 Pharmacology & Pharmacy, 2011, 2, 73-81 doi:10.4236/pp.2011.22009 Published Online April 2011 (http://www.SciRP.org/journal/pp) Copyright © 2011 SciRes. PP 73 Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings Nirmeen A. Sabry1, Emad El-Din Omar2 1Clinical Pharmacy Department, Faculty of Pharmacy, Cairo University, Cairo, Egypt; 2Department of Critical Care Medicine, Fac- ulty of Medicine, Cairo University, Cairo, Egypt. Email: papers.sabry@gmail.com Received February 2nd, 2011; revised February 12th, 2011; accepted February 21st, 2011. ABSTRACT Background: Pneumonia is the most common cause of community-acquired infection requiring ICU admission. 60% - 87% of pa ti en ts with sever e community ac qu ir ed pneumoni a (CAP) admitted to the ICU develops res piratory failure and require mechanical ventilation (MV). Objectives: To assess the efficacy and safety of adjunctive low dose hydrocortisone infusion treatment in Egyptian ICU patients with CAP. Methods: Hospitalized patients, clinically and radiologically diagnosed with CAP, were rand omized t o re ceive hydr ocor tisone 12.5 mg/h IV infusion for 7 days or placeb o, along with antibiotics. The end-points of the study were improvement in PaO2:FIO2 (PaO2:FIO2 > 300 or ≥ 100 increase from st udy entry) and SOFA score by study day 8 and the development of delayed septic shock. Results: 80 patients were recruited, 40 of them received hydrocortisone and the remaining 40 received placebo. By study day 8, hydrocortisone treated pa- tients showed a significant improvement in PaO2:FIO2 and chest radiograph score, and a significant reduction in C-reactive protein (CRP) levels, Sepsis-related Organ Failure Assessment (SOFA) score, and delayed septic shock compared to the control group. Hydro cortisone treatment wa s associated with a sign ificant reduc tion in the duration of MV. However, hydrocortisone infusion did not show significant difference in the ICU mortality. Conclusions: adjunctive 7-day course of low dose hydrocortisone IV in patients with CAP hastens recovery of pneumonia and prevents the de- velopment of sepsis related comp lications with a significant reduction in duration of the mechanical ventilation. Keywords: Community-Acquired Pneumonia, Corticosteroids, Hydrocort isone, Pneumonia 1. Introduction Community acquired pneumonia (CAP), which is a very common reason for hospital admission, represents a po- tentially life-threatening condition [1]. CAP is the first infectious cause of death in developed countries [2], with an estimate of 10% - 25% of CAP patients do not cure in a timely manner [3]. The main reasons behind this delay are: 1) development of complications e.g., post-obstructive pneumonia, empyema, or lung abscess; 2) false percep- tion of treatment failure. This perception may be attrib- uted to the slow recovery in patients have superimposed problems, e.g., malignancy, inflammations, heart failure, or hospital-acquired infection [4]. Patients diagnosed with severe CAP normally require admission to intensive care unit (ICU). More than 50% of the patients admitted to the ICU develop respiratory failure and require mechanical ventilation (MV) with a high mortality rate [5]. Several studies have shown increased pulmonary and circulating inflammatory cytokine levels in patients with severe CAP [6-9]. The levels of these mediators were strongly associated with the presence of pneumonia [6], bacteremia, and need for MV [7], Acute Physiology and Chronic Health Evaluation (APACHE) II and multiple organ dysfunction syndrome (MODS) scores [6,8,9]. It was shown that there is a direct correlation between CAP’s severity and the levels of circulating inflammatory cytokines in CAP patients [6-8,10]. One of the main factors controlling the progression of pneumonia is the host inflammatory response, which in- creases excessively in non-responding pneumonia [11,12]. Many researchers were highly motivated to study the relationship between host response and the level of stress. These studies had a great focus on the role of intact hy- pothalamic-pituitary-adrenal response to prevent the dis- semination of pro-inflammatory storm from one organ to another following local infection [13]. Corticosteroids, the most important natural inhibitors of inflammation, are not always effective in suppressing  Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings 74 life-threatening systemic inflammation. The presence of systemic inflammation-induced tissue resistance to glu- cocorticoids and/or inadequate adrenal output might ex- plain why older clinical trials found no efficacy with a time-limited course of massive doses of glucocorticoids [14,15]. On the other hand, other studies have shown that prolonged treatment with moderate doses of corticoster- oids might improve intracellular down-regulation of in- flammatory cytokine transcription and hasten the resolu- tion of the illness [16,17]. In a multicentre study conducted on CAP patients ad- mitted to the ICU and received antibiotic treatment. The patients were randomly assigned to