 Pharmacology & Pharmacy, 2011, 2, 47-55 doi:10.4236/pp.2011.22006 Published Online April 2011 (http://www.SciRP.org/journal/pp) Copyright © 2011 SciRes. PP 47 Antitrypanosomal Activity of a Semi-Purified Subfraction Rich in Labdane Sesquiterpenes, Obtained from Flowers of Anthemis tinctoria, against Trypanosoma cruzi Nilza de Lucas Rodrigues Bittencourt1, Tânia Ueda-Nakamura1,2, Benedito Prado Dias Filho1,2, Celso Vataru Nakamura1,2 1Programa de Pós-graduação em Ciências Farmacêuticas; 2Departamento de Ciências Básicas da Saúde, Laboratório de Inovação Tecnológica no Desenvolvimento de Fármacos e Cosméticos, Universidade Estadual de Maringá, Maringá, Paraná, Brazil. Email: cvnakamura@gmail.com Received September 27th, 2010; revised December 15th, 2010; accepted December 29th, 2010. ABSTRACT In Brazil and several other Latin American countries, Chagas’ disease still constitutes a serious medical and social problem, and there is a need to develop new, more-potent drugs with fewer side effects to effectively treat this disease. We investigated the antitrypanosomal effect of a crude extract, fractions, and a semi-purified subfraction rich in a mix- ture of isomeric labdane sesquiterpenes, obtained from flowers of Anthemis tinctoria, against Trypanosoma cruzi. In epimastigote forms, the aqueous crude extract, dichloromethane fraction, and semi-purified subfraction showed a dose-dependent inhibitory activity, with IC50 of 2.3 μg/ml, 1.8 μg/ml, and 0.2 μg/ml, respectively. In the interaction in- dex, the semi-purified subfraction showed a reduction in both the percentage of infected LLCMK2 cells and the mean number of amastigotes per infected cell. The cytotoxicity evaluation demonstrated that the cytotoxic concentrations of the semi-purified subfraction were higher for LLCMK2 cells than for the protozoans, with a selectivity index of 35.0. Epimastigote forms treated with the semi-purified subfraction showed ultrastructural and morphological alterations such as rounding of the cells and bleb formation in the flagellum and cytoplasmic membrane. These results show that the flowers from A. tinctoria may be a source of new drugs with antiprotozoal activity. However, additional in vitro and in vivo studies are needed to validate the use of A. tinctoria in the treatment of Chagas’ disease. Keywords: Antiprotozoan Activity, Medicinal Plants, Trypanosoma cruzi, Ultrastructure Alterations 1. Introduction About 65% - 80% of the population in developing countries essentially depends on plants for primary health care. Some 25% of all modern medicines are derived directly or indirectly from plants [1]. Many plants from Brazilian ecosystems such as the savanna, and the Atlantic and Amazon forests are used in traditional medi- cine [2]. Also, many exotic plants that were introduced into Brazil and incorporated into traditional medicine display curative properties [3]. Various studies have demonstrated a strong correlation between popular use and experimenttally demonstrated pharmacological acti- vity. Many plant extracts and essential oils have been shown to exert in vitro and in vivo activity, which jus- tifies research on plants used in traditional medicine [4]. Plants produce a variety of compounds with antimi- crobial properties, which have led to the development of new drugs for treatment of infectious diseases [5]. The family Compositae is one of the most species-rich among the flowering plants. Anthemis L. is the second- largest genus in this family with approximately 25 000 species, widely distributed in subtropical and temperate areas. Species of Anthemis are widely used in the phar- maceutical, cosmetic and food industries. Anthemis tinc- toria L. is a perennial herb cultivated in Mediterranean countries, and several secondary metabolites have been identified in this species, such as volatile oils, triterpenes, polyacetylenes, and flavonoids [6]. In traditional medi- cine, this plant is used to treat liver problems and jaun- dice [7]. Its flowers have well-known antiseptic and me- dicinal properties, derived from flavonoids as well as es-  Antitrypanosomal Activity of a Semi-Purified Subfraction Rich in Labdane Sesquiterpenes, Obtained 48 from Flowers of Anthemis tinctoria, against Trypanosoma cruzi sential oils [8]. In Europe, extracts, dyes, teas, and oint- ments are used as anti-inflammatory, antibacterial, anti- spasmodic, and