin-5’-ylidne]-2,3- dihydro-1,3,4-thiadiazole derivatives 15a-h and 2- 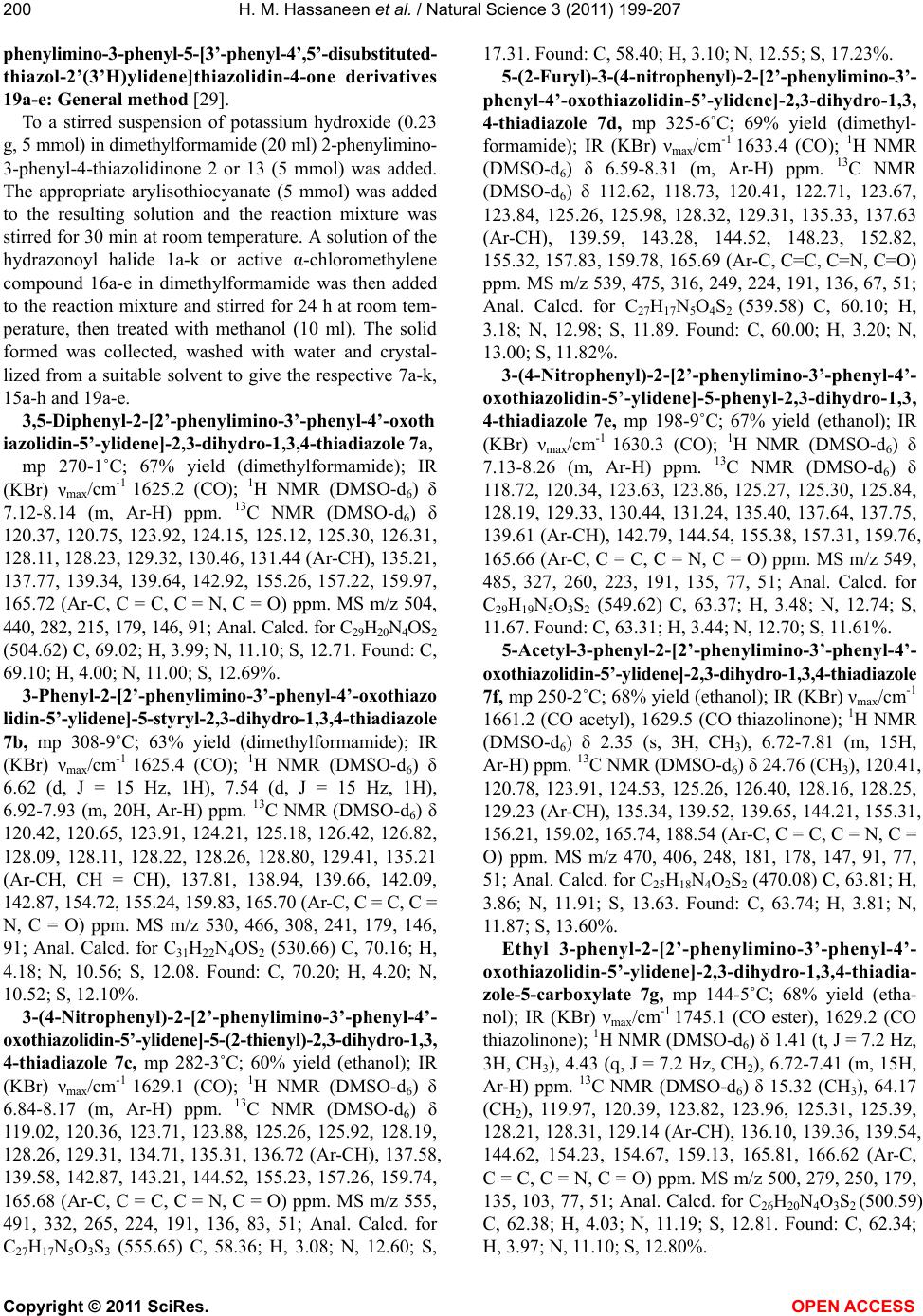 H. M. Hassaneen et al. / Natural Science 3 (2011) 199-207 Copyright © 2011 SciRes. OPEN ACCESS 200 phenylimino-3-phenyl-5-[3’-phenyl-4’,5’-disubstituted- thiazol-2’(3’H)ylidene]thiazolidin-4-one derivatives 19a-e: General method [29]. To a stirred suspension of potassium hydroxide (0.23 g, 5 mmol) in dimethylformamide (20 ml) 2-phenylimino- 3-phenyl-4-thiazolidinone 2 or 13 (5 mmol) was added. The appropriate arylisothiocyanate (5 mmol) was added to the resulting solution and the reaction mixture was stirred for 30 min at room temperature. A solution of the hydrazonoyl halide 1a-k or active α-chloromethylene compound 16a-e in dimethylformamide was then added to the reaction mixture and stirred for 24 h at room tem- perature, then treated with methanol (10 ml). The solid formed was collected, washed with water and crystal- lized from a suitable solvent to give the respective 7a-k, 15a-h and 19a-e. 3,5-Diphenyl-2-[2’-phenylimino-3’-phenyl-4’-oxoth iazolidin-5’-ylidene]-2,3-dihy dro-1,3,4-thiadiazole 7a, mp 270-1˚C; 67% yield (dimethylformamide); IR (KBr) νmax/cm-1 1625.2 (CO); 1H NMR (DMSO-d6) δ 7.12-8.14 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 120.37, 120.75, 123.92, 124.15, 125.12, 125.30, 126.31, 128.11, 128.23, 129.32, 130.46, 131.44 (Ar-CH), 135.21, 137.77, 139.34, 139.64, 142.92, 155.26, 157.22, 159.97, 165.72 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 504, 440, 282, 215, 179, 146, 91; Anal. Calcd. for C29H20N4OS2 (504.62) C, 69.02; H, 3.99; N, 11.10; S, 12.71. Found: C, 69.10; H, 4.00; N, 11.00; S, 12.69%. 3-Phenyl-2-[2’-phenylimino-3’-phenyl-4’-oxothiazo lidin-5’-ylidene]-5-styryl-2,3-dihydro-1,3,4-thiadiazole 7b, mp 308-9˚C; 63% yield (dimethylformamide); IR (KBr) νmax/cm-1 1625.4 (CO); 1H NMR (DMSO-d6) δ 6.62 (d, J = 15 Hz, 1H), 7.54 (d, J = 15 Hz, 1H), 6.92-7.93 (m, 20H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 120.42, 120.65, 123.91, 124.21, 125.18, 126.42, 126.82, 128.09, 128.11, 128.22, 128.26, 128.80, 129.41, 135.21 (Ar-CH, CH = CH), 137.81, 138.94, 139.66, 142.09, 142.87, 154.72, 155.24, 159.83, 165.70 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 530, 466, 308, 241, 179, 146, 91; Anal. Calcd. for C31H22N4OS2 (530.66) C, 70.16; H, 4.18; N, 10.56; S, 12.08. Found: C, 70.20; H, 4.20; N, 10.52; S, 12.10%. 3-(4-Nitrophenyl)-2-[2’-phenylimino-3’-phenyl-4’- oxothiazolidin-5’-ylidene]-5-(2-thienyl)-2,3-dihydro-1,3, 4-thiadiazole 7c, mp 282-3˚C; 60% yield (ethanol); IR (KBr) νmax/cm-1 1629.1 (CO); 1H NMR (DMSO-d6) δ 6.84-8.17 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 119.02, 120.36, 123.71, 123.88, 125.26, 125.92, 128.19, 128.26, 129.31, 134.71, 135.31, 136.72 (Ar-CH), 137.58, 139.58, 142.87, 143.21, 144.52, 155.23, 157.26, 159.74, 165.68 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 555, 491, 332, 265, 224, 191, 136, 83, 51; Anal. Calcd. for C27H17N5O3S3 (555.65) C, 58.36; H, 3.08; N, 12.60; S, 17.31. Found: C, 58.40; H, 3.10; N, 12.55; S, 17.23%. 5-(2-Furyl)-3-(4-nitrophenyl)-2-[2’-phenylimino-3’- phenyl-4’-oxothiazolidin-5’-ylidene]-2,3-dihydro-1,3, 4-thiadiazole 7d, mp 325-6˚C; 69% yield (dimethyl- formamide); IR (KBr) νmax/cm-1 1633.4 (CO); 1H NMR (DMSO-d6) δ 6.59-8.31 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 112.62, 118.73, 120.41, 122.71, 123.67, 123.84, 125.26, 125.98, 128.32, 129.31, 135.33, 137.63 (Ar-CH), 139.59, 143.28, 144.52, 148.23, 152.82, 155.32, 157.83, 159.78, 165.69 (Ar-C, C=C, C=N, C=O) ppm. MS m/z 539, 475, 316, 249, 224, 191, 136, 67, 51; Anal. Calcd. for C27H17N5O4S2 (539.58) C, 60.10; H, 3.18; N, 12.98; S, 11.89. Found: C, 60.00; H, 3.20; N, 13.00; S, 11.82%. 3-(4-Nitrophenyl)-2-[2’-phenylimino-3’-phenyl-4’- oxothiazolidin-5’-ylidene]-5-phenyl-2,3-dihydro-1,3, 4-thiadiazole 7e, mp 198-9˚C; 67% yield (ethanol); IR (KBr) νmax/cm-1 1630.3 (CO); 1H NMR (DMSO-d6) δ 7.13-8.26 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 118.72, 120.34, 123.63, 123.86, 125.27, 125.30, 125.84, 128.19, 129.33, 130.44, 131.24, 135.40, 137.64, 137.75, 139.61 (Ar-CH), 142.79, 144.54, 155.38, 157.31, 159.76, 165.66 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 549, 485, 327, 260, 223, 191, 135, 77, 51; Anal. Calcd. for C29H19N5O3S2 (549.62) C, 63.37; H, 3.48; N, 12.74; S, 11.67. Found: C, 63.31; H, 3.44; N, 12.70; S, 11.61%. 