receive either hydro- cortisone or placebo for 7 days. Treated patients showed a significant improvement in PaO2:FIO2 and chest radio- graph score. Also, a significant reduction in C-reactive protein (CRP) levels, MODS score, and delayed septic shock were observed, with a significant decline in the periods the patients spent in hospital and mortality [18]. Salluh and co-authors investigated four studies fo- cused on the use of corticosteroid in the management of CAP [5]. They showed that, the administration of corti- costeroids demonstrated an improvement in both the physiological and clinical status without evidence for any increased harm. This issue is not new, as the use of steroids as an ad- junct therapy in the management of pneumonia has been recommended for more than 60 years [19]. The encour- aging results of the recent low-dose hydrocortisone trials together with the fact that, till the time of writing this manuscript, glucocorticoids are not regularly used as a treatment of pneumonia in the ICUs in the Egyptian hos- pitals, generated the aim of the present study and evoked a reassessment of the role of glucocorticoids in CAP in the Egyptian ICUs. 2. Aim The aim of the study was to assess the efficacy and safety of adjunctive low dose hydrocortisone infusion treatment in Egyptian ICU patients with CAP. 3. Methodology 3.1. Patients and Settings This study was conducted between July 2010 and Janu- ary 2011 where eighty adult subjects were enrolled in a randomized, double blind, interventional study. The study was conducted at critical care department, 3rd unit, Cairo University, and the National Institute of Chest Diseases, and Intensive Care Unit of Ain-Shams Hospital, Ain- Shams University. Each institutional review board approved the study protocol, and the protocol of the study was registered in ClinicalTrials.gov (ref. number: NCT01228110). Patients to be eligible for this study should be meeting the following criteria (1) presence of CAP, including two minor or one major 1998 American Thoracic Society (ATS) criterion for severe pneumonia which is modified in 2007 [20]. Minor criteria included [18] • Respiratory rate > 30 bpm on admission; • Ratio of PaO2 to fraction of inspired oxygen (PaO2:FIO2) < 250; • Chest radiograph showing bilateral involvement or multilobar involvement; • Systolic blood pressure < 90 mm Hg; or diastolic blood pressure < 60 mm Hg. Major criteria included [18] • Requirement of MV; • Increase in the size of opacities on chest radiograph of 50% at 48 hours; • Requirement of vasopressors > 4 hours; or • Serum creatinine 2 mg/dl or more. Exclusion criteria: • Children • Aspiration or hospital acquired pneumonia; • Discharge from hospital within the previous 14 days; • Transferred from another hospital; • Immunosuppressed patients; • Chronic chest disease; TB, obstructive pneumonia; cystic fibrosis, bronchiectasis; • Concomitant infections (e.g., sinusitis, urinary tract infections); • Congestive heart failure (CHF); • Chronic renal or hepatic disease; • Acute burn injury; • Malignancy; • Pregnancy; and • Major gastrointestinal bleed within 3 months of the current hospitalization. 3.2. Study Design Intervention group patients received maximal conven- tional therapy plus intravenous hydrocortisone (loading dose of 200 mg over 30 minutes, followed by 300 mg in 500 ml 0.9% saline at a rate of 12.5 mg/hr) for 7 days. The Control group subjects were entitled to receive the maximal conventional therapy plus equal volume of in- travenous normal saline solution as placebo. Patients continued to receive standard treatment after Day 7, and in the incidences of hemodynamic instabili- ties, hydrocortisone was administered according to the physicians’ clinical judges. The following variables were recorded: 1) demographic data (age, sex, DOB); Copyright © 2011 SciRes. PP  Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings Copyright © 2011 SciRes. PP 75 2) comorbidities; 3) routine laboratory screen and arterial blood gas (ABG) at least once daily; 4) cause of pneumonia; 5) administered antibiotic regimen; 6) chest X-ray at least on admission and at day 8; and 7) Sepsis-related Organ Failure Assessment (SOFA) score daily 8) days from diagnosis to death. The primary outcomes were improvement in PaO2:FIO2 (PaO2:FIO2 > 300 or ≥ 100 increase from study entry), SOFA score by day 8 and the development of delayed septic shock. The adopted SOFA score was proposed by Vincent et al. [21]. The number of MV-free days was defined as “the number of days after ventilation wa s discontinued ” up to study day 8. Shock was defined as “requirement of vaso- pressors”. ARDS was defined by consensus criteria [22]. 