sedative agents [9]. The antimicrobial ac- tivity of essential oils and extracts from different species of Anthemis has been studied previously [10,11]. Trypanosoma cruzi is the etiologic agent of Chagas’ disease, which in Brazil and several other Latin Ameri- can countries constitutes, a serious social and medical problem [12]. Transmission to vertebrates occurs through feces of hemipteran insects contaminated with metacyclic trypomastigotes, the infective stage of the parasite [13]. The acute phase of Chagas’ disease is frequently asymp- tomatic, and the chronic phase usually develops 10 to 20 years after the infection, affecting about 10% to 30% of infected individuals [14]. In spite of the impressive progress in the understand- ing of the biology of T. cruzi, the drugs available (nifur- timox and benzonidazole) are active on the acute stage of Chagas’ disease, with about 80% effectiveness; but have limited utility against the established chronic disease [15]. The side effects of both compounds can be quite severe [16]. Therefore, trypanocidal drugs with less-serious side effects are necessary. In this context, plants are a reser- voir of chemical and biological diversity that has led to the development of hundreds of pharmaceutical drugs [17]. The objective of the present study was to investi- gate the activity of the extracts, fractions, and a sequiter- pene-rich semi-purified subfraction, obtained from flow- ers of A. tinctoria, against T. cruzi. 2. Materials and Methods 2.1. General Experimental Procedures The NMR spectra were obtained in VARIAN GEMINI 300 (7.05 T) spectrometers, using deuterated solvent, TMS as the internal standard and a constant temperature of 298 K. Sephadex LH-20; Silica gel 60 (70 - 230 and 230 - 400 mesh); TLC: silica gel plates F254 (0.25 mm thickness). 2.2. Collections of the Plant Flowers of Anthemis tinctoria were collected in Novem- ber 2004 at the “Profa. Irenice Silva” Garden of Medici- nal Plants of the State University of Maringá. The plant was identified through authentic comparison by Dr. Cir- ino Correia Júnior, and a voucher specimen (No. HUM 1133) is deposited at the Herbarium of the State Univer- sity of Maringá, Paraná, Brazil. 2.3. Separation of the Components Dried flowers of A. tinctoria (130 g) were extracted with ethanol:water (9:1 v/v) by maceration for 8 days at room temperature. The solvent was removed in a rotating evaporator, to give an aqueous extract and a dark-green residue. The aqueous extract was lyophilised (21.0 g) and the water-insoluble residue was diluted with ethyl-acetate, yielding the ethyl-acetate extract (4.71 g). The aqueous and ethyl-acetate extracts were assayed against the epi- mastigote form of T. cruzi. The aqueous extract (13 g) - was submitted to vacuum-column chromatography (32 g silica gel) and eluted with hexane, dichloromethane, ethyl acetate, methanol, and methanol/water (9:1 v/v). Each fraction (hexane, F1; dichloromethane, F2; ethyl acetate, F3; methanol, F4; and methanol/water 9:1, F5) was as- sayed for antitrypanosomal activity. The dichloromethane fraction (500 g), which showed the highest inhibitory activity, was chromatographed by column chromatography in Sephadex-LH-20 and eluted with chloroform/methanol (1:1 v/v). This process yielded 8 subfractions, denomi- nated F2a, F2b, F2c, F2d, F2e, F2f, F2g, and F2h. Sub- fraction F2 g (140 g) was chromatographed in Sephadex phase with movable phase, chloroform/methanol (1:1 v/v), yielding the subfraction (11 g). The subfraction was identified as a mixture of isomeric labdane ses- quiterpenes by analyses of spectral data of 1H and 13C (Chart 1). 2.4. Parasites Epimastigote forms of T. cruzi Y strain were cultured in LIT (Liver Infusion Triptone broth) [18] with 10% fetal bovine serum (SFB) (Gibco Invitrogen Corporation, New York, USA) at 28˚C for 96 h. Amastigote and trypomas- tigote forms were grown in monolayers of LLCMK2 cells in DMEM medium (Gibco Invitrogen) with 10% SFB in 5% CO2 at 37˚C. 2.5. Antiproliferative Activity against Epimastigote Forms The aqueous and ethyl-acetate crude extracts, fractions (hexane, dichloromethane, ethyl acetate, methanol, and methanol/water ) and the semi-purified subfraction were dissolved in dimethyl sulphoxide (DMSO, Sigma Chemi- cal Co., St. Louis, Missouri, USA) at a final concentra- tion not exceeding 