 J. Biomedical Science and Engineering, 2009, 2, 200-207 Published Online June 2009 in SciRes. http://www.scirp.org/journal/jbise JBiSE Effects of granulocyte colony-stimulating factor and stem cell factor, alone and in combination, on the biological behaviours of bone marrow mesenchymal stem cells Feng-Ping Tang1, Xing-Huo Wu2, Xi-Lin Yu1, Shu-Hua Yang 2, Wei-Hua Xu 2, Jin Li 2 1Wuhan Medical & Health Center for Women and Children, Wuhan, China; 2Department of Orthopaedics, Union Hospital, Tongji Medical College, Science and Technology of Huazhong University, Wuhan, China; Corresponding author: Xing-Huo Wu, Fax: 086-027-85351627. Email: wuxinghuo_71@yahoo.com.cn Received 6 January 2009; revised 20 March 2009; accepted 9 April 2009. ABSTRACT Aim: The effects of granulocyte colony- stimu- lating factor (G-CSF) and stem cell factor (SCF) on the proliferation and osteogenic differentia- tion capacity of bone marrow mesenchymal stem cells (MSCs) were studied in the experi- ment. Methods: Bone marrow MSCs were col- lected from rabbits successfully, and treated with various concentrations of G-CSF, SCF or a combination of the two. Flow cytometric ana- lyse, MTT test, CFU-F assay, and alkaline phosphatase (ALP) activity measurement were employed. Results: The results of flow cytome- try showed that immunophenotype of the cells were CD29+/CD45-, CD105+/ CD34–, CD90+/ HLADR–. MSCs were shown to constitutively express low levels of c-kit which could be en- hanced by SCF. G-CSF and SCF had an obvious facilitative effect on the proliferation of MSCs in a dose-dependent fashion. In addition, G-CSF and SCF would be effective in reversibly pre- venting their differentiation, as showed by the decrease of ALP activity, leading to self-renewal rather than differentiative cell divisions. The effects of G-CSF were superior to SCF. And cells in the group treated with combination of G-CSF and SCF showed more powerful effects than the groups treated with G-CS, SCF, or non e of the two. Conclusion: On the whole, these studies demonstrated that MSCs responsed to G-CSF, SCF, and to G-CSF plus SCF in a manner that suppressed differentiation, and promotes proliferation and self-renewal, and support the view that the se fac tors cou ld act sy nergisti cally . Keywords: Granulocyte Colony-Stimulating Factor; Stem Cell Factor; Synergistic Effect; Bone Marrow Mesenchymal Stem Cells 1. INTRODUCTION Bone marrow is composed of various types of cells of specific phenotypes and function. Bone marrow cells can be transplanted either as total, unfractionated bone mar- row or as a well-defined subpopulation of bone marrow mesenchymal stem cells (MSCs) [1,2]. MSC is a group of multipotent cells that can expand, self-replicate, and differentiate into many cell types under appropriate con- ditions [3,4]; their progeny includes chondrocytes, ten- don cells, haematopoiesis-support stromal cells, adipo- cytes and osteoblasts [5,6,7]. MSCs, similar to other stem cells, have an essential role in the regeneration/ maintenance of the adult tissues submitted to physio- logical modelling/turnover or following injury. At pre- sent, MSCs show great promise for use in a variety of cell-based therapies, include repair of defects in cardio- vascular muscle, spinal cord , bone, and cartilage. Recently, enhancement of bone repair in the necrotic zone by bone marrow MSCs has been highlighted for the treatment of osteonecrosis before collapse of the head [8,9]. MSCs can be delivered into the injured tissue ei- ther by invasive or by noninvasive means. Of primary importance to the success of such a strategy is the pro- duction of viable, reproducible protocols for stem cell population expansion. Invasive method is done on a sur- gically exposed necrotic head. Isolated primary mesen- chymal stem cells are low in numbers, in vitro expansion is necessary. Although it is known that adult bone mar- row MSCs can be rapidly expanded in vitro, migrate, and differentiate into multiple tissues in vivo. However, the expansion potential is limited and in vitro aging leads to loss of multipotency and replicative senescence. In addition, many transplanted cells die shortly after implantation as a result of physical stress from the im- plantation procedure itself, inflammation, or hypoxia. Under consideration of noninvasive methods of targeting  F. P. Tang et al. / J. Biomedical Science and Engineering 2 (2009) 202-207 201 SciRes Copyright © 2009 JBiSE the injured tissue with stem cells that take advantage of endogenous mechanisms. Recent studies in a rat model showed that endogenous signaling via cytokines can enhance mobilization, homing, and transdifferentiation of stem cells [10]. MSCs can be mobilized from the bone marrow (central pool of stem cells) and directed to the injured tissue or organ. Currently, little is known about the signals involved in the mobilization and