3.3. Statistical Analysis Patients who were placed on systemic steroid therapy concomitant were compared with those not receiving steroids. Descriptive statistics were generated, specifically means, standard deviations and ranges. To minimize type I error, a p value equal to or less than 0.05 was taken as cut-off level for the level of significance. The main tests being used to analyze data extracted from this study are the Mann-Whitney for nominal continuous data. Wilcoxon signed rank test was also used to compare the observed mean difference of the data arising when the same individuals are studied more than once. The chi-squared (X2) test for categorical data used to identify by how much the two observations differ and also whether this difference is more than might reasonably be expected to occur in sampling. Statistical Package for Social Science (SPSS) was used for data analysis. 4. Results In total, 80 patients hospitalized with severe CAP were recruited to be allocated to one of the two previously described groups. Of these 80 subjects, 72.25% were male with a mean (range) age of 62.23 (50 - 72) years. At baseline, the 2 groups were comparable regarding the general characteristics including age, sex, initial body temperature, blood pressure, the diagnosis of underlying diseases, severity of illness, MV and vasopressors re- quirements, and microbiological results. The two groups were also comparable regarding the respiratory rate > 30 bpm, PaO2:FIO2 < 200, chest X-ray assessment, and other parameters at the beginning of the study (Table 1). S. pneumoniae was the most common offending mi- croorganism, followed by L. pneumophila, but the causa- tive pathogen could not be identified in many of the pa- tients as the culture specimen was sampled post antibiot- ics initiation. Almost all patients were initially treated empirically with intravenous antibiotic agents. When the two study groups were compared, a signifi- cant elevation in PaO2:FIO2 was recorded within the hy- drocortisone group by the first day, and within the placebo group by the 4th day (p = 0.027 and 0.01 respectively). By day 8, a PaO2:FIO2 ≥ 300 was observed in 10 (25%) pla- cebo group patients versus 28 (70%) patients in the steroid group with a level of significance of p = 0.003. PaO2:FIO2 improved by ≥ 100 from study entry was observed in 36 (90%) of the study group compared to 12 (30%) patients in the placebo group (p < 0.001) (Table 2). Table 1. Subject baseline characte ristic s in the tw o main groups. Parameter Placebo (N = 40) Hydrocortisone (N = 40) p* Male Distribution (%) 28 (70) 30 (75) 0.723** Average Age in years (SD)† 62.5 (4.26) 61.95 (6.97) 0.850‡ Age range in years 55-70 50 - 72 Average SOFA score (SD) 8.2 (1.46) 8.5 (1.52) 0.75‡ Average Body Temperature, °C (SD) 38.210 (0.43) 37.910 (0.61) 0.114‡ Average WBC count x 109/L (SD) 16.5 (4.11) 17.1 (3.97) 0.232‡ Number of patients on MV (%) 34 (85%) 26 (65%) 0.144** Average PaO2:FIO2 (SD) 182 (52) 178 (48) 0.061‡ Number of patients with PaO2:FIO2 < 200 (%) 28 (70) 32 (80) 0.465** Number of patients with catecholamine-dependent septic shock (%) 4 (10) 8 (20) 0.376** Average CRP (mg/dl) 58.7 (10.44) 55 (15.38) 0.438‡ Average Chest radiograph score (SD) 2.5 (0.58) 2.8 (0.71) 0.0.712‡ *p is the level of significance. ** Chi-square test was used to compare the groups at level of significance of < 0.05. †SD: Standard Deviation. ‡Mann Whitney test was used to compare the groups at level of significance of < 0.05.  Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings 76 Table 2. Clinical and physiological characteristics by study day 8. Parameter Placebo (N = 40) Hydrocortisone (N = 40) p* Mean SOFA score (SD) 3.0 (0.90) 1.1 (0.53) 0.003‡ Number of patients on mechanical ventilation (%) 26 (65%) 10 (25%) 0.011** Mean time from weaning of ventilated patients up to day 8 (SD) 1.2 (0.42) 3.4 (0.58) 0.01‡ Mean PaO2:FIO2 (SD) 243 (45) 338 (39) 0.0008‡ Number of patients with PaO2:FIO2 ≥ 300 (%) 10 (25) 28 (70) 0.004** Number of patients with PaO2:FIO2 improvement ≥ 100 from study entry (%) 12 (30) 36 (90) 0.000** Mean Chest radiograph score (SD) 2.7 (1.34) 1.3 (0.63) 0.0001‡ Number of patients with improvement in chest radiograph score from Day 1 to 8 (%) 10 (25) 36 (90) 0.000** Number of patients with MODS (%) 26 (65) 12 (30) 0.027** Number of patients with Delayed septic shock (%) 14 (35) 2 (5) 0.018** Number of patients with New ARDS (%) 6 (15%) 2 (5%) 0.292** Mean CRP (mg/dl) (range) 36 (3 - 231) 17 (2 - 49) 0.0001‡ Number of Survival by day 8 (%) 34 (85%) 38 (95%) 0.633** * p is the level of significance. ‡Mann Whitney test was used to compare the groups at level of significance of < 0.05. ** Chi-square test was used to compare e groups at level of significance of < 0.05. th At study day 1, 34 versus 26 patients in placebo and hydrocortisone groups respectively were placed on MV (p = 0.18), while at day 8, 26 versus 10 patients in pla- cebo and hydrocortisone groups respectively were still ventilated (p = 0.01). Also, the hydrocortisone group showed a significant reduction (p = 0.002) in the duration of MV by day 8 (Figure 1). group and 95% in the hydrocortisone group with no sig- nificant difference between the two groups (p = 0.66). All non-survivors required MV and had progression of ra- diographic densities on chest radiograph. All (95%) but 2 patients developed pressor-dependent shock. The 2 pa- tients without shock had