1% [19] and assayed against the epi- mastigote form of T. cruzi. The experiments were per- formed on 24-well polystyrene plates containing 1 ml of diluted compound at different concentrations (from 1.0 to 1,000 μg/ml). The starting inoculum consisted of 106 parasites in logarithmic growth phase per well. The cells were incubated at 28˚C and the growth was determined by counting the parasites with a Neubauer hemocytome- ter (Improved Double Neubauer Ruling) every 24 h over a 7-day period. Benznidazole (N-benzyl-2-nitro-1-imida- zolacetamide, Roche Pharmaceuticals, Rio de Janeiro, Copyright © 2011 SciRes. PP 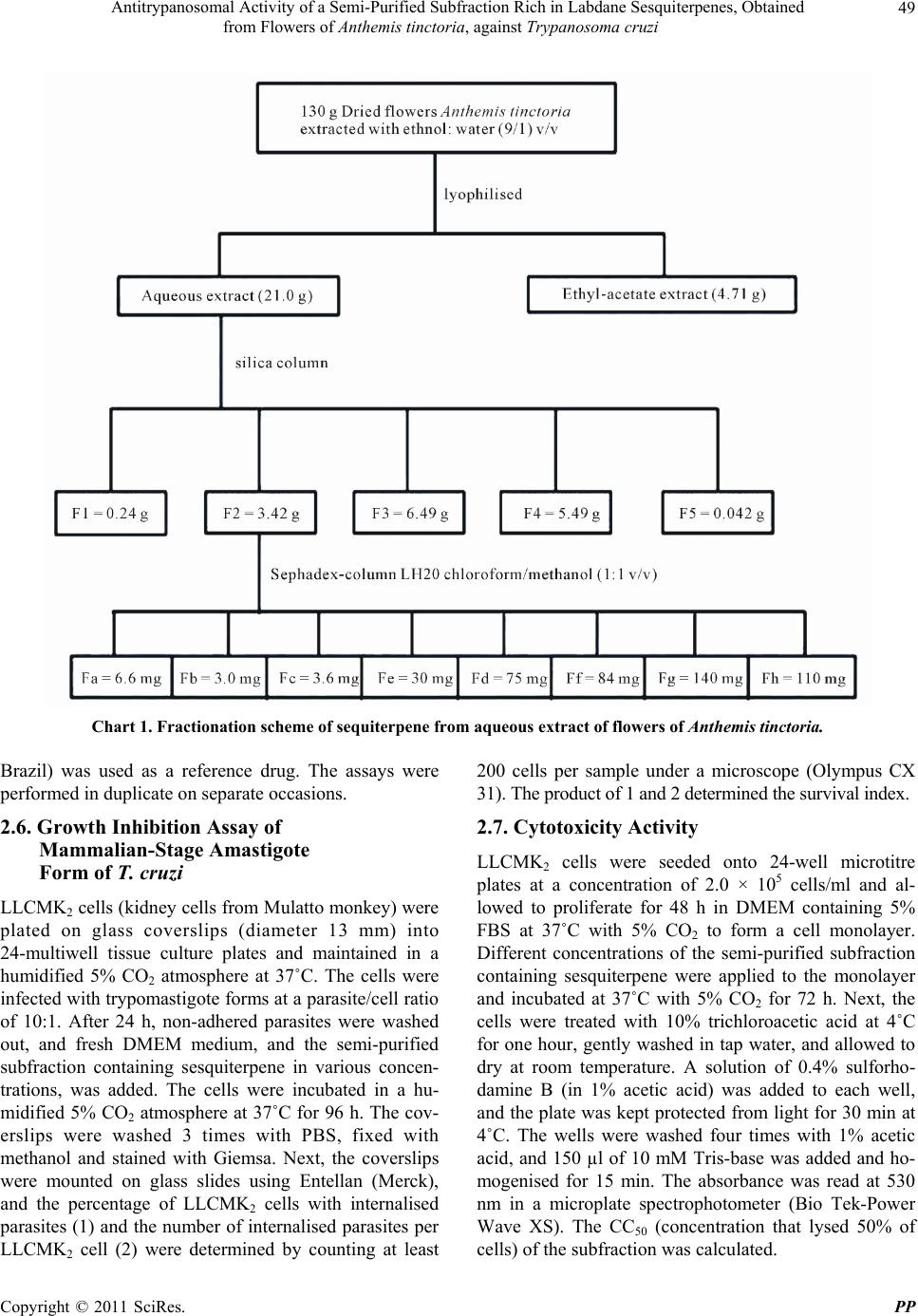 Antitrypanosomal Activity of a Semi-Purified Subfraction Rich in Labdane Sesquiterpenes, Obtained from Flowers of Anthemis tinctoria, against Trypanosoma cruzi Copyright © 2011 SciRes. PP 49 Chart 1. Fractionation scheme of sequiterpene from aqueous extract of flowers of Anthe m is t inctoria. Brazil) was used as a reference drug. The assays were performed in duplicate on separate occasions. 2.6. Growth Inhibition Assay of Mammalian-Stage Amastigote Form of T. cruzi LLCMK2 cells (kidney cells from Mulatto monkey) were plated on glass coverslips (diameter 13 mm) into 24-multiwell tissue culture plates and maintained in a humidified 5% CO2 atmosphere at 37˚C. The cells were infected with trypomastigote forms at a parasite/cell ratio of 10:1. After 24 h, non-adhered parasites were washed out, and fresh DMEM medium, and the semi-purified subfraction containing sesquiterpene in various concen- trations, was added. The cells were incubated in a hu- midified 5% CO2 atmosphere at 37˚C for 96 h. The cov- erslips were washed 3 times with PBS, fixed with methanol and stained with Giemsa. Next, the coverslips were mounted on glass slides using Entellan (Merck), and the percentage of LLCMK2 cells with internalised parasites (1) and the number of internalised parasites per LLCMK2 cell (2) were determined by counting at least 200 cells per sample under a microscope (Olympus CX 31). The product of 1 and 2 determined the survival index. 2.7. Cytotoxicity Activity LLCMK2 cells were seeded onto 24-well microtitre plates at a concentration of 2.0 × 105 cells/ml and al- lowed to proliferate for 48 h in DMEM containing 5% FBS at 37˚C with 5% CO2 to form a cell monolayer. Different concentrations of the semi-purified subfraction containing sesquiterpene were applied to the monolayer and incubated at 37˚C with 5% CO2 for 72 h. Next, the cells were treated with 10% trichloroacetic acid at 4˚C for one hour, gently washed in tap water, and allowed to dry at room temperature. A solution of 0.4% sulforho- damine B (in 1% acetic acid) was added to each well, and the plate was kept protected from light for 30 min at 4˚C. The wells were washed four times with 1% acetic acid, and 150 µl of 10 mM Tris-base was added and ho- mogenised for 15 min. The absorbance was read at 530 nm in a microplate spectrophotometer (Bio Tek-Power Wave XS). The CC50 (concentration that lysed 50% of cells) of the subfraction was calculated.  Antitrypanosomal Activity of a Semi-Purified Subfraction Rich in Labdane Sesquiterpenes, Obtained 50 from Flowers of Anthemis tinctoria, against Trypanosoma cruzi 2.8. Evaluation of Morphological Alterations by Scanning Electron Microscopy Epimastigote forms treated with IC50 (0.2 µg/ml) or IC90 (1.0 µg/ml) of the semi-purified sesquiterpene subfrac- tion for 96 h were collected by centrifugation, washed in PBS and fixed with 2.5% glutaraldehyde in 0.1 M so- dium cacodylate buffer, pH 7.2, containing 1.0 mM CaCl2 at 4˚C. After fixation, small drops of the sample were placed on a specimen support with poly-L-lysine. Subsequently, the samples were dehydrated in graded ethanol, critical-point dried in CO2, sputter-coated with gold and observed in a SHIMADZU SS-550 Scanning electron microscope. 2.9. Evaluation of the Ultrastructural Alterations in T. cruzi by Transmission Electron Microscopy After treatment with IC50 or IC90 of the semi-purified sesquiterpene subfraction for 96 h, epimastigote forms were washed in 0.01 M phosphate-buffered saline and fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2. The cells were postfixed in a solution containing 1% OsO4 and 0.8% potassium ferrocyanide in 0.1 M cacodylate buffer, dehydrated in acetone, and em- bedded in Epon. Thin sections were collected on a cop- per grid (300 mesh), stained with uranyl acetate and lead citrate, and observed in a Zeiss 900 transmission electron microscope. 