5-Acetyl-3-phenyl-2-[2’-phenylimino-3’-phenyl-4’- oxothiazolidin-5’-ylidene]-2,3-dihydro-1,3,4-thiadiazole 7f, mp 250-2˚C; 68% yield (ethanol); IR (KBr) νmax/cm-1 1661.2 (CO acetyl), 1629.5 (CO thiazolinone); 1H NMR (DMSO-d6) δ 2.35 (s, 3H, CH3), 6.72-7.81 (m, 15H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 24.76 (CH3), 120.41, 120.78, 123.91, 124.53, 125.26, 126.40, 128.16, 128.25, 129.23 (Ar-CH), 135.34, 139.52, 139.65, 144.21, 155.31, 156.21, 159.02, 165.74, 188.54 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 470, 406, 248, 181, 178, 147, 91, 77, 51; Anal. Calcd. for C25H18N4O2S2 (470.08) C, 63.81; H, 3.86; N, 11.91; S, 13.63. Found: C, 63.74; H, 3.81; N, 11.87; S, 13.60%. Ethyl 3-phenyl-2-[2’-phenylimino-3’-phenyl-4’- oxothiazolidin-5’-ylidene]-2,3-dihydro-1,3,4-thiadia- zole-5-carboxylate 7g, mp 144-5˚C; 68% yield (etha- nol); IR (KBr) νma x /c m-1 1745.1 (CO ester), 1629.2 (CO thiazolinone); 1H NMR (DMSO-d6) δ 1.41 (t, J = 7.2 Hz, 3H, CH3), 4.43 (q, J = 7.2 Hz, CH2), 6.72-7.41 (m, 15H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 15.32 (CH3), 64.17 (CH2), 119.97, 120.39, 123.82, 123.96, 125.31, 125.39, 128.21, 128.31, 129.14 (Ar-CH), 136.10, 139.36, 139.54, 144.62, 154.23, 154.67, 159.13, 165.81, 166.62 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 500, 279, 250, 179, 135, 103, 77, 51; Anal. Calcd. for C26H20N4O3S2 (500.59) C, 62.38; H, 4.03; N, 11.19; S, 12.81. Found: C, 62.34; H, 3.97; N, 11.10; S, 12.80%.  H. M. Hassaneen et al. / Natural Science 3 (2011) 199-207 Copyright © 2011 SciRes. OPEN ACCESS 201201 3-Phenyl-5-phenylaminoca rbonyl-2-[2’-phenylimino- 3’-phenyl-4’-oxothiazolidin-5’-ylidene]-2,3-dihydro- 1,3,4-thiadiazole 7h, mp 307-8˚C; 70% yield (ethanol); IR (KBr) νmax /c m-1 1661.9 (broad CO); 1H NMR (DMSO-d6) δ 7.34-8.41 (m, NH, Ar-H) ppm. 13C NMR (DMSO-d6) δ 120.36, 121.12, 122.38, 122.79, 124.12, 124.54, 124.97, 125.61, 128.18, 128.31, 128.75, 128.91 (Ar-CH), 135.41, 139.42, 139.67, 140.66, 146.68, 153.14, 157.87, 159.18, 165.32, 165.45 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 547, 325, 258, 178, 147, 103, 91, 77; Anal. Calcd. for C30H21N5O2S2 (547.65) C, 65.78; H, 3.87; N, 12.79; S, 11.71. Found: C, 65.81; H, 3.94; N, 12.83; S, 11.66%. 5-Benzoyl-3-phenyl-2-[2’-phenylimino-3’-phenyl-4’- oxothiazolidin-5’-ylidene]-2,3-dihydro-1,3,4-thiadiazole 7i, mp 256-7˚C; 65% yield (ethanol); IR (KBr) νmax/cm-1 1674.3 (CO benzoyl), 1629.9 (CO thiazolinone); 1H NMR (DMSO-d6) δ 7.16-8.34 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 120.32, 120.39, 123.98, 124.54, 125.21, 126.39, 128.24, 128.27, 129.12, 129.19, 129.81, 133.47 (Ar-CH), 135.41, 136.46, 139.43, 139.49, 143.92, 155.61, 156.21, 158.93, 165.80, 182.96 (Ar-C, C=C, C = N, C = O) ppm. MS m/z 532, 310, 243, 178, 163, 147, 103, 91, 77; Anal. Calcd. for C30H20N4O2S2 (532.63) C, 67.65; H, 3.78; N, 10.52; S, 12.04. Found: C, 67.70; H, 3.81; N, 10.48; S, 12.00%. 3-Phenyl-2-[2’-phenylimino-3’-phenyl-4’-oxothiazo- lidin-5’-ylidene]-5-(2-thienoyl)-2,3-dihydro-1,3,4-thia- diazole 7j, mp 122-3˚C; 66% yield (ethanol); IR (KBr) νmax/cm-1 1674.4 (CO thienoyl), 1625.6 (CO thiazoli- none); 1H NMR (DMSO-d6) δ 6.92-8.34 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 119.84, 120.40, 124.31, 124.45, 125.30, 125.87, 128.19, 128.21, 128.32, 129.21, 135.11, 135.42 (Ar-CH), 137.19, 139.39, 139.48, 143.89, 144.12, 154.31, 155.91, 158.94, 165.28, 175.95 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 538, 316, 249, 179, 91, 77; Anal. Calcd. for C28H18N4O2S3 (538.66) C, 62.43; H, 3.37; N, 10.40; S, 17.86. Found: C, 62.40; H, 3.35; N, 10.36; S, 17.90%. 5-(2-Naphthoyl)-3-phenyl-2-[2’-phenylimino-3’- phenyl-4’-oxothiazolidin-5’-ylidene]-2,3-dihydro-1,3, 4-thiadiazole 7k, mp 118-9˚C; 68% yield (ethanol); IR (KBr) νmax/cm-1 1638.7 (CO naphthoyl), 1629.9 (CO thiazolinone); 1H NMR (DMSO-d6) δ 7.15-8.39 (m, 21H, Ar-H), 8.85 (s, 1H, naphthoyl α-H) ppm. 13C NMR (DMSO-d6) δ 119.68, 120.32, 123.68, 124.16, 125.23, 125.49, 126.40, 126.58, 127.76, 127.84, 128.10, 128.18, 129.23, 129.29, 129.87, 132.28 (Ar-CH), 132.51, 132.86, 135.50, 135.61, 139.44, 142.26, 144.18, 155.29, 155.63, 159.13, 165.82, 183.60 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 582, 360, 293, 213, 181, 155, 103, 91, 77; Anal. Calcd. for C34H22N4O2S2 (582.69) C, 70.08; H, 3.81; N, 9.62; S, 11.01. Found: C, 70.10; H, 3.80; N, 9.57; S, 11.00%. 