L. pneumonia that progressed to ARDS. Other complications included two (5%) liver failure cases, and two (5%) lung abscess cases in the placebo group, and two (5%) drug-induced hepatitis con- ditions in the hydrocortisone-treated group (Figure 3). A progressive reduction in CRP values was observed in the hydrocortisone group. A significant difference (p = 0.0001) from the control group was reported by day 8 (Figure 2). Adverse events were few and the two groups were comparable. When the two groups were compared with respect to the major complications experienced by the recruited pa- tients, it was found that 6 (15%) placebo patients developed ARDS, 2 (5%) died by day 5; the other 4 (10%) died by day 7. Survival to ICU discharge was 85% in the placebo 0 10 20 30 40 50 60 70 80 Day 0Day 2Day 4Day 6Day 8 Time (Day) Placebo Group Steroid Group CRP 0 50 100 150 200 250 300 350 Day 0Day 1Day 2Day 3Day 4Day 5Day 6Day 7Day 8 Time (Day) Steroid Group Placebo Group PO2/FIO2 Figure 1. PaO2/FIO2 ratio curve during the study. Figure 2. CRP values during the study. Copyright © 2011 SciRes. PP 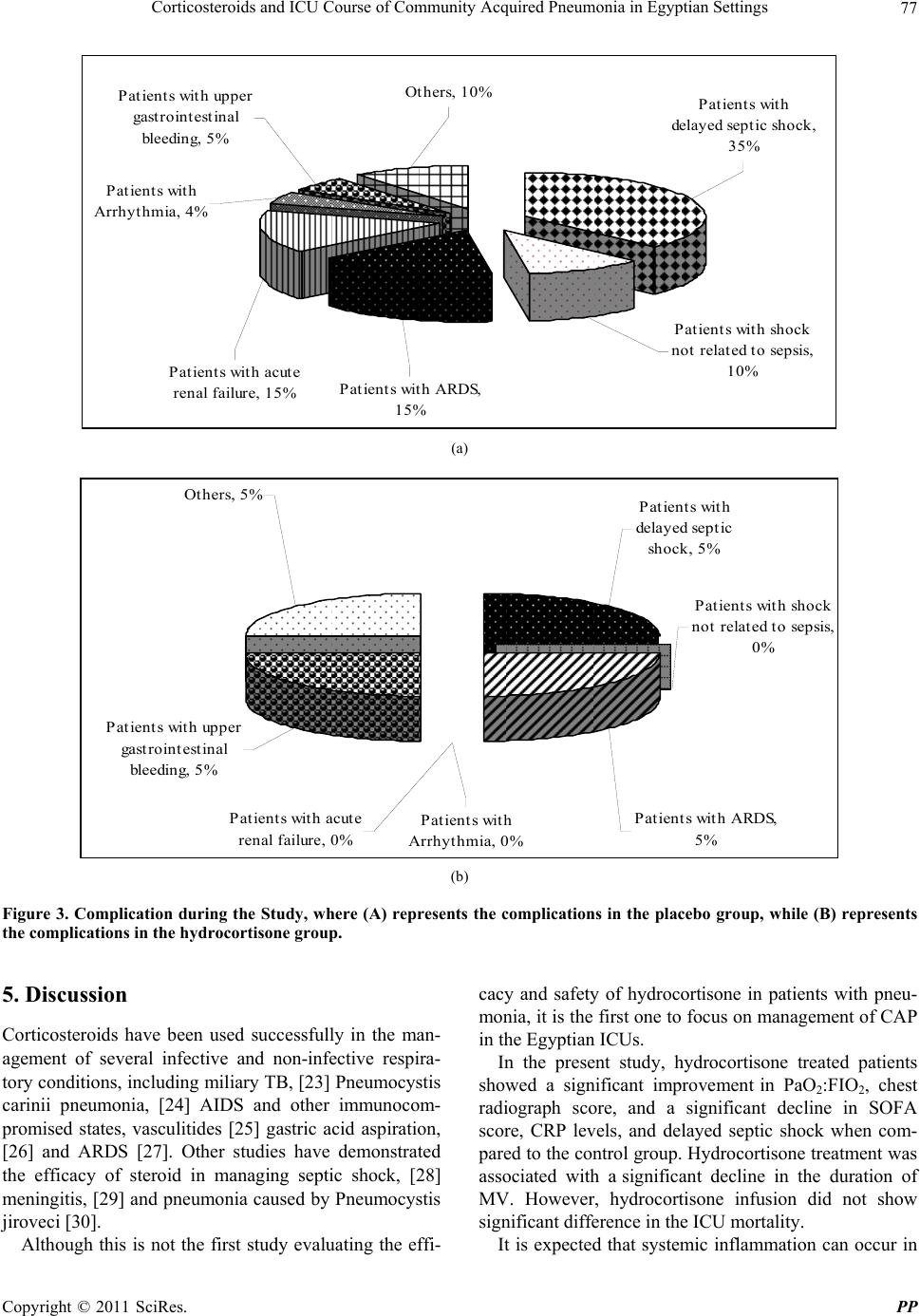 Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings77 Patients with delayed septic shock, 35% Patients with shock not related to sepsis, 10% Patients with ARDS, 15% Patients with acute renal failure, 15% Patients with upper gastrointestinal bleeding, 5% Patients with Arrhythmia, 4% Others, 10% (a) Patients with delayed septic shock, 5% Patients with shock not related to sepsis, 0% Patients with ARDS, 5% Patients with Arrhythmia, 0% Patients with acute renal failure, 0% Patients with upper gastrointestinal bleeding, 5% Others, 5% (b) Figure 3. Complication during the Study, where (A) represents the complications in the placebo group, while (B) represents the complications in the hydrocortisone group. 5. Discussion Corticosteroids have been used successfully in the man- agement of several infective and non-infective respira- tory conditions, including miliary TB, [23] Pneumocystis carinii pneumonia, [24] AIDS and other immunocom- promised states, vasculitides [25] gastric acid aspiration, [26] and ARDS [27]. Other studies have demonstrated the efficacy of steroid in managing septic shock, [28] meningitis, [29] and pneumonia caused by Pneumocystis jiroveci [30]. Although this is not the first study evaluating the effi- cacy and safety of hydrocortisone in patients with pneu- monia, it is the first one to focus on management of CAP in the Egyptian ICUs. In the present study, hydrocortisone treated patients showed a significant improvement in PaO2:FIO2, chest radiograph score, and a significant decline in SOFA score, CRP levels, and delayed septic shock when com- pared to the control group. Hydrocortisone treatment was associated with a significant decline in the duration of MV. However, hydrocortisone infusion did not show significant difference in the ICU mortality. It is expected that systemic inflammation can occur in Copyright © 2011 SciRes. PP  Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings 78 patients with severe CAP. Several studies have reported increased local and systemic inflammatory cytokine lev- els in CAP patients as the first step of the immune re- sponse. These studies recommended the presence of a fine balance between cytokine pro-inflammatory and anti-inflammatory activities, which is very critical in the host response [6,7,31]. Sibila et al. explained in a study conducted on severe pneumonia patients, that despite the inflammatory response initially being compartmentalised, elevation in plasma cytokine levels is detected and was strongly connected to poor prognosis [32]. Yende et al. in 2005, has demonstrated that patients with increased levels of inflammatory cytokines prior to infection were at higher risk of suffering from CAP. This could be attributed to the up-regulated adherence of bac- teria to human alveolar epithelial cells. This happens by increasing expression of receptors that bind to these or- ganisms suggesting an essential role of the inflammatory response in the pathogenicity of the infection [33]. Evidence recommends that early administration of corticosteroids may modify the inflammatory response [34]. It is believed that corticoids inhibit the action of many cytokines associated with pneumonia. At the mo- lecular level, corticosteroids inhibits the transcription of genes coding for cytokines, results in a decrease in the release of macrophage-derived pro-inflammatory cyto- kines [35], which in turn inhibits the T-cells and eosino- phil functions. Production of leukotrienes and pros- taglandins is also inhibited [36]. It is also possible that corticosteroids may favorably alter pulmonary heamo- dynamics [37]. The response to hydrocortisone treatment observed in the present study and in other randomized trials of pa- tients with septic shock [28,38-41] and severe pneumo- cystis pneumonia [42] contrasts with the negative findings of older trials. These negative finding may be related to the short duration of treatment [14,15]. Among a number of studies in support of this hypothesis [14], a randomized trial of patients with severe CAP found that a single dose of hydrocortisone (10 mg/kg) before antibi- otic administration showed no effect on plasma TNF- levels [43]. On the other hand, Monton and coworkers [44] dem- onstrated that the co-administration of steroids with anti- biotic treatment reduces the lung inflammatory responses in severe pneumonia and on MV, which supports the results of the present study. In 2006, Mikami et al. studied the effect of low dose steroids on Japanese adult CAP patients, who received 40 mg intravenous prednisolone for 7 days. The steroids group demonstrated faster stabilization, which lead to the conclusion that, the administration of corticosteroids in moderate-severe CAP, resolve the clinical symptoms and shorten the duration of intravenous antibiotic therapy [45]. The same dose of prednisolone was used for the same duration in a study conducted by Snijders et al., in 2010 and opposing to what was expected, the results were dif- ferent. Although patients on prednisolone had faster de- fervescence and faster decline in serum CRP levels com- pared with placebo, data analysis did not show differences in clinical outcome. Late failure was significantly more common in the patients who had prednisolone than those on placebo. So, it was concluded that 40 mg prednisolone daily for one week does not improve the outcomes in hospitalized patients diagnosed with CAP [46]. Keh et al. [28] confirmed the beneficial effects of low-dose glucocorticoids, where rapid haemodynamic stabilization was achieved in patients with septic shock who received low doses of hydrocortisone. They con- firmed that hydrocortisone attenuates the inflammatory response, but does not have any effect on macrophages and monocytes. The correlation between organ failure, assessed by MODS, and systemic inflammation, assessed by CRP, suggests that a good clinical course is associated with resolution of the inflammatory process whereas a clinical deterioration is associated with ongoing inflammation. The justification of the clinical improvement shown in the present study can be based on what was explained by Confalonieri et al., which was based on possible immu- nomodulatory effects of the steroid infusion, thus accel- erating the development of acute lung injury and multi- organ failure [18]. It was also observed in the present study that, no evi- dence for any increased risk of superinfection, bleeding, or neuromuscular weakness, whereas hypernatremia and hyperglycaemia occurred more frequently in the studied subjects. Sprung et al., showed an increased incidence of superinfection, including new episodes of sepsis or septic shock, in the hydrocortisone group [47]. This variation in the results may be attributed to the variation in the doses used and the duration of the treatment, where in Sprung et al. study, hydrocortisone was used for the longer treat- ment period. Previous studies with high-dose corticoster- oids have shown similar findings to the present study, whereas the study conducted by the Acute Respiratory Distress Syndrome (ARDS) Network [48] using higher doses of corticosteroids and meta-analyses of studies that used low doses [15,49] did not report higher rates of in- fectious complications. Despite these results, it is still important for physicians to adopt prophylactic measures and to screen the patients daily for possible complications. Studies involving critically ill patients have reported an association between corticosteroid therapy and the incidence of neuromuscular weakness [48,50]. We did Copyright © 2011 SciRes. PP  Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings79 not see this in the present study, although electrophysio- logical testing was not performed. Regarding the mortality, in contrast to what was found in the present study, two studies found a significant re- duction in mortality for patients with severe CAP treated with corticosteroids [9,18]. Garcia-Vidal and colleagues [9] evaluated 308 patients in a large retrospective sin- gle-center cohort study and demonstrated that the use of corticosteroids was strongly associated with a lower mor- tality. The discrepancies between the two studies may be attributed to the difference in the type of the study and the adopted type of corticosteroid and the treatment dura- tion period, were these patients were treated with a me- dian dose of 45 mg/day of methylprednisolone for a mean period of 11.4 days. The study of Confalonieri and colleagues [18], showed an impressive reduction in hospital mortality with a 7-day continuous infusion of hydrocortisone (240 mg/day). This difference may be attributed to the difference in the sample size used in the present study (80 patients) and in Confalonieri study (46 patients). Based upon the clinical observations, it is believed that low dose of corticosteroid should be considered in addi- tion to the antibacterial therapy together with an appro- priate supportive care in the management of pneumonia. This study should form the nucleus for other randomized studies in Egypt as no other relevant studies are available nationally. 