2.10. Statistical Analysis Statistical analysis was performed with the program GraphPad Prism 4 (GraphPad Software, San Diego, California, USA). One-Way Anova was applied, and a p- value less than 0.05 was regarded as significant. 3. Results and Discussion In the attempt to develop new drugs for treatment of in- fectious diseases, studies are carried out with compounds of both natural and synthetic origin. Many studies have demonstrated that crude extracts, fractions, and com- pounds isolated from medicinal plants exhibit antiproton- zoal activity [20-26]. In this study, we evaluated the anti- trypanosomal activity of the crude extract, fractions, and a subfraction rich in a mixture of isomeric labdane ses- quiterpenes, obtained from flowers of A. tinctoria, against epimastigote, and amastigote forms of T. cruzi. The analyses of the 1H and 13C NMR spectrum of the semi-purified subfraction showed a signal of a mixture of isomers of cross-conjugated terpenoid ketones derived from labdane sesquiterpenes that are frequently found in the family Asteraceae [27]. Three types of classes of secondary metabolites have been detected in Anthemis: polyacetylenes [28], flavonoids [29], and sesquiterpene lactones [30]. Previous chemical studies of species of Anthemis have shown the presence of sesquiterpene lac- tones [31-37]. The three major types of sesquiterpene lactones are germacrolides, eudesmanolides, and guaia- nolides [38]. Recently it was demonstrated that anthecu- larin, a sesquiterpene lactone isolated from Anthemis auriculata, shows antimalarial activity, and also antitry- panosomal activity against Trypanosoma brucei [39]. The hydroalcoholic crude extracts, fractions, and semi- purified subfraction obtained from A. tinctoria flowers were used in order to investigate the antiprotozoal activ- ity of this plant against T. cruzi. A progressive increase in the antitrypanosomal effect was observed in the course of the purification process. Figure 1(a) shows the per- centage of growth inhibition of the epimastigote form treated with the aqueous phase of the crude extract for 96 h of incubation at 28˚C. This extract showed a dose-de- pendent inhibitory activity of 77.4% and 91.9% at 5 and 10 μg/ml, respectively. In the same concentrations, the ethyl-acetate extract showed an inhibitory activity of 31.7% and 76.8%. The 50% inhibitory concentration (IC50) of the crude extract aqueous phase was 2.3 g/ml. On the basis of this finding, the crude aqueous extract was fractionated on silica gel into five fractions: hexane (F1), dichloromethane (F2), ethyl-acetate (F3), methanol (F4), and methanol:water (F5). The F2 fraction showed an IC50 of 1.8 g/ml (Figure 1(b)), and the F3 fraction an IC50 of 5.0 g/ml (data not shown). The other fractions (F1, F4, and F5) showed lower inhibitory activity, with IC50 of 26.8, 23.6, and 145 g/ml, respectively. Subsequently, the F2 fraction was submitted to the Sephadex column, yielding 8 subfractions: F2a, F2b, F2c, F2d, F2e, F2f, F2g, and F2h. Figure 1(c) illustrates the inhibitory ac- tiveity of the F2g subfraction, with a percentage of inhi- bition of growth of the epimastigote form above 90% for all concentrations used (1 - 1000 µg/ml). The F2g sub- fraction, which was rich in a mixture of isomeric labdane sesquiterpenes, showed an IC50 at 0.2 g/ml. In com- parison, Luize et al. [40] investigated the in vitro anti- proliferative effect of eupomatenoid-5, a sesquiterpene isolated from leaves of Piper regnellii var. pallecens, a plant of the same family (Compositae) as A. tinctoria, against T. cruzi. Our results obtained with a mixture of sesquiterpenes also showed that this mixture was more active than Benzonidazole. The effect of the semi-purified subfraction obtained from flowers of A. tinctoria on the interaction of T. cruzi with LLCMK2 cells was evaluated. The treatment of LLCMK2 cells infected with the amastigote form showed that the semi-purified subfraction had a dose-dependent Copyright © 2011 SciRes. PP 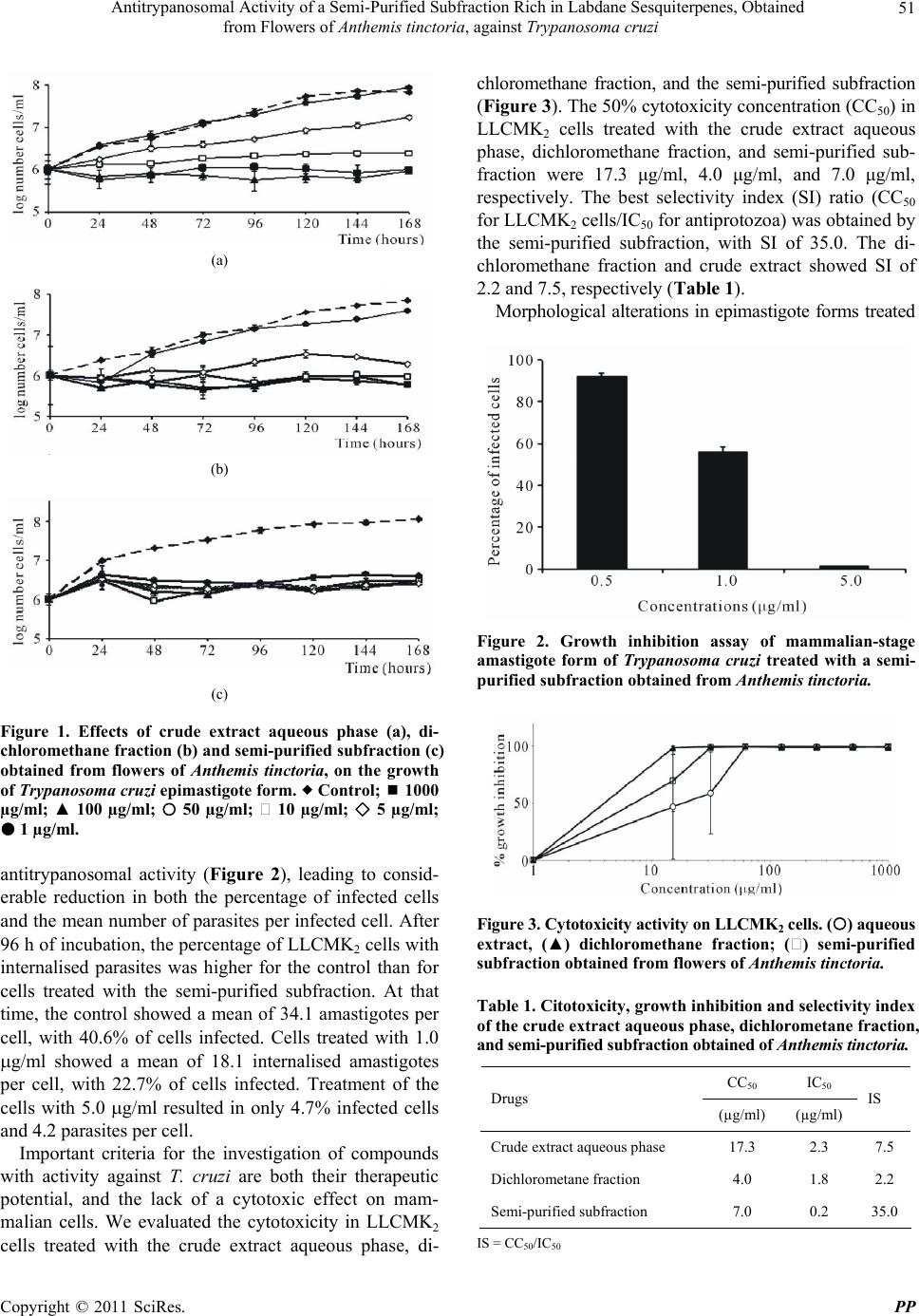 Antitrypanosomal Activity of a Semi-Purified Subfraction Rich in Labdane Sesquiterpenes, Obtained 51 from Flowers of Anthemis tinctoria, against Trypanosoma cruzi (a) (b) (c) Figure 