3,5-diphenyl-2-[1’,3’-diphenyl-2’-thioxo-4’-oxoimid- azolidin-5’-ylidne]-2,3-dihydro-1,3,4-thiadiazole 15a, mp 136-8˚C; 63% yield (ethanol); IR (KBr) νmax/cm-1 1670.1 (CO); 1H NMR (DMSO-d6) δ 7.12-8.19 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 120.31, 120.40, 120.73, 123.84, 123.98, 124.10, 125.31, 127.93, 128.10, 128.21, 130.36, 131.43 (Ar-CH), 137.71, 138.92, 139.41, 139.52, 142.93, 156.17, 157.22, 165.74, 178.62 (Ar-C, C = C, C = N, C = O, C = S) ppm. MS m/z 504, 341, 274, 238, 206, 135, 103, 91, 77; Anal. Calcd. for C29H20N4OS2 (504.62) C, 69.02; H, 3.99; N, 11.10; S, 12.71. Found: C, 69.00; H, 4.00; N, 11.00; S, 12.65%. 3-Phenyl-2-[1’,3’-diphenyl-2’-thioxo-4’-oxoimidazol- idin-5’-ylidne]-5-styryl-2,3-dihydro-1,3,4-thiadiazole 15b, mp 151-2˚C; 65% yield (ethanol); IR (KBr) νmax/ cm-1 1675.3 (CO); 1H NMR (DMSO-d6) δ 6.81 (d, J = 15 Hz, 1H), 7.63 (d, J = 15 Hz, 1H), 7.26-7.83 (m, 20H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 119.64, 119.85, 120.61, 123.78, 123.94, 124.12, 126.78, 127.92, 128.10, 128.15, 128.19, 128.23, 128.71, 137.76 (Ar-CH, CH = CH), 138.96, 139.12, 140.12, 142.01, 143.26, 154.34, 155.31, 165.74, 178.51 (Ar-C, C = C, C = N, C = O, C = S) ppm. MS m/z 530, 367, 276, 206, 135, 103, 91, 77; Anal. Calcd. for C31H22N4OS2 (530.66) C, 70.16; H4.18, ; N, 10.56; S, 12.08. Found: C, 70.10; H, 4.15; N, 10.50; S, 12.00%. 3-(4-Nitrophenyl)-2-[1’,3’-diphenyl-2’-thioxo-4’- oxoimidazolidin-5’-ylidne]-5-(2-thienyl)-2,3-dihydro- 1,3,4-thiadiazole 15c, mp 144-5˚C; 70% yield (ethanol); IR (KBr) νmax/cm-1 1670.9 (CO); 1H NMR (DMSO-d6) δ 6.85-8.16 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 119.67, 120.03, 120.16, 123.76, 123.92, 124.18, 127.73, 128.13, 128.21, 134.80, 136.64, 137.61 (Ar-CH), 139.54, 139.62, 143.14, 143.34, 144.32, 155.31, 157.32, 165.73, 178.43 (Ar-C, C = C, C = N, C = O, C = S) ppm. MS m/z 555, 420, 392, 251, 136, 90, 77, 51; Anal. Calcd. for C27H17N5O3S3 (555.65) C, 58.36; H, 3.08; N, 12.60; S, 17.31. Found: C, 58.43; H, 3.05; N, 12.54; S, 17.27%. 5-Acetyl-3-phenyl-2-[1’,3’-diphenyl-2’-thioxo-4’- oxoimidazolidin-5’-ylidne]-2,3-dihydro-1,3,4-thiadi- azole 15d, mp 184-5˚C; 70% yield (ethanol); IR (KBr) νmax/cm-1 1734.2 (CO acetyl), 1680.8 (CO imidazoline); 1H NMR (DMSO-d6) δ 2.42 (s, 3H, CH3), 6.63-8.12 (m, 15H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 24.84 (CH3), 119.23, 120.26, 120.52, 124.18, 124.32, 124.51, 128.19 , 128.26, 128.34 (Ar-CH), 139.36, 139.48, 139.67, 144.32, 155.29, 156.51, 165.69, 179.63, 184.26 (Ar-C, C = C, C = N, C = O, C = S) ppm. MS m/z 470, 406, 307, 240, 135, 103, 91, 77, 43; Anal. Calcd. for C25H18N4O2S2 (470.56) C, 63.81; H, 3.86; N, 11.91; S, 13.63. Found: C, 63.80; H, 3.82; N, 11.90; S, 13.60% Ethyl 3-Phenyl-2-[1’,3’-diphenyl-2’-thioxo-4’-oxo- 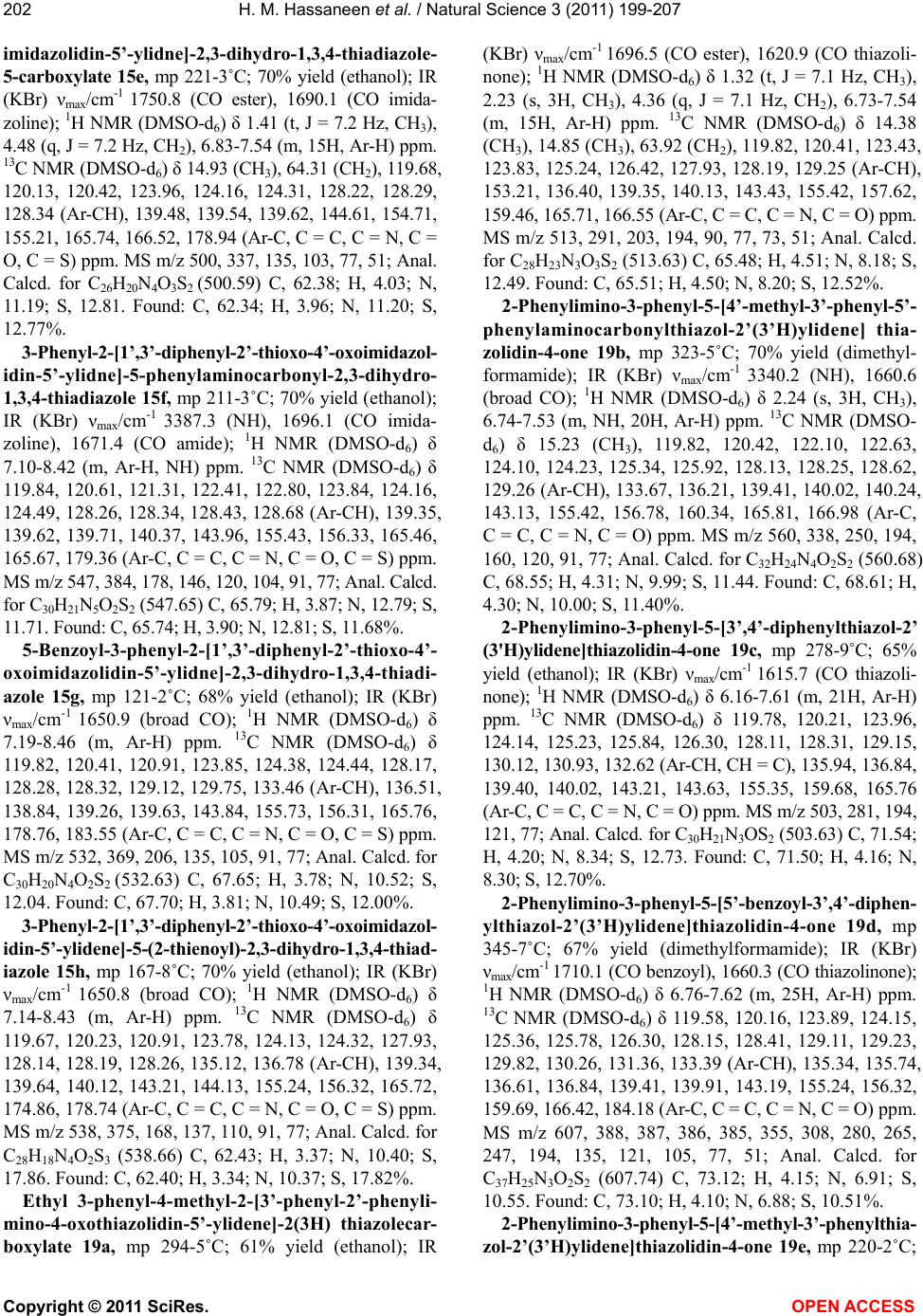 H. M. Hassaneen et al. / Natural Science 3 (2011) 199-207 Copyright © 2011 SciRes. OPEN ACCESS 202 imidazolidin-5’-ylidne]-2,3-dihydro-1,3,4-thiadiazole- 5-carboxylate 15e, mp 221-3˚C; 70% yield (ethanol); IR (KBr) νmax/cm-1 1750.8 (CO ester), 1690.1 (CO imida- zoline); 1H NMR (DMSO-d6) δ 1.41 (t, J = 7.2 Hz, CH3), 4.48 (q, J = 7.2 Hz, CH2), 6.83-7.54 (m, 15H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 14.93 (CH3), 64.31 (CH2), 119.68, 120.13, 120.42, 123.96, 124.16, 124.31, 128.22, 128.29, 128.34 (Ar-CH), 139.48, 139.54, 139.62, 144.61, 154.71, 155.21, 165.74, 166.52, 178.94 (Ar-C, C = C, C = N, C = O, C = S) ppm. MS m/z 500, 337, 135, 103, 77, 51; Anal. Calcd. for C26H20N4O3S2 (500.59) C, 62.38; H, 4.03; N, 11.19; S, 12.81. Found: C, 62.34; H, 3.96; N, 11.20; S, 12.77%. 3-Phenyl-2-[1’,3’-diphenyl-2’-thioxo-4’-oxoimidazol- idin-5’-ylidne]-5-phenylaminocarbonyl-2,3-dihydro- 1,3,4-thiadiazole 15f, mp 211-3˚C; 70% yield (ethanol); IR (KBr) νmax/cm-1 3387.3 (NH), 1696.1 (CO imida- zoline), 1671.4 (CO amide); 1H NMR (DMSO-d6) δ 7.10-8.42 (m, Ar-H, NH) ppm. 13C NMR (DMSO-d6) δ 119.84, 120.61, 121.31, 122.41, 122.80, 123.84, 124.16, 124.49, 128.26, 128.34, 128.43, 128.68 (Ar-CH), 139.35, 139.62, 139.71, 140.37, 143.96, 155.43, 156.33, 165.46, 165.67, 179.36 (Ar-C, C = C, C = N, C = O, C = S) ppm. MS m/z 547, 384, 178, 146, 120, 104, 91, 77; Anal. Calcd. for C30H21N5O2S2 (547.65) C, 65.79; H, 3.87; N, 12.79; S, 11.71. Found: C, 65.74; H, 3.90; N, 12.81; S, 11.68%. 