6. Study Limitation The small sample size was the main weak point in pre- sent study. This can be attributed to the self-funding na- ture of the study. This limited sample size may have bi- ased the estimate of the treatment effect on mortality, a larger randomized trial is recommended to support the mortality findings of this trial. Secondly, old weaning guidelines were used and NIV was not adopted in the two studied centers. However, because of the parallel control nature of the study, the two designed groups were treated in the same way, where any difference in the outcomes between the two studied groups could be attributed to the corticosteroid intervention. Finally, in developing countries, due to limited access to expensive diagnostic procedures, it was difficult to assess the adrenal gland functions and the endogenous steroids. 7. Conclusion Among the hospitalized patients with CAP, hydrocorti- sone was associated with significant clinical improvement, fastens recovery of pneumonia and prevented the devel- opment of sepsis related complications with a significant reduction in MV duration, but it does not affect the mor- tality. At least for now, corticosteroids should routinely be used as adjunctive therapy for CAP in the ICU. 8. Acknowledgements This was a self-funded study. The authors would like to acknowledge the great efforts made by the staff of the ICUs in the study hospitals. Special thanks for Dr. Manal El-Attar for her great help. REFERENCES [1] M. Woodhead, “Community-Acquired Pneumonia,” In: J. Franklin, Ed., Horizons in Medicine, Royal College of Physicians, London, 2004, pp. 165-173. [2] “Statistical Abstract of the United States,” 104th Edition, Washington DC, US Government Printing Office, 1984. [3] S. H. Feinsilver, A. M. Fein, M. S. Niederman, D. E. Schultz and D. H. Faegenburg “Utility of Fiberoptic Bronchoscopy in Nonresolving Pneumonia,” Chest, Vol. 98, No. 6, 1990, pp. 1322-1326. doi:10.1378/chest.98.6.1322 [4] M. S. Niederman, L. A. Mandell, A. Anzueto, J. B. Bass, W. A. Broughton, G. D. Campbell, N. Dean, T. File, M. J. Fine, P. A. Gross, F. Martinez, T. J. Marrie, J. F. Plouffe, J. Ramirez, G. A. Sarosi, A. Torres, R. Wilson and V. L. Yu, “Guide Lines for the Management of Adults with Community-Acquired Pneumonia. Diagnosis, Assessment of Severity, Antimicrobial Therapy, and Prevention,” American Journal of Respiratory and Critical Care Medicine, Vol. 163, No. 7, 2001, pp. 1730-1754. [5] J. Salluh, P. Póvoa, M. Soares, H. Castro-Faria-Neto, F. Bozza and P. Bozza, “The Role of Corticosteroids in Se- vere Community-Acquired Pneumonia: A Systematic Review,” Critical Care, Vol. 12, No. 3, 2008, pp. 76-82. doi:10.1186/cc6922 [6] C. Monton, A. Torres, M. El-Ebiary, X. Filella, A. Xaubet and J. P. De la Bellacasa, “Cytokine Expression in Severe Pneumonia: A Bronchoalveolar Lavage Study,” Critical Care Medicine, Vol. 27, No. 9, 1999, pp. 1745- 1753. doi:10.1097/00003246-199909000-00008 [7] S. Fernandez-Serrano, J. Dorca, M. Coromines, J. Car- ratala, F. Gudiol and F. Manresa, “Molecular Inflamma- tory Responses Measured in Blood of Patients with Se- vere Community-Acquired Pneumonia,” Clinical and Diagnostic Laboratory Immunology, Vol. 10, No. 5, 2003, pp. 813-820. [8] G. Antunes, S. A. Evans, J. L. Lordan and A. J. Frew, “Systemic Cytokine Levels in Community-Acquired Pneumonia and Their Association with Disease Severity,” European Respiratory Journal, Vol. 20, No. 4, 2002, pp. 990-995. doi:10.1183/09031936.02.00295102 [9] C. Garcia-Vidal, E. Calbo, V. Pascual, C. Ferrer, S. Quintana and J. Garau, “Effects of Systemic Steroids in Patients with Severe Community-Acquired Pneumonia,” European Respiratory Journal, Vol. 30, No. 5, 2007, pp. Copyright © 2011 SciRes. PP  Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings 80 951-956. doi:10.1183/09031936.00027607 [10] H. Schutte, J. Lohmeyer, S. Rosseau, S. Ziegler, C. Siebert, H. Kielisch, H. Pralle, F. Grimminger, H. Morr, and W. Seeger, “Bronchoalveolar and Systemic Cytokine Profiles in Patients with ARDS, Severe Pneumonia and Cardiogenic Pulmonary Oedema,” European Respiratory Journal, Vol. 9, No. 9, 1996, pp. 1858-1867. doi:10.1183/09031936.96.09091858 [11] R. Mene´ndez, A. Torres, R. Zalacain, J. Aspa, J. J. Mar- tin Villasclaras, L. Borderias, J. M. Benitez Moya, J. Ruiz-Manzano, F. Rodriguez de Castro, J. Blanquer, D. Perez, C. Puzo, F. Sanchez Gascon, J. Gallardo, C Alva- rez and L. Molino, “Predictive Factors of Clinical Stabil- ity in Community-Acquired Pneumonia,” Thorax, Vol. 59, 2004, pp. 960-965. [12] M. Ioanas, M. Ferrer, M. Cavalcanti, .R Ferrer, S. Ewig, X. Filella, J. P. dela Bellacasa and A. Torres, “Causes and Predictors of Nonresponse to Treatment of ICU-Acquired Pneumonia,” Critical Care Medicine, Vol. 32, No. 4, 2004, pp. 938-945. doi:10.1097/01.CCM.0000114580.98396.91 [13] G. P. Chrouso, “Stressors, Stress, and Neuroendocrine Integration of the Adaptive