1. Effects of crude extract aqueous phase (a), di- chloromethane fraction (b) and semi-purified subfraction (c) obtained from flowers of Anthemis tinctoria, on the growth of Trypanosoma cruzi epimastigote form. Control; 1000 µg/ml; ▲ 100 µg/ml; 50 µg/ml; 10 µg/ml; 5 µg/ml; 1 µg/ml. antitrypanosomal activity (Figure 2), leading to consid- erable reduction in both the percentage of infected cells and the mean number of parasites per infected cell. After 96 h of incubation, the percentage of LLCMK2 cells with internalised parasites was higher for the control than for cells treated with the semi-purified subfraction. At that time, the control showed a mean of 34.1 amastigotes per cell, with 40.6% of cells infected. Cells treated with 1.0 g/ml showed a mean of 18.1 internalised amastigotes per cell, with 22.7% of cells infected. Treatment of the cells with 5.0 g/ml resulted in only 4.7% infected cells and 4.2 parasites per cell. Important criteria for the investigation of compounds with activity against T. cruzi are both their therapeutic potential, and the lack of a cytotoxic effect on mam- malian cells. We evaluated the cytotoxicity in LLCMK2 cells treated with the crude extract aqueous phase, di- chloromethane fraction, and the semi-purified subfraction (Figure 3). The 50% cytotoxicity concentration (CC50) in LLCMK2 cells treated with the crude extract aqueous phase, dichloromethane fraction, and semi-purified sub- fraction were 17.3 μg/ml, 4.0 μg/ml, and 7.0 μg/ml, respectively. The best selectivity index (SI) ratio (CC50 for LLCMK2 cells/IC50 for antiprotozoa) was obtained by the semi-purified subfraction, with SI of 35.0. The di- chloromethane fraction and crude extract showed SI of 2.2 and 7.5, respectively (Table 1). Morphological alterations in epimastigote forms treated Figure 2. Growth inhibition assay of mammalian-stage amastigote form of Trypanosoma cruzi treated with a semi- purified subfraction obtained from Anthemis tinctoria. Figure 3. Cytotoxicity activity on LLCMK2 cells. () aqueous extract, (▲) dichloromethane fraction; () semi-purified subfraction obtained from flowers of Anthemis tinctoria. Table 1. Citotoxicity, growth inhibition and selectivity index of the crude extract aqueous phase, dichlorometane fraction, and semi-purified subfraction obtained of Anthemis tinctoria. CC50 IC50 Drugs (µg/ml) (µg/ml) IS Crude extract aqueous phase 17.3 2.3 7.5 Dichlorometane fraction 4.0 1.8 2.2 Semi-purified subfraction 7.0 0.2 35.0 IS = CC50/IC50 Copyright © 2011 SciRes. PP 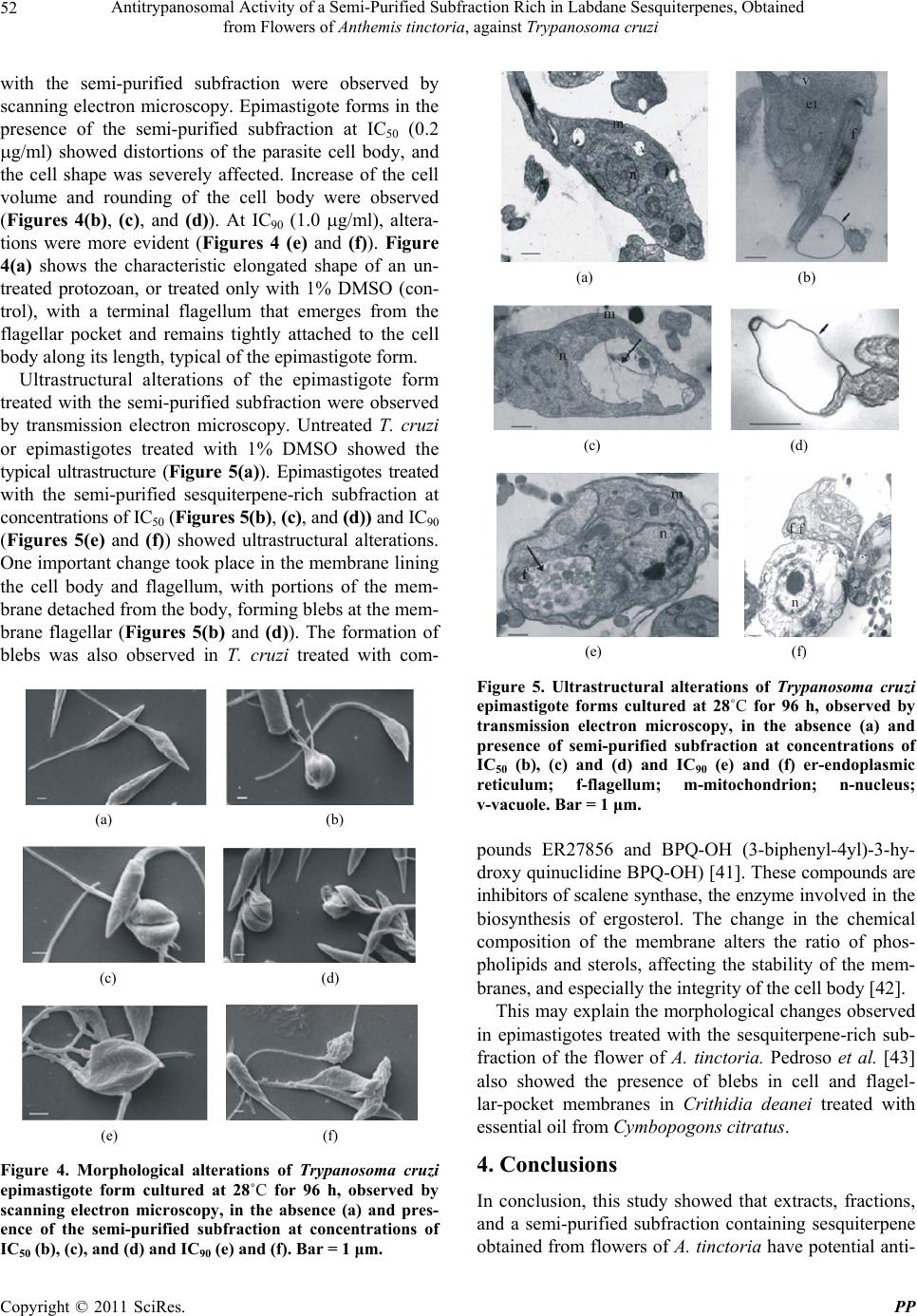 Antitrypanosomal Activity of a Semi-Purified Subfraction Rich in Labdane Sesquiterpenes, Obtained 52 from Flowers of Anthemis tinctoria, against Trypanosoma cruzi with the semi-purified subfraction were observed by scanning electron microscopy. Epimastigote forms in the presence of the semi-purified subfraction at IC50 (0.2 g/ml) showed distortions of the parasite cell body, and the cell shape was severely affected. Increase of the cell volume and rounding of the cell body were observed (Figures 4(b), (c), and (d)). At IC90 (1.0 g/ml), altera- tions were more evident (Figures 4 (e) and (f)). Figure 4(a) shows the characteristic elongated shape of an un- treated protozoan, or treated only with 1% DMSO (con- trol), with a terminal flagellum that emerges from the flagellar pocket and remains tightly attached to the cell body along its length, typical of the epimastigote form. Ultrastructural alterations of the epimastigote form treated with the semi-purified subfraction were observed by transmission electron microscopy. Untreated T. cruzi or epimastigotes treated with 1% DMSO showed the typical ultrastructure (Figure 5(a)). Epimastigotes treated with the semi-purified sesquiterpene-rich subfraction at concentrations of IC50 (Figures 5(b), (c), and (d)) and IC90 (Figures 5(e) and (f)) showed ultrastructural alterations. One important change took place in the membrane lining the cell body and flagellum, with portions of the mem- brane detached from the body, forming blebs at the mem- brane flagellar (Figures 5(b) and (d)). The formation of blebs was also observed in T. cruzi treated with com- (a) (b) (c) (d) (e) (f) Figure 4. Morphological alterations of Trypanosoma cruzi epimastigote form cultured at 28˚C for 96 h, observed by scanning electron microscopy, in the absence (a) and pres- ence