5-Benzoyl-3-phenyl-2-[1’,3’-diphenyl-2’-thioxo-4’- oxoimidazolidin-5’-ylidne]-2,3-dihydro-1,3,4-thiadi- azole 15g, mp 121-2˚C; 68% yield (ethanol); IR (KBr) νmax/cm-1 1650.9 (broad CO); 1H NMR (DMSO-d6) δ 7.19-8.46 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 119.82, 120.41, 120.91, 123.85, 124.38, 124.44, 128.17, 128.28, 128.32, 129.12, 129.75, 133.46 (Ar-CH), 136.51, 138.84, 139.26, 139.63, 143.84, 155.73, 156.31, 165.76, 178.76, 183.55 (Ar-C, C = C, C = N, C = O, C = S) ppm. MS m/z 532, 369, 206, 135, 105, 91, 77; Anal. Calcd. for C30H20N4O2S2 (532.63) C, 67.65; H, 3.78; N, 10.52; S, 12.04. Found: C, 67.70; H, 3.81; N, 10.49; S, 12.00%. 3-Phenyl-2-[1’,3’-diphenyl-2’-thioxo-4’-oxoimidazol- idin-5’-ylidene]-5-(2-thienoyl)-2,3-dihydro-1,3,4-thiad- iazole 15h, mp 167-8˚C; 70% yield (ethanol); IR (KBr) νmax/cm-1 1650.8 (broad CO); 1H NMR (DMSO-d6) δ 7.14-8.43 (m, Ar-H) ppm. 13C NMR (DMSO-d6) δ 119.67, 120.23, 120.91, 123.78, 124.13, 124.32, 127.93, 128.14, 128.19, 128.26, 135.12, 136.78 (Ar-CH), 139.34, 139.64, 140.12, 143.21, 144.13, 155.24, 156.32, 165.72, 174.86, 178.74 (Ar-C, C = C, C = N, C = O, C = S) ppm. MS m/z 538, 375, 168, 137, 110, 91, 77; Anal. Calcd. for C28H18N4O2S3 (538.66) C, 62.43; H, 3.37; N, 10.40; S, 17.86. Found: C, 62.40; H, 3.34; N, 10.37; S, 17.82%. Ethyl 3-phenyl-4-methyl-2-[3’-phenyl-2’-phenyli- mino-4-oxothiazolidin-5’-ylidene]-2(3H) thiazolecar- boxylate 19a, mp 294-5˚C; 61% yield (ethanol); IR (KBr) νmax/cm-1 1696.5 (CO ester), 1620.9 (CO thiazoli- none); 1H NMR (DMSO-d6) δ 1.32 (t, J = 7.1 Hz, CH3), 2.23 (s, 3H, CH3), 4.36 (q, J = 7.1 Hz, CH2), 6.73-7.54 (m, 15H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 14.38 (CH3), 14.85 (CH3), 63.92 (CH2), 119.82, 120.41, 123.43, 123.83, 125.24, 126.42, 127.93, 128.19, 129.25 (Ar-CH), 153.21, 136.40, 139.35, 140.13, 143.43, 155.42, 157.62, 159.46, 165.71, 166.55 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 513, 291, 203, 194, 90, 77, 73, 51; Anal. Calcd. for C28H23N3O3S2 (513.63) C, 65.48; H, 4.51; N, 8.18; S, 12.49. Found: C, 65.51; H, 4.50; N, 8.20; S, 12.52%. 2-Phenylimino-3-phenyl-5-[4’-methyl-3’-phenyl-5’- phenylaminocarbonylthiazol-2’(3’H)ylidene] thia- zolidin-4-one 19b, mp 323-5˚C; 70% yield (dimethyl- formamide); IR (KBr) νmax/cm-1 3340.2 (NH), 1660.6 (broad CO); 1H NMR (DMSO-d6) δ 2.24 (s, 3H, CH3), 6.74-7.53 (m, NH, 20H, Ar-H) ppm. 13C NMR (DMSO- d6) δ 15.23 (CH3), 119.82, 120.42, 122.10, 122.63, 124.10, 124.23, 125.34, 125.92, 128.13, 128.25, 128.62, 129.26 (Ar-CH), 133.67, 136.21, 139.41, 140.02, 140.24, 143.13, 155.42, 156.78, 160.34, 165.81, 166.98 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 560, 338, 250, 194, 160, 120, 91, 77; Anal. Calcd. for C32H24N4O2S2 (560.68) C, 68.55; H, 4.31; N, 9.99; S, 11.44. Found: C, 68.61; H, 4.30; N, 10.00; S, 11.40%. 2-Phenylimino-3-phenyl-5-[3’,4’-diphenylthiazol-2’ (3'H)ylidene]thiazolidin-4-one 19c, mp 278-9˚C; 65% yield (ethanol); IR (KBr) νmax/cm-1 1615.7 (CO thiazoli- none); 1H NMR (DMSO-d6) δ 6.16-7.61 (m, 21H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 119.78, 120.21, 123.96, 124.14, 125.23, 125.84, 126.30, 128.11, 128.31, 129.15, 130.12, 130.93, 132.62 (Ar-CH, CH = C), 135.94, 136.84, 139.40, 140.02, 143.21, 143.63, 155.35, 159.68, 165.76 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 503, 281, 194, 121, 77; Anal. Calcd. for C30H21N3OS2 (503.63) C, 71.54; H, 4.20; N, 8.34; S, 12.73. Found: C, 71.50; H, 4.16; N, 8.30; S, 12.70%. 2-Phenylimino-3-phenyl-5-[5’-benzoyl-3’,4’-diphen- ylthiazol-2’(3’H)ylidene]thiazolidin-4-one 19d, mp 345-7˚C; 67% yield (dimethylformamide); IR (KBr) νmax/cm-1 1710.1 (CO benzoyl), 1660.3 (CO thiazolinone); 1H NMR (DMSO-d6) δ 6.76-7.62 (m, 25H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 119.58, 120.16, 123.89, 124.15, 125.36, 125.78, 126.30, 128.15, 128.41, 129.11, 129.23, 129.82, 130.26, 131.36, 133.39 (Ar-CH), 135.34, 135.74, 136.61, 136.84, 139.41, 139.91, 143.19, 155.24, 156.32, 159.69, 166.42, 184.18 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 607, 388, 387, 386, 385, 355, 308, 280, 265, 247, 194, 135, 121, 105, 77, 51; Anal. Calcd. for C37H25N3O2S2 (607.74) C, 73.12; H, 4.15; N, 6.91; S, 10.55. Found: C, 73.10; H, 4.10; N, 6.88; S, 10.51%. 