Response,” The 1997 Hans Selye Memorial Lecture, Annals New York Academy of Sciences, Vol. 851, 1998, pp. 311-335. doi:10.1111/j.1749-6632.1998.tb09006.x [14] G. U. Meduri, “An Historical Review of Glucocorticoid Treatment in Sepsis. Disease Pathophysiology and the Design of Treatment Investigation,” Sepsis, Vol. 3, 1999, pp. 21-38. doi:10.1023/A:1009870524359 [15] P. C. Minneci, K. J. Deans, S. M. Banks, P. Q. Eichacker, and C. Natanson, “Meta-Analysis: The Effect of Steroids on Survival and Shock during Sepsis Depends on the Dose,” Annals of International Medicine, Vol. 141, No. 1, 2004, pp. 47-56. [16] P. E. Marik, S. M. Pastores, D. Annane, G. U. Meduri, W. Arlt, C. L. Sprung, D. Keh, J. Briegel, A. Beishuizen, I. Dimopoulou, S. Tsagarakis, M. Singer, G. P. Chrousos, G. Zaloga, F. Bokhari and M. Vogeser, “Clinical Practice Guidelines for the Diagnosis and Management of Corti- costeroid Insufficiency in Critical Illness: Recommenda- tions of an International Task Force,” Critical Care Medicine, Vol. 36, No. 6, 2008, pp. 1937-1949. doi:10.1097/CCM.0b013e31817603ba [17] M. Mer, and G. A. Richards, “Pneumonia Corticosteroids in Life-Threatening Varicella,” Chest, Vol. 114, No. 2, 1998, pp. 426-431. doi:10.1378/chest.114.2.426 [18] M. Confalonieri, R. Urbino, A. Potena, M. Piattella, P. Parigi, G. Puccio, R. Della Porta, C. Giorgio, F. Blasi, R. Umberger and G. U. Meduri, “Hydrocortisone Infusion for Severe Community-Acquired Pneumonia: A Prelimi- nary Randomized Study,” American Journal of Respira- tory and Critical Care Medicine, Vol. 171, No. 3, 2005, pp. 242-248. [19] D. Perla and J. Marmorston, “Suprarenal Cortical Hor- mone and Salt in the Treatment of Pneumonia and Other Severe Infections,” Endocrinology, Vol. 27, No. 3, 1940, pp. 367-374. doi:10.1210/endo-27-3-367 [20] S. Ewig, M. Ruiz, J. Mensa, M. A. Marcos, J. A. Marti- nez, F. Arancibia, M. S. Niederman and A. Torres, “Se- vere Community-Acquired Pneumonia: Assessment of Severity Criteria,” American Journal of Respiratory and Critical Care Medicine, Vol. 158, No. 4, 1998, pp. 1102 -1108. [21] J. L. Vincent, R. Moreno, J. Takala, S. Willatts, A. De Mendonça, H. Bruining, C. K. Reinhart, P. M. Suter, and L. G. Thijs. “The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Fai- lure,” Intensive Care Medicine, Vol. 22, No. 7, 1996, pp. 707-710. doi:10.1007/BF01709751 [22] G. R. Bernard, A. Artigas, K. L. Brigham, J. Carlet, K. Falke, L. Hudson, M. Lamy, J. R. LeGall, A. Morris and R. Spragg, “The American-European Consensus Confer- ence on ARDS: Definitions, Mechanisms, Relevant Out- comes, and Clinical Trial Coordination,” American Jour- nal of Respiratory and Critical Care Medicine, Vol. 149, No. 3, 1994, pp. 818-24. [23] T. Senderovitz and K. Viskun, “Corticosteroids in Tu- berculosis,” Respiratory Medicine, Vol. 88, 1994, pp. 561-565. doi:10.1016/S0954-6111(05)80002-2 [24] S. A. Bozette, F. R. Sattler and J. Chiu, “A Controlled Trial of Early Adjunctive Treatment with Corticosteroids for Pneumocystis Carinii Pneumonia in the Acquired Immunodeficiency Syndrome,” New England Journal of Medicine, Vol. 323, No. 21, 1990, pp. 1451-1457. doi:10.1056/NEJM199011223232104 [25] R. H. Leavitt and A. S. Fauci, “Pulmonary Vasculitis,” American Review of Respiratory Diseases, Vol. 134, No. 1, 1986, pp. 149-166 [26] W. K. Bannister, A. J. Sattilano and R. D. Otis, “Thera- peutic Aspects of Aspiration Pneumonitis in Experimen- tal Animals,” Anesthiology, Vol. 23, 1961, pp. 440-445. doi:10.1097/00000542-196105000-00017 [27] C. U. Meduri, S. Headley, S. Carson, et al. “Methylpred- nisolone Treatment of Late ARDS,” American Journal of Respiratory and Critical Care Medicine, Vol. 155, 1997, p. A391. [28] D. Keh, T. Boehnke, S. Weber-Cartens, C. Schulz, O. Ahlers, S. Bercker, H. Volk, W. Doecke, K. J. Falke and H. Gerlach, “Immunologic and Hemodynamic Effects of ‘Low-Dose’ Hydrocortisone in Septic Shock. A Double Blind, Randomized, Placebo-Controlled, Crossover Study,” American Journal of Respiratory and Critical Care Medicine, Vol. 167, No. 4, 2003, pp. 512-520. doi:10.1164/rccm.200205-446OC [29] L. A. Mandell, R. G. Wunderink, A Anzueto, J. G. Bart- lett, G. D. Campbell, A. Torres and C. G. Whitney, “Guidelines for the Initial Management of Adults with Community-Acquired Pneumonia: Diagnosis, Assess- ment of Severity, and Initial Antimicrobial Therapy. American Thoracic Society. Medical Section of the American Lung Association,” American Review of Res- piratory Diseases, Vol. 148, No. 5, 2007, pp. 1418-1426. [30] M. Briel, R. Boscacci, H. Furrer and H. C. Bucher, “Ad- junctive Corticosteroids for Pneumocystis Jiroveci Pneu- monia in Patients with HIV infection: A Meta-Analysis of Copyright © 2011 SciRes. PP  Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings Copyright © 2011 SciRes. PP 81 Randomized Controlled Trials,” BMC Infectious Diseases, Vol. 5, 2005, p. 101. doi:10.1186/1471-2334-5-101 [31] J. Puren, C. Feldman, N. Savage, P. J. Becker and C. Smith, “Patterns of Cytokine Expression in Community- Acquired Pneumonia,” Chest, Vol. 