of the semi-purified subfraction at concentrations of IC50 (b), (c), and (d) and IC90 (e) and (f). Bar = 1 μm. (a) (b) (c) (d) (e) (f) Figure 5. Ultrastructural alterations of Trypanosoma cruzi epimastigote forms cultured at 28˚C for 96 h, observed by transmission electron microscopy, in the absence (a) and presence of semi-purified subfraction at concentrations of IC50 (b), (c) and (d) and IC90 (e) and (f) er-endoplasmic reticulum; f-flagellum; m-mitochondrion; n-nucleus; v-vacuole. Bar = 1 μm. pounds ER27856 and BPQ-OH (3-biphenyl-4yl)-3-hy- droxy quinuclidine BPQ-OH) [41]. These compounds are inhibitors of scalene synthase, the enzyme involved in the biosynthesis of ergosterol. The change in the chemical composition of the membrane alters the ratio of phos- pholipids and sterols, affecting the stability of the mem- branes, and especially the integrity of the cell body [42]. This may explain the morphological changes observed in epimastigotes treated with the sesquiterpene-rich sub- fraction of the flower of A. tinctoria. Pedroso et al. [43] also showed the presence of blebs in cell and flagel- lar-pocket membranes in Crithidia deanei treated with essential oil from Cymbopogons citratus. 4. Conclusions In conclusion, this study showed that extracts, fractions, and a semi-purified subfraction containing sesquiterpene obtained from flowers of A. tinctoria have potential anti- Copyright © 2011 SciRes. PP  Antitrypanosomal Activity of a Semi-Purified Subfraction Rich in Labdane Sesquiterpenes, Obtained 53 from Flowers of Anthemis tinctoria, against Trypanosoma cruzi proliferative activity in vitro against T. cruzi. Therefore, natural products may be a source of new drugs with anti- protozoal activity that could be used to treat parasitic infectious diseases such as Chagas’ disease. 5. Acknowledgements This study was supported through grants from DECIT /SCTIE/MS and MCT by Conselho Nacional de Desen- volvimento Científico e Tecnológico (CNPq), Financia- dora de Estudos e Projetos (FINEP), Programa de Núcleos de Excelência (PRONEX/Fundação Araucária), and Pro- grama de Pós-graduação em Ciências Farmacêuticas da Universidade Estadual de Maringá. REFERENCES [1] N. R. Farnsworth and R. W. Morris, “Higher Plants— The Sleeping Gian of Drug Development,” The American Journal of Pharmaceutical Education, Vol. 148, 1976, pp. 46-52. [2] T. M. A. Alves, A. F. Silva, M. Brandão, T. S. M. Grandi, E. F. Smânia, A. Smânia Jr. and C. L. Zani, “Biological Screening of Brazilian Medicinal Plants,” Memórias do Instituto Oswaldo Cruz ,Vol. 95, No. 3, 2000, pp. 367-373. doi:10.1590/S0074-02762000000300012 [3] T. C. M. Duarte, M. G. Figueira, A. Sartoratto, G. L. V. Rehder and C. Delarmelina, “Anti-Candida Activity of Brazilian Medicinal Plants,” Journal of Ethnopharma- cology, Vol. 97, No. 2, 2005, pp. 305-311. doi:10.1016/j.jep.2004.11.016 [4] M. J. Martinez, J. Betancourt, N. Alonso-González and A. Jauregui, “Screening of Some Cuban Madicinal Plants for Antimicrobial Activity,” Journal of Ethnopharmacology, Vol. 52, No. 3, 1996, pp. 171-174. doi:10.1016/0378-8741(96)01405-5 [5] I. Ahmad and A. Z. Beg, “Antimicrobial and Phyto- chemical Studies on 45 Indian Medicinal Plants against Multi-Drug Resistant Human Pathogens,” Journal of Ethnopharmacology, Vol. 74, No. 2, 2001, pp. 113-123. [6] I. Masterová, D. Grancai, Z. Grancoivá, M. Pour and K. Ubik, “A New Flavonoid: Tinctosid from Anthemis tinc- toria L,” Short Communications Pharmazie, Vol. 60, No. 12, 2005, pp. 956-957. [7] P. M. Liszt and L. Hörhammer, “Hagers Handbuch der Pharmazeutischen Praxis,” Springer Verlag Heidelberg III, Vol. 113, 1972. [8] S. Vaverkova, M. Haban and K. Eerna, “Qualitive Prop- erties of Anthemis tinctoria and Anthemis nobilis (Cha- maemelum nobile) under Different Environmental Condi- tions,” Ecophysiology of Plant Production Processes in Stress Conditions, Abstracts of the fourth International Conference, Račkova dolina, Slovakia. Vol. 02, No. 1-2, 2001. [9] C. Mann and E. Staba, “Spices and Medical Plants. Re- cent Advances in Botany, Horticulture and Pharmacol- ogy,” Food Products Press, 1986, pp. 235-280. [10] M. H. Grace, “Chemical Composition and Biological Activity of the Volatiles of Anthemis melampodina and Pluchea discoridis,” Phytoterapy Research, Vol. 16, No. 3, 2002, pp. 183-185. [11] M. Holla, S. Svajdlenka, B. Zibrunova, J. Tekel