2-Phenylimino-3-phenyl-5-[4’-methyl-3’-phenylthia- zol-2’(3’H)ylidene]thiazolidin-4-one 19e, mp 220-2˚C; 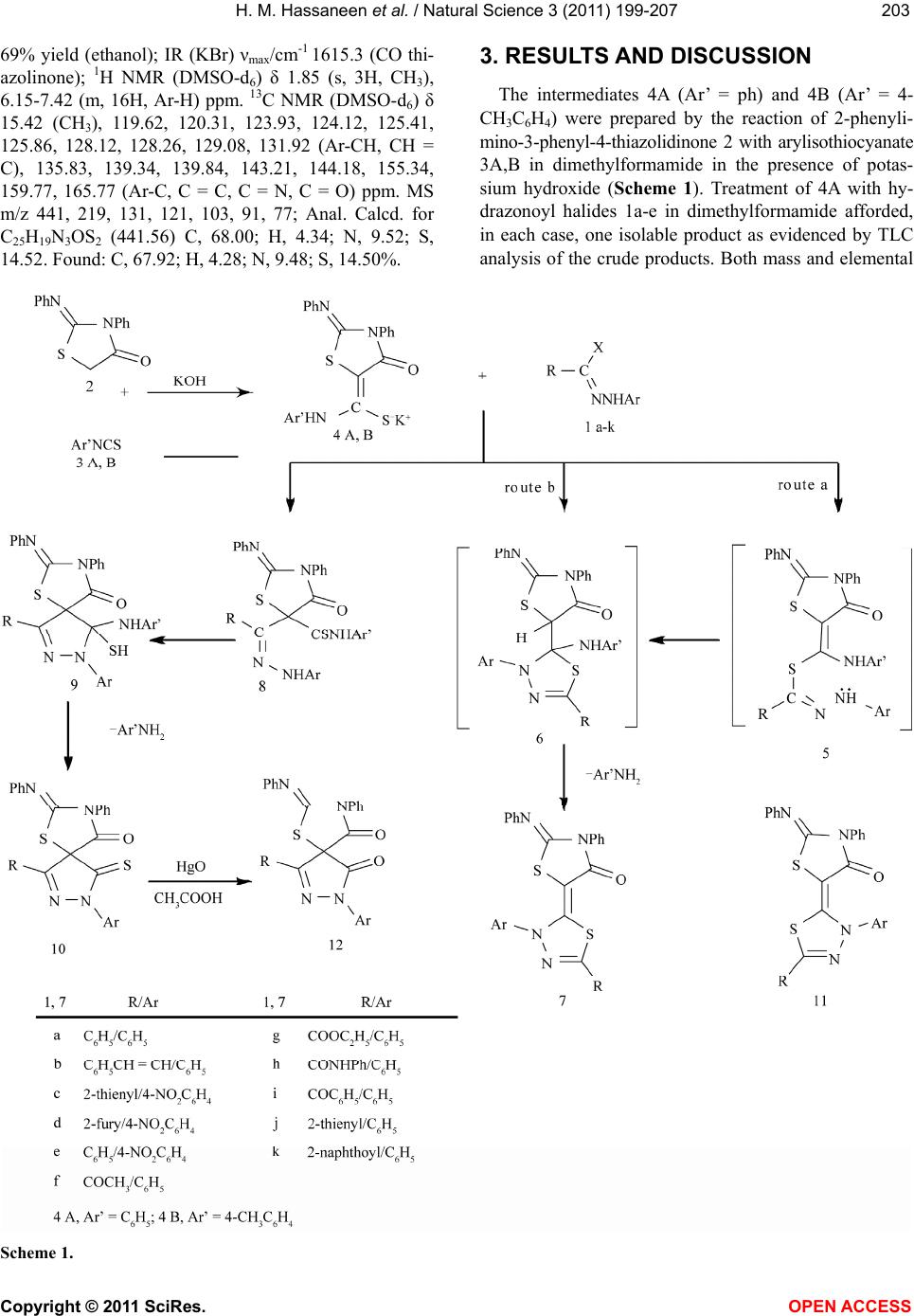 H. M. Hassaneen et al. / Natural Science 3 (2011) 199-207 Copyright © 2011 SciRes. OPEN ACCESS 203203 69% yield (ethanol); IR (KBr) νmax/cm-1 1615.3 (CO thi- azolinone); 1H NMR (DMSO-d6) δ 1.85 (s, 3H, CH3), 6.15-7.42 (m, 16H, Ar-H) ppm. 13C NMR (DMSO-d6) δ 15.42 (CH3), 119.62, 120.31, 123.93, 124.12, 125.41, 125.86, 128.12, 128.26, 129.08, 131.92 (Ar-CH, CH = C), 135.83, 139.34, 139.84, 143.21, 144.18, 155.34, 159.77, 165.77 (Ar-C, C = C, C = N, C = O) ppm. MS m/z 441, 219, 131, 121, 103, 91, 77; Anal. Calcd. for C25H19N3OS2 (441.56) C, 68.00; H, 4.34; N, 9.52; S, 14.52. Found: C, 67.92; H, 4.28; N, 9.48; S, 14.50%. 3. RESULTS AND DISCUSSION The intermediates 4A (Ar’ = ph) and 4B (Ar’ = 4- CH3C6H4) were prepared by the reaction of 2-phenyli- mino-3-phenyl-4-thiazolidinone 2 with arylisothiocyanate 3A,B in dimethylformamide in the presence of potas- sium hydroxide (Scheme 1). Treatment of 4A with hy- drazonoyl halides 1a-e in dimethylformamide afforded, in each case, one isolable product as evidenced by TLC analysis of the crude products. Both mass and elemental Scheme 1. 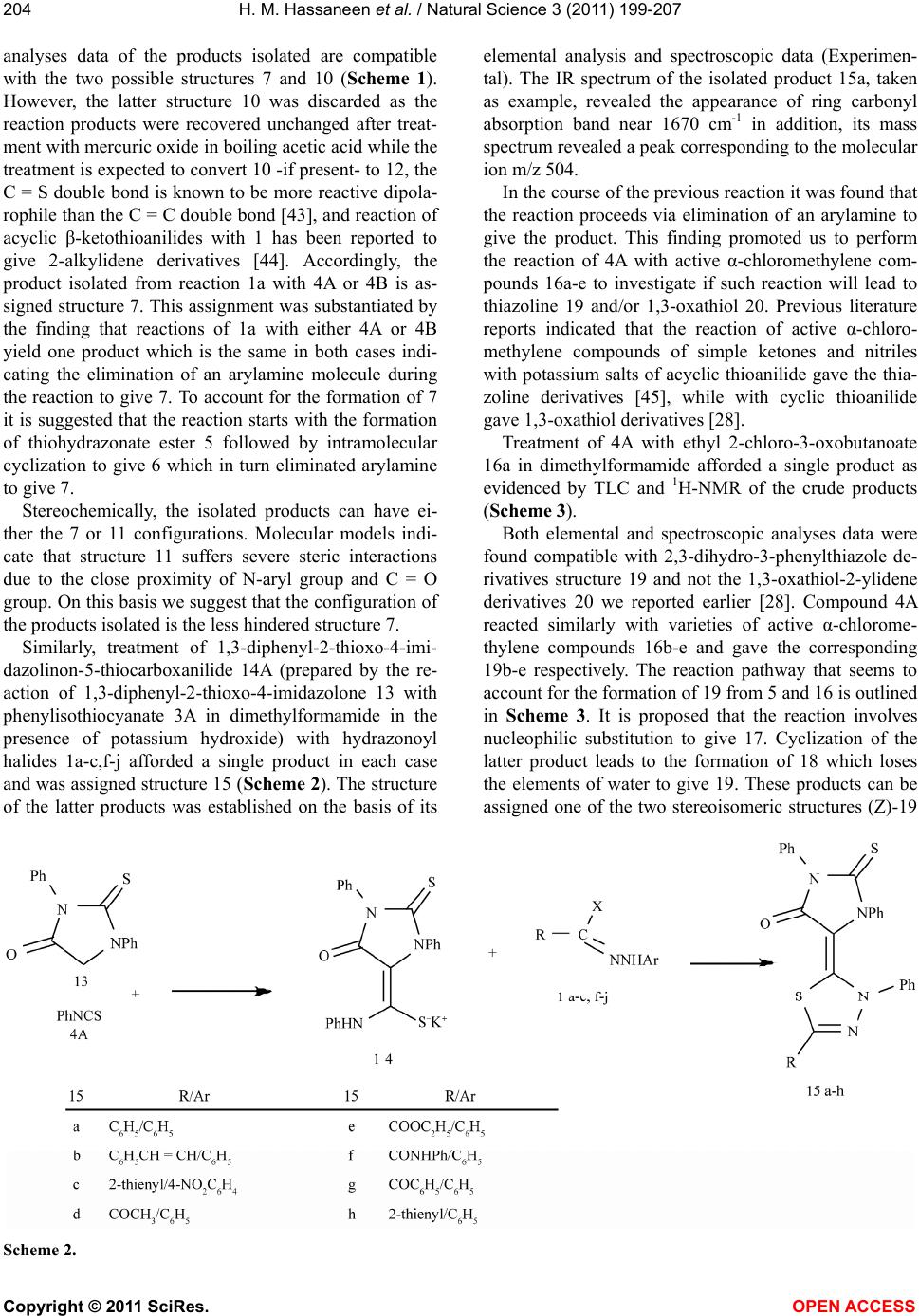 H. M. Hassaneen et al. / Natural Science 3 (2011) 199-207 Copyright © 2011 SciRes. OPEN ACCESS 204 analyses data of the products isolated are compatible with the two possible structures 7 and 10 (Scheme 1). However, the latter structure 10 was discarded as the reaction products were recovered unchanged after treat- ment with mercuric oxide in boiling acetic acid while the treatment is expected to convert 10 -if present- to 12, the C = S double bond is known to be more reactive dipola- rophile than the C = C double bond [43], and reaction of acyclic β-ketothioanilides with 1 has been reported to give 2-alkylidene derivatives [44]. Accordingly, the product isolated from reaction 1a with 4A or 4B is as- signed structure 7. This assignment was substantiated by the finding that reactions of 1a with either 4A or 4B yield one product which is the same in both cases indi- cating the elimination of an arylamine molecule during the reaction to give 7. To account for the formation of 7 it is suggested that the reaction starts with the formation of thiohydrazonate ester 5 followed by intramolecular cyclization to give 6 which in turn eliminated arylamine to give 7. Stereochemically, the isolated products can have ei- ther the 7 or 11 configurations. Molecular models indi- cate that structure 11 suffers severe steric interactions due to the close proximity of N-aryl group and C = O group. On this basis we suggest that the configuration of the products isolated is the less hindered structure 7. Similarly, treatment of 1,3-diphenyl-2-thioxo-4-imi- dazolinon-5-thiocarboxanilide 14A (prepared by the re- action of 1,3-diphenyl-2-thioxo-4-imidazolone 13 with phenylisothiocyanate 3A in dimethylformamide in the presence of potassium hydroxide) with hydrazonoyl halides 1a-c,f-j afforded a single product in each case and was assigned structure 15 (Scheme 2). The structure of the latter products was established on the basis of its elemental analysis and spectroscopic data (Experimen- tal). The IR spectrum of the isolated product 15a, taken as example, revealed the appearance of ring carbonyl absorption band near 1670 cm-1 in addition, its mass spectrum revealed a peak corresponding to the molecular ion m/z 504. In the course of the previous reaction it was found that the reaction proceeds via elimination of an arylamine to give the product. This finding promoted us to perform the reaction of 4A with active α-chloromethylene com- pounds 16a-e to investigate if such reaction will lead to thiazoline 19 and/or 1,3-oxathiol 20. Previous literature reports indicated that the reaction of active α-chloro- methylene compounds of simple ketones and nitriles with potassium salts of acyclic thioanilide gave the thia- zoline derivatives [45], while with cyclic thioanilide