107, No. 5, 1995, pp. 1342-1349. doi:10.1378/chest.107.5.1342 [32] Sibila, C. Agusti and A. Torres, “Corticosteroids in Se- vere Pneumonia,” European Respiratory Journal, Vol. 32, No. 2, 2008, pp. 259-264. doi:10.1183/09031936.00154107 [33] S. Yende, E. I. Tuomanen, R. Wunderink, A. Kanaya, A. B. Newman, T. Harris, N. De Rekeneire and S. B. Kritchevsky, “Preinfection Systemic Inflammatory Mark- ers and Risk of Hospitalization Due to Pneumonia,” American Journal of Respiratory and Critical Care Medicine, Vol. 172, No. 11, 2005, pp. 1440-1446. doi:10.1164/rccm.200506-888OC [34] C. U. Meduri, “The Role of the Host Defense Response in the Progression and Outcome of ARDS: Pathophysi- ological Correlations and Response to Gluococorticoid Treatment,” European Respiratory Journal, Vol. 9, No. 12, 1996, pp. 2650-2670. doi:10.1183/09031936.96.09122650 [35] P. J. Barnes and I. Adcock, “Anti-Inflammatory Actions of Steroids: Molecular Mechanisms,” Trends in Pharma- cological Sciences, Vol. 14, No. 12, 1993, pp. 436-441. doi:10.1016/0165-6147(93)90184-L [36] S. Hong and L. Levine, “Inhibition of Arachidonic Acid Release from Cells as the Biochemical Actions of Anti-Inflammatory Steroids,” Proceedings of the Na- tional Academy of Sciences USA, Vol. 73, No. 5, 1976, pp. 1730-1733. doi:10.1073/pnas.73.5.1730 [37] T. J. K. Toung, D. Bordos, D. W. Benson, D. Carter, G. D. Zuidema, S. Permutt and L. Cameron, “Aspiration Pneu- monia: Experimental Evaluation of Albumin and Steroid Therapy,” Annals of Surgery, Vol. 183, No. 2, 1976, pp. 179-184. doi:10.1097/00000658-197602000-00016 [38] P. E. Bollaert, C. Charpentier, B. Levy, M. Debouverie, G. Audibert and A. Larcan, “Reversal of Late Septic Shock with Supraphysiologic Doses of Hydrocortisone,” Criti- cal Care Medicine, Vol. 26, No. 4, 1998, pp. 645-650. doi:10.1097/00003246-199804000-00010 [39] K. Chawla, Y. Kupfer, I. Goldman and S. Tessler, “Hy- drocortisone Reverses Refractory Septic Shock,” Critical Care Medicine, Vol. 27, No. 1, 1999, p. A33. doi:10.1097/00003246-199901001-00022 [40] Yildiz, M. Doganay, B. Aygen, M. Guven, F. Keleutimur and A. Tutuu, “Physiological-Dose Steroid Therapy in Sepsis,” Critical Care, Vol. 6, No. 3, 2002, pp. 251-259. doi:10.1186/cc1498 [41] D. Annane, V. Sebille, C. Charpentier, P. E. Bollaert, B. Francois, J. M. Korach, G. Capellier, Y. Cohen, E. Azou- lay, G. Troche, et al., “Effect of Treatment with Low Doses of Hydrocortisone and fludrocortisone on Mortality in Patients with Septic Shock,” Journal of the American Medical Association, Vol. 288, No. 7, 2002, pp. 862-871. doi:10.1001/jama.288.7.862 [42] M. A. Jantz and S. A. Sahn, “Corticosteroids in Acute Respiratory Failure,” American Journal of Respiratory and Critical Care Medicine, Vol. 160, No. 4, 1999, pp. 1079-1100. [43] P. Marik, P. Kraus, J. Sribante, I. Havlik, J. Lipman and D. W. Johnson, “Hydrocorisone and Tumor Necrosis Fac- tor in Severe Community-Acquired Pneumonia: A Ran- domized Controlled Study,” Chest, Vol. 104, No. 2, 1993, pp. 389-392. doi:10.1378/chest.104.2.389 [44] C. Monton, S. Ewig, A. Torres, M. El-Ebiary, X. Filella, A. Rano and A. Xaubet, “Role of Glucocorticoids on In- flammatory Response in Nonimmunosuppressed Patients with Pneumonia: A Pilot Study,” European Respiratory Journal, Vol. 14, No. 1, 1999, pp. 218-220. doi:10.1034/j.1399-3003.1999.14a37.x [45] K. Mikami, M. Suzuki, H. Kitagawa, M. Kawakami, N. Hirota, H. Yamaguchi, O. Narumoto, Y. Kichikawa, M. Kawai, H. Tashimo, H. Arai, T. Horiuchi and Y. Saka- moto, “Efficacy of Corticosteroids in the Treatment of Community-Acquired Pneumonia Requiring Hospitaliza- tion,” Annals of International Medicine, Vol. 141, 2006, pp. 47-56. [46] D. Snijders, M. Johannes, A. Daniels, S. Casper, S. De Graaff, S. Tjip, F. Van der Wer, G. Wim and A. Boersma, “Efficacy of Corticosteroids in Community-acquired Pneumonia,” American Journal of Respiratory and Criti- cal Care Medicine, Vol. 181, No. 9, 2010, pp. 975-982. doi:10.1164/rccm.200905-0808OC [47] C. L. Sprung, D. Annane, D. Keh, R. Moreno, M. Singer, K. Freivogel, Y. G. Weiss, J. Benbenishty, A. Kalenka, H. Forst, P. F. Laterre, K. Reinhart, B. H. Cuthbertson, D. Payen and J. Briegel, “Hydrocortisone Therapy for Pa- tients with Septic Shock,” New England Journal of Medi- cine, Vol. 358, No. 2, 2008, pp. 111-124. doi:10.1056/NEJMoa071366 [48] D. Annane, E. Bellissant, P. E. Bollaert, J. Briegel, D. Keh and Y. Kupfer, “Corticosteroids for Severe Sepsis and Septic Shock: A Systematic Review and Meta- Analysis,” British Medical Association Journal, Vol. 329, No. 7464, 2004, pp. 480-488. [49] K. P. Steinberg, L. D. Hudson, R. B. Goodman, et al., “Efficacy and Safety of Corticosteroids for Persistent Acute Respiratory Distress Syndrome,” New England Journal of Medicine, Vol. 354, No. 16, 2006, pp. 1671- 1684. [50] B. De Jonghe, T. Sharshar, J. P. Lefaucheur, et al., “Pare- sis Acquired in the Intensive Care Unit: A Prospective Multicenter Study,” Journal of the American Medical Association, Vol. 288, No. 22, 2002, pp. 2859-2867. doi:10.1001/jama.288.22.2859
|