and E. Havranek, “Composition of the Oil from the Flowertheds of Anthemis tinctoria L. Cultived in Slovak Republic,” Journal ofEssential Oil Research, Vol. 12, No. 6, 2000, pp. 714-716. doi:10.1002/ptr.872 [12] World Health Organization, “Chagas Disease,”2008, http://www.who.int/tdr/diseases/chagas/files/chagasposter .pdf. [13] W. De Souza, “Cell Biology of Trypanosoma cruzi,” International Review of Cytology, Vol. 86, No. 5, 1984, pp. 197-283. doi:10.1016/S0074-7696(08)60180-1 [14] R. Viotti, C. Vigliano, H. Armenti and E. Segura, “Treatment of Chronic Chagas Disease with Benznida- zole: Clinical and Serological Evolution of Patients with Long Term Follow-Up,” American Heart Journal, Vol. 127, No. 1, 1994, pp. 151-162. doi:10.1016/0002-8703(94)90521-5 [15] E. S. Sosa, “Efficacy of Chemotherapy with Benzonida- zole in Children in the Determinate Phase of Chagas Dis- ease,” American Journal of Tropical Medicine and Hy- giene, Vol. 59, No. 4, 1998, pp. 526-529. [16] L. S. Croft, P. M. Barret and J. A. Urbina, “Chemo- therapy of Trypanosomiases and Leishmaniasis,” Trends in Parasi- tology, Vol. 21, 2005, pp. 508-512. doi:10.1016/j.pt.2005.08.026 [17] J. B. Calixto, “Efficacy, Safety, Quality Control, Market- ing and Regulatory Guidelines for Herbal Medicines (Phytotherapeutical Agents),” Brazilian Journal of Medi- cal Biology Research, Vol. 48, 2000, pp. 179-189. doi:10.1590/S0100-879X2000000200004 [18] E. C. Camargo, “Growth Differentiation in Trypanosoma cruzi. Origin of Metacyclic Trypanosomes in Liquid Me- dia,” Revista Instituto Medicina Tropical, Vol. 06, 1964, pp. 93-100. [19] V. Yong, V. Schmitz and M. A. Vannier-Santos, “ Al- tered Expression of Cruzipain and a Cathepsis B-like Terget in a Trypanosoma cruzi Cell Line Displaying Re- sistance to Synthetic Inhibitors of Cysteine-Proteinases,” Molecular Biochemical Parasitology, Vol. 109, 2000, pp. 47-59. doi:10.1016/S0166-6851(00)00237-1 [20] B. Weninger, S. Robledo, G. J. Arango, E. Deharo, R. Aragón, V. Munoz, J. Callapa, A. Lobstein and R. Anton, “Antiprotozoal Activities of Colombian Plants,” Journal of Ethnopharmacology, Vol. 78, No. 1-2, 2001, pp. 193- 200. doi:10.1016/S0378-8741(01)00346-4 [21] F. Abe, S. Nagafugi, T. Yamauchi, H. Okabe, J. Maki, H. Higo, H. Akahane, A. Aguiar, M. Jiménez-Estrada and R. Reyes-Chilpa, “Trypanocidal Constituints in Plants 1. Evaluation of Some Mexican Plants for Their Trypano- cidal Activity and Active Constituents in Guaco, Root of Aristolochia taliscana,” Biological Pharmaceutical Bul- Copyright © 2011 SciRes. PP  Antitrypanosomal Activity of a Semi-Purified Subfraction Rich in Labdane Sesquiterpenes, Obtained 54 from Flowers of Anthemis tinctoria, against Trypanosoma cruzi letin, Vol. 25, No. 9, 2001, pp. 1188-1191. doi:10.1016/j.ejmech.2007.10.031 [22] P. S. Luize, T. S. Tiuman, L. G. Morello, P. K. Maza, T. Ueda-Nakamura, B. P. Dias Filho, D. A. G. Cortez, J. C. P. Mello and C. V. Nakamura, “Effects of Medicinal Plant Extracts on Growth of Leishmania (L.) amazonensis and Trypanosoma cruzi,” Brazilian Journal of Pharma- ceutical Science, Vol. 41, No. 1, 2005, pp. 85-94. [23] M. L. Lavaggi, G. Aguirre, L. Boiani, L. Orelli, B. García, H. Cerecetto and M. González, “Pyrimido [1,2-a] Quino- xaline 6-Oxide and Phenazine 5,10-Dioxide Derivatives and Related Compounds as Growth Inhibitors of Try- panosoma cruzi,” European Journal of Medicinal Che- mistry, Vol. 43, No. 8, 2008, pp. 1737-1741. [24] G. F. Santoro, M. G. Cardoso, L. G. L. Guimarães, L. Z. Mendonça and M. J. Soares, “Trypanosoma cruzi: Ac- tivity of Essential Oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on Epimastigotes and Trypomastigotes,” Experimental Para- sitology, Vol. 116, No. 3, 2007, pp. 283-290. doi:10.1016/j.exppara.2007.01.018 [25] E. Izumi, L. G. Morello, T. Ueda-Nakamura, S. F. Ya- mada-Ogatta, B. P. Dias Filho, D. A. G. Cortez, I. C. P. Ferreira, J.A. Morgado-Díaz and C. V. Nakamura, “Try- panosoma cruzi: Antiprotozoal Activity of Parthenolide Obtained from Tanacetum parthenium (L.) Schultz Bip. (Asteraceae, Compositae) against Epimastigote and Amastigote Forms,” Experimental Parasitology, Vol. 118, No. 3, 2008, pp. 324-330. doi:10.1016/j.exppara.2007.08.015 [26] C. Salas, R. A. Tapia, K. Ciudad, V. Armstrong, M. Orellana, U. Kemmerling, J. Ferreira, J. D. Maya and A. Morello, “Trypanosoma cruzi: Activities of Lapachol