gave 1,3-oxathiol derivatives [28]. Treatment of 4A with ethyl 2-chloro-3-oxobutanoate 16a in dimethylformamide afforded a single product as evidenced by TLC and 1H-NMR of the crude products (Scheme 3). Both elemental and spectroscopic analyses data were found compatible with 2,3-dihydro-3-phenylthiazole de- rivatives structure 19 and not the 1,3-oxathiol-2-ylidene derivatives 20 we reported earlier [28]. Compound 4A reacted similarly with varieties of active α-chlorome- thylene compounds 16b-e and gave the corresponding 19b-e respectively. The reaction pathway that seems to account for the formation of 19 from 5 and 16 is outlined in Scheme 3. It is proposed that the reaction involves nucleophilic substitution to give 17. Cyclization of the latter product leads to the formation of 18 which loses the elements of water to give 19. These products can be assigned one of the two stereoisomeric structures (Z)-19 Scheme 2. 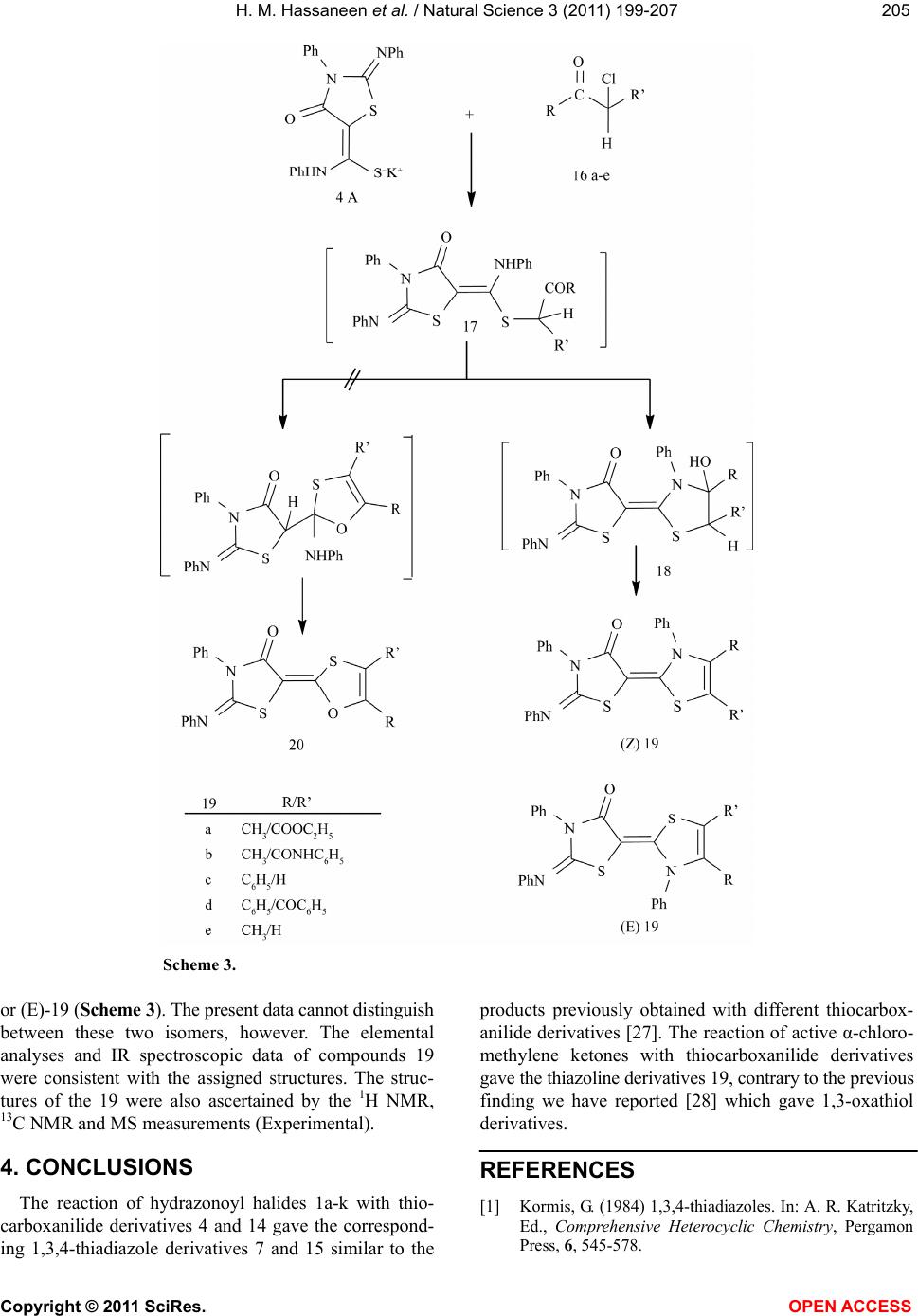 H. M. Hassaneen et al. / Natural Science 3 (2011) 199-207 Copyright © 2011 SciRes. OPEN ACCESS 205205 Scheme 3. or (E)-19 (Scheme 3). The present data cannot distinguish between these two isomers, however. The elemental analyses and IR spectroscopic data of compounds 19 were consistent with the assigned structures. The struc- tures of the 19 were also ascertained by the 1H NMR, 13C NMR and MS measurements (Experimental). 4. CONCLUSIONS The reaction of hydrazonoyl halides 1a-k with thio- carboxanilide derivatives 4 and 14 gave the correspond- ing 1,3,4-thiadiazole derivatives 7 and 15 similar to the products previously obtained with different thiocarbox- anilide derivatives [27]. The reaction of active α-chloro- methylene ketones with thiocarboxanilide derivatives gave the thiazoline derivatives 19, contrary to the previous finding we have reported [28] which gave 1,3-oxathiol derivatives. REFERENCES [1] Kormis, G. (1984) 1,3,4-thiadiazoles. In: A. R. Katritzky, Ed., Comprehensive Heterocyclic Chemistry, Pergamon Press, 6, 545-578.  H. M. Hassaneen et al. / Natural Science 3 (2011) 199-207 Copyright © 2011 SciRes. OPEN ACCESS 206 [2] Hussein, M.A., Kafafy, A.H.N., Abdel-Moty, S.G. and Abou-Ghadir, O.M.F. (2009) Synthesis and biological activities of new substituted thiazoline-quinoline deriva- tives. Acta Pharmaceutica, 59, 365-382. doi:10.2478/v10007-009-0033-8 [3] Pintilie, O., Profire, L., Sunel, V., Popa, M. and Pui, A. (2007) Synthesis and antimicrobial activity of some new 1,3,4-thiadiazole and 1,2,4-triazole compounds having a D,L-methionine moiety. Molecules, 12, 103-113. doi:10.3390/12010103 [4] Faroumadi, A., Mirzaei, M. and Shafiee, A. (2001) Anti- tubercular agents, I: Synthesis and antituberculosis activ- ity of 2-aryl-1,3,4-thiadiazole derivatives. Pharmazie, 56, 610-612. [5] Mamolo, M.G., Falagiani, V., Zanpier, D., Vio, L. and Banfi, F. (2001) Synthesis and antimycobacterial activity of [5- (pyridin-2-yl)-1,3,4-thiadiazol-2-ylthio]acetic acid arylidene- hydrazide derivatives. II Farmaco, 56, 587- 592. doi:10.1016/S0014-827X(01)01097-7 [6] Zou, X.J., Lai, L.H., Jin, G.Y. and Zhang, Z.X. (2002) Synthesis, fungicidal activity, and 3D-QSAR of pyridazi- none-substituted 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. Journal of Agricultural and Food Chemistry, 50, 3757-3760. doi:10.1021/jf0201677 [7] Chen, H., Li, Z. and Han, Y. (2000) Synthesis and fungi- cidal activity against rhizoctonia solani of 2-alkyl (alkyl- thio)-5-pyrazolyl-1,3,4-oxadiazoles (thiadiazoles). Jour- nal of Agricultural and Food Chemistry, 48, 5312-5315. doi:10.1021/jf991065s [8] Clerici, F., Pocar, D., Guido, M., Loche, A., Perlini, V. and Brufani, M. (2001) Synthesis of 2-amino-5-sulfanyl- 1,3,4-thiadiazole derivatives and evaluation of their anti- depressant and anxiolytic activity. Journal of Medicinal Chemistry, 44, pp. 931-936. doi:10.1021/jm001027w [9] Palaska, E., Sahin, G., Kelincen, P., Durlu, N.T. and Altionax, G. (2002 ) Synthesis and anti-inflammatory ac- tivity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3, 4-thiadiazoles and 1,2,4-triazole-3-thiones. II Farmaco, 57, 101-107. doi:10.1016/S0014-827X(01)01176-4 [10] Shenone, S., Bruno, O., Ranise, A., Bondavalli, W., Fal- cone, G., Giordano, L. and Vitelli, M. (2001) 