and - and -Lapachone Derivatives against Epimastigote and Trypomastigote Forms,” Bioorganic Medicinal Che- mistry, Vol. 15, No. 2, 2008, pp. 668-674. doi:10.1016/j.bmc.2007.10.038 [27] P. S. Kalsi, O. S. Singh and B. R. Chhabra, “Cross Con- jugated Terpenoid Ketones: A New Group of Plant Growth Regulators,” Phytochemistry, Vol. 17, No. 3, 1978, pp. 576-577. doi:10.1016/S0031-9422(00)89380-2 [28] L. Christensen, “Acetylenes D Related Compounds,” An- themidae Phytochemistry, Vol. 31, No. 1, 1992, pp. 7-49. doi:10.1016/S0305-1978(01)00039-4 [29] C. A. Williams and J. Greenham, “The Role of Lipophilic and Polar Flavonoids in the Classification of Temperate Members of the Anthemidae. Biochemical Systematic Ecology, Vol. 29, No. 9, 2001, pp. 929-945. doi:10.1016/S0305-1978(01)00039-4 [30] V. Bulatovic, “Comparative Examination of chemical Constituets of Species Anthemis carpatica and Anthemis montana,” PhD Thesis, Faculty of Chemistry, University of Belgrade, Vol. 61, No. 1,1998, pp. 57-66. [31] R. N. Baruah, F. Bohlmann and R. M. King, “Novel Ses- quiterpene Lactones from Anthemis Cotula,” Planta Me- dica, Vol. 51, 1985, pp. 531-532. doi:10.1055/s-2007-969590 [32] M. Bruno, A. Maggio, N. A. Arnold, J. G. Díaz and W. Herz, “(1998) Sesquiterpene Lactones from Anthemis plutonia,” Phytochemistry, Vol. 49, 1998, pp. 1739-1740. doi:10.1016/S0031-9422(98)00259-3 [33] V. Vajs, N. Todorovic, V. B. Bulatovic, N. Menkovic, S. Macura, N. Juranic and S. Miolosavjevic, “Further Ses- quiterpene Lactones from Anthemis carpatica,” Phyto- chemistry, Vol. 54, 2000, pp. 625-633. doi:10.1016/S0031-9422(00)00160-6 [34] M. Konstantinopoulou, A. Karioti, S. Skaltsa and H. Skaltsa, “Sesquiterpene Lactones from Anthemis altis- sima and Their Anti-Helicobacter pylori Activity,” Journal of Natural Product, Vol. 66, No. 5, 2003, pp. 699-702. doi:10.1021/np020472m [35] V. Saroglou, N. Dorizas, Z. Kypriotakis and H. Skaltsa, “Analysis of Essential Oil Composition of Eight Anthe- mis species from Greece,” Journal of Chromatog- raphy, Vol. 1104, No. 1-2, 2006, pp. 313-322. doi:10.1016/j.chroma.2005.11.087 [36] J. D. Staneva, M. N. Todorova and L. N. Evstatieva, “Sesquiterpene Lactones as Chemotaxonomic Markers in Genus Anthemis,” Phytochemistry, Vol. 69, No. 3, 2008, pp. 607-618. doi:10.1016/j.phytochem.2007.07.021 [37] M. Todorova, J. Staneva, P. Denkova and L. Evstatieva, “Irregular Linear Sesquiterpene Dilactones from Anthemis Auriculata Boiss,” Natural Product Research, Vol. 22, No. 10, 2008, pp. 907-914. doi:10.1080/14786410701642730 [38] V. Vajs, V. Bulatovic, K. Fodulovic-Savikin, N. Men- tovic, S. Macura, N. Juranic and S. Milosavijevic, “Highly Oxigenated Guaianolides from Anthemis cretica Subsp. Cretica,” Phytochemistry, Vol. 50, No. 2, 1999, pp. 287-291. doi:10.1016/S0031-9422(98)00504-4 [39] A. Karioti, H. Skaltsa, A. Linden, R. Perozzo, R. Brun and D. Tasdemir, “Anthecularin: A Novel Sesquiterpene Lactone from Anthemis auriculata with antiprotozoal ac- tivity,” Journal Organic Chemistry, Vol. 72, No. 21, 2007, pp. 8103-8106. doi:10.1021/jo701751w [40] P. S. Luize, T. Ueda-Nakamura, B. P. Dias Filho, D. A. G. Cortez and C. V. Nakamura, “Activity of Neolignans Isolated from Piper regnellii (MIQ.) C. DC. var. pallescens (C. DC.) Yunk against Trypanosoma cruzi,” Biological Pharmaceutical Bulletin, Vol. 29, No. 10, 2006, pp. 2126-2130. doi:10.1248/bpb.29.2126 [41] M. V. Braga, J. A. Urbina and W. De Souza, “Effects of Squalene Synthase Inhibitors on the Growth and Ultrastructure of Trypanosoma cruzi,” International Journal of Antimicrobial Agents, Vol. 24, No. 1, 2004, pp. 72-78. doi:10.1016/j.ijantimicag.2003.12.009 [42] P. W. Denny, M. C. Field and D. F. Smith, “GPI-An- chored Proteins and Glycoconjugates Segregat into Pipid Rafs in Kinetoplastid,” FEBS Letters, Vol. 491, No. 1-2, 2001, pp. 148-153. doi:10.1016/S0014-5793(01)02172-X Copyright © 2011 SciRes. PP  Antitrypanosomal Activity of a Semi-Purified Subfraction Rich in Labdane Sesquiterpenes, Obtained from Flowers of Anthemis tinctoria, against Trypanosoma cruzi Copyright © 2011 SciRes. PP 55 [43] R. B. Pedroso, T. Ueda-Nakamura, B. P. Dias Filho, D. A. G. Cortez, L. E. R. Cortez, J. A. Morgado-Díaz and C. V. Nakamura, “Biological Activities of Essential Oil Ob- tained from Cymbopogon citratus on Crithidia deanei,” Acta Protozoologica, Vol. 45, No. 3, 2006, pp. 231-240.
|