3-Arylsul- phonyl-5-arylamino-1,3,4-thiadiazol-2(3H) ones as anti- inflammatory and analgesic agents. Bioorganic & Me- dicinal Chemistry, 9, 2149-2153. doi:10.1016/S0968-0896(01)00121-3 [11] Onko., T. Cakir, B. and Sahin, M.F. (2004) Synthesis and antinociceptive activity of 2-[(2-Oxobenzothiazolin-3-yl) methyl]-5-aminoalkyl/aryl-1,3,4-thiadiazole. Turkish Jour- nal of Chemistry, 28, 461-468. [12] Azab, M.E., El-Hag Ali, G.A.M. and Abd El-Wahab, A.H.F. (2003) Studies on thiazolopyridines: A novel syn- thesis of bisthiazolopyridines as promising antimicrobial agents. Acta Pharmaceutica, 53, 213-221. [13] Sondhi, S.M., Singh, N., Lahoti, A.M., Bajaj, K., Kumar, A., Lozach, O. and Meijer, L. (2005) Synthesis of acrid- inyl-thiazolino derivatives and their evaluation for anti- nflammatory, analgesic and kinase inhibition activities. Bioorganic & Medicinal Chemistry, 13, 4291-4299. doi:10.1016/j.bmc.2005.04.017 [14] Mahler, G., Serra, G., Dematteis, S., Saldana, J., Dominguez, L. and Manta, E. (2006) Synthesis and bio- logical evaluation of simplified mycothiazole analogues. Bioorganic & Medicinal Chemistry Letters, 16, 1309- 1311. doi:10.1016/j.bmcl.2005.11.072 [15] Shih, M. and Ke, F. (2004) Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidi- none and thiazoline derivatives. Bioorganic & Medicinal Chemistry Letters, 12, 4633-4643. doi:10.1016/j.bmc.2004.06.033 [16] Godefroi, E.F. and Platje, J.Th. (1972) DL-1-(.alpha.-Me- thylbenzyl)-2- methylimidazole-5-carboxylate esters. Syn- thesis and pharmacological properties. Journal of Me- dicinal Chemistry, 15, 336-337. doi:10.1021/jm00273a035 [17] Harfenist, M., Soroko, F.E. and Mckenzie, G.M. (1978) 2-(Alkoxyaryl)-2-imidazoline monoamine oxidase in- hibitors with antidepressant activity. Journal of Medici- nal Chemistry, 21, 405-409. doi:10.1021/jm00202a021 [18] Kornicka, A., Hudson, A.L. and Bednarski, P.J. (2009) Synthesis and biological activity of some 2-imida- zolinylhydrazone derivatives. Acta Poloniae Pharmaceu- tica-Drug Research, 66, 523-534. [19] Desai, N.C., Bhavsar, A.M. and Baldaniya, B.B. (2009) Synthesis and antimicrobial activity of 5-imidazolinone derivatives. Journal of Postgraduate Medicine, 71, 90- 94. [20] Solankee, A., Kapadia, K., Thakor, I., Patel, J. and Lad, S. (2004) Synthesis and Antimicrobial Activity of 1-(4´ -Trifluoro methylphenyl and- 2-phenyl-4-(benzylidene/ substituted Benzylidene/2’ furylidene/2’-thienylidene)- imidazolin-5-ones. Asian Journal of Chemistry, 16, 917- 920. [21] Mor, M., Bordi, F., Silva, C., Rivara, S., Zuliani, V., Vacodio, F., Morini, G., Barocelli, E., Ballabeni, V., Im- picciatore, M. and Plazzi, P.V. (2000) Synthesis and bio- logical assays of new H3-antagonists with imidazole and imidazoline polar groups. II Farmaco, 55, 27-34. doi:10.1016/S0014-827X(99)00115-9 [22] Giorgioni, G., Claudi, F., Ruggieri, S., Ricciutelli, M., Palmieri, G.F., Di-Stefano, A., Sozio, P., Cerasa, L.S., Chiavaroli, A., Ferrante, C., Orlando and G., Glennon, (2010) Design, synthesis, and preliminary pharmacol- ogical evaluation of new imidazolinones as l-DOPA prodrugs. Bioorganic & Medicinal Chemistry Letters, 18, 1834-1843. doi:10.1016/j.bmc.2010.01.041 [23] Abunada, N.M., Hassaneen, H.M., Kandile, N.G. and Miqdad, O.A. (2008) Synthesis and biological activity of some new pyrazoline and pyrrolo[3,4-c]pyrazole-4,6- dione derivatives: reaction of nitrilimines with some di- polarophiles. Molecules, 13, 1011-1024. doi:10.3390/molecules13041011 [24] Abunada, N.M., Hassaneen, H.M., Kandile, N.G. and Miqdad, O.A. (2008) Synthesis and antimicrobial activity of some new pyrazole, fused pyrazolo[3,4-d]-pyrimidine and pyrazolo[4,3-e][1,2,4]-triazolo[1,5-c]pyrimidine de- rivatives. Molecules, 13, 1501-1517. doi:10.3390/molecules13071501 [25] Abunada, N.M., Hassaneen, H.M., Abu Samaha, A.M. and Miqdad, O.A. (2009) Synthesis and antimicrobial evaluation of some new pyrazole, pyrazoline and chromeno [3,4-c]pyrazole derivatives. Journal of Brazil- ian Chemical Society, 20, 975-987.  H. M. Hassaneen et al. / Natural Science 3 (2011) 199-207 Copyright © 2011 SciRes. OPEN ACCESS 207207 doi:10.1590/S0103-50532009000500024 [26] Hassaneen, H.M., Shawali, A.S., Khalil, M.S. and Ab- dallah, T.A.A. (1993) One step synthesis of benzimi- dazo[2,1-c] [1,2,4]triazole derivatives using hydrazonoyl halides. Heterocycles, 36, 1775-1781. doi:10.3987/COM-93-6344 [27] Hassaneen, H.M., Abbas, I.M., Abdelhadi, H.A., Abdal- lah, T.A. and Algharib, M.S. (1994) Reactions of 5-oxo- 2-pyrazolin-4-thiocarboxanilides and 5-oxo-2-oxazolin- 4-thiocarboxanilides with hydrazonoyl halides. Sulfur Letters, 17, 295-307. [28] Hassaneen, H.M., Elwan, N.M., Abdelhadi, H.A. and Abdallah, T.A. (1995) Reactions of thioanilides with ac- tive chloromethylene compounds. Synthesis of 1,3- oxathiol-2-ylidene derivatives. Sulfur Letters, 18, 121- 128. [29] Hassaneen, H.M., Harhash, A.H.E., Abunada, N.M., Ab- dallah, T.A. and Algharib, M.S. (1993) Cyano-(1-me- thylbenzimidazol-2-yl)thioacetanilide in the synthesis of 2,3-dihydro-1,3,4-thiadiazole derivatives. The Journal of Chemical Research, 194-195. [30] Argyropoulou, E.C. and Thessalonikeos, E. (1990) Reac- tions of nitrile oxides and nitrile imines with 4-arylidene- 2-phenyl-5(4H)-thiazolones. Justus Liebigs Annalen der Chemie, 1097-1100. doi:10.1002/jlac.1990199001198 [31] Johnson, T.B. and Hadley, S.E. (1915) Researches on hydantoins. XXIX. Geometrical isomerism in the hydan- toin series. Journal of American Chemical Society, 37, 171-177. doi:10.1021/ja02270a017 [32] Wolkoff, P. (1975) A new method of preparing hydra- zonyl halides. Canadian Journal of Chemistry, 53 , 1333- 1335. doi:10.1139/v75-183 [33] Hassaneen, H.M., Elwan, N.M., Harhash, A. and Shawali, A.S. (1984) The regioselectivity in the formation of pyrazolines and pyrazoles from nitrile imines. Journal of Heterocyclic Chemistry, 21, 1013-1016. doi:10.1002/jhet.5570210417 [34] Hassaneen, H.M., Mousa, H.A.H., Abed, N.M. and Sha- wali, A.S. (1988) Chemistry of C-Heteroarylhydrazidoyl halides. Synthesis and reactions of n-(p-nitrophenyl)-c- (2-thienyl)-formohydrazidoyl halides. Heterocycles, 27, 695-706. doi:10.3987/COM-87-4381 [35] Shawali, A.S., Hassaneen, H.M. and Ibrahim, H.A. (1990) Synthesis and cycloaddition reactions of N-aryl-2-furo- hydrazonoyl chlorides. Archives of Pharmacal Research, 13, 126-131. doi:10.1007/BF02857788 [36] Aylward, J.B. and Scott, F.L. (1969) Preparation and sovolysis of N1-arylbenzohydrazonyl bromides. Journal of Chemical Society (B), 1969, 1080-1084. doi:10.1039/j29690001080 [37] Eweiss, N.F. and Osman, A. (1980) Synthesis of hetero- cycles. Part II. New routes to acetylthiadiazolines and alkylazothiazoles. Journal of Heterocyclic Chemistry, 17, 1713-1717. doi:10.1002/jhet.5570170814 [38] Shawali, A.S., Eweiss, N.F., Hassaneen, H.M. and Al- gharib, M.S. (1975) Synthesis and rearrangement of ethyl aryloxyglyoxalate arylhydrazones. Bulletin of the Chemical Society of Japan, 48, 365-366. doi:10.1246/bcsj.48.365 [39] Shawali, A.S. and Osman, A. (1971) Synthesis and reac- tions of phenylcarbamoylarylhydrazidic chlorides. Tet- rahedron, 27, 2517-2528. doi:10.1016/S0040-4020(01)90753-7 [40] Shawali, A.S. and Abdelhamid, A.O. (1976) Reaction of dimethylphenacylsulfonium bromide with N-nitro- soacetarylamides and reactions of the products with nu- cleophiles. Bulletin of the Chemical Society of Japan, 49, 321-324. doi:10.1246/bcsj.49.321 [41] Farag, A.M. and Algharib, M.S. (1988) Synthesis and reactions of C-(2-thenoyl)-N-arylformahydrazidoyl bro- mides. Organic Preparations and Procedures Interna- tional, 20, 521-526. doi:10.1080/00304948809356298 [42] Hassaneen, H.M., Shawali, A.S., Elwan, N.M. and Abunada, N.M. (1992) Reaction of 1-(2-naphthoyl) methyl-2-dimethylsulfonium bromide with N-nitroso-N- arylacetamides and reactions of the products with some nucleophiles. Sulfur Letters, 13, 273-285. [43] Huisgen, R., Grashey, R., Seidel, M., Knupfer, H. and Schmidt, R. (1962) 1,3-Dipolare Additionen, III. Um- setzungen des Diphenylnitrilimins mit Carbonyl und Thiocarbonyl-Verbindungen. Justus Liebigs Annalen der Chemie, 658, 169-180. doi:10.1002/jlac.19626580115 [44] Abdelhamid, A.O. and Abdou, S.E. (1987) Reactions of cyanothioacetamide with hydrazidoyl halides: A novel synthesis of some hydrazidoyl sulfides and triazole de- rivatives. Sulfur Letters, 6, 41-47. [45] Mohareb, R.M., Sherif, S.M., Abdel-Aal, F.A.M. and Sayed, N.I.A. (1990) One-pot synthesis of polyfunction- ally substituted 2,3-dihydrothiazoles and thiazolidinones. Justus Liebigs Annalen der Chemie, 1143-1146.
|