homing of stem cells to the injured tissue. It is believed that the cytokines SCF, G-CSF, and stromal-cell-derived factor-1 (SDF-1) and their receptors play a major role (1). MSCs constitutively expressed mRNA for interleukin (IL), colony-stimulating factor (CSF), and stem cell fac- tor (SCF) [11]. G-CSF is a polypeptide hematopoietic factor that stimulates survival, proliferation, and matura- tion of neutrophilic granulocyte progenitors and en- hances their functions. Stem cell factor (SCF) is a potent costimulatory molecule for many cytokines. Its synergy with granulocyte colony-stimulating factor (G-CSF) re- sults in important biologic and clinical effects, although the mechanism by which this occurs remains poorly un- derstood [12]. Notwithstanding, cytokine-induced mobi- lization of bone marrow stem / progenitor cells in the necrotic foci may represent a promising strategy for re- placing necrotic bone. A better understanding of the ki- netics of MSC and MSC derived progenitor cell prolif- eration and differentiation is of great current interest from both a clinical and tissue engineering perspective [13,14,15]. Consequently, in the present study, we inves- tigated the biological effects of G-CSF and SCF, alone and in combination, on proliferation and osteogenic dif- ferentiation capacity of bone marrow MSCs. 2. MATERIALS AND METHODS 2.1. Generation of Rabbit BM-MSCs The BM-MSCs were prepared as described previously with slight modification [16]. Bone marrow cells were harvested from iliac crest aspirates from healthy 3-month old New Zealand white rabbits, and the proce- dures were used in accordance with the procedures ap- proved by the animal experimentation and ethics com- mittees of Tongji Medical College. Approximately 10 ml of iliac bone marrow was aspirated and suspended in 20 ml of DMEM-LG medium (Gibco) containing 2000 U of heparin sodium. Mononuclear cells (MNCs) were separated on Ficoll-Paque density gradient (1.077g/mL) and washed in PBS. Then MNCs were seeded at a den- sity of 1x106 cells/cm2 in growth medium containing DMEM- LG and 10% FBS (HyClone) and incubated at 37°C in 5% CO2/95% air. Medium was changed first after 24 h and then every 3 days. MSCs were used at passage 3 to 4. 2.2. Flow Cytometric Analyses To evaluate the lineage and surface marker phenotype of passage 3 cultures of MSCs, cells were detached and incubated in phosphate-buffered saline containing 1% bovine serum albumin with the following fluorescent antibodies: anti-human CD29 (integrin b1 chain)–PE, anti-huaman CD90 (Thy-1)-FITC, anti-human CD105 (endoglin)-PE, anti-human CD34-FITC, anti-human CD45-FITC, anti-human HLA-DR-PE(Santa Cruze, USA), and were analyzed by FACS caliber flow cy- tometry (BD, USA). To examine the expression of G- CSFR(G-CSF receptor) and c-kit (SCF receptor) on the cultured MSCs, PE- conjugated mouse anti-human G-CSFR (CD114) monoclonal antibody (Becton- Dick- inson, America), mouse anti-human c-kit(CD117) monoclonal antibody and secondary antibodies conju- gated with FITC (Zymed,America)were used. Isotype- identical antibodies served as controls. Meanwhile, im- munofluorescence staining was used to test cultured MSCs as well. Primary antibody was anti-vimentin, and secondary antibody was anti-gout polyvalent-Cy3 con- jugate (Sigma). 2.3. Cell Proliferation of MSCs MSCs of passage 3 were harvested by treatment of the cells with Trysin/EDTA and washed twice with DMEM. The cells were then resuspended (1×104 cells per ml) in DMEM containing 10% FBS and plated in 96-well culture plates (100ul /well). After 48 h culture, culture medium and nonadherent cells were removed. Every experimental group was given 100ul growth medium containing DMEM-LG, 2% FBS, and various concentration of SCF and G-CSF, and cultured in the CO2 incubator. Assessment of cell proliferation was measured in terms of optical absorbance (OD) per well by a semi-automated tetrazolium- based colorimetric assay using MTT. The growth rate was calculated ac- cording to the formula: (OD treated/OD control -1)×100%. And the cell growth curves were drawn with the cul- ture time (d) as the abscissa and the mean OD valu e as the ordinate. 2.4. Colony-Forming Unit-Fibroblast (CFU-F) Assay The frequency of CFU-F was measured using the method of Castro-Malaspina with slight modification [17]. BMSCs (at 5×105 cells/ml) were suspended in growth medium containing DMEM-LG, 10% FBS, anti- biotics, and various concentration of SCF and G-CSF (0.1,1,10,100, and 1000 ng/mL), and cultur ed in the CO2 incubator. Each flask contained 1×106 cells. The me- dium was completely renewed every 3 days. The fibro- blast colonies were counted on day 12 of culture. Cell clusters containing > 50 cells were scored as CFU-F colonies. Based on the number of colonies generated in the various concentrations of CSF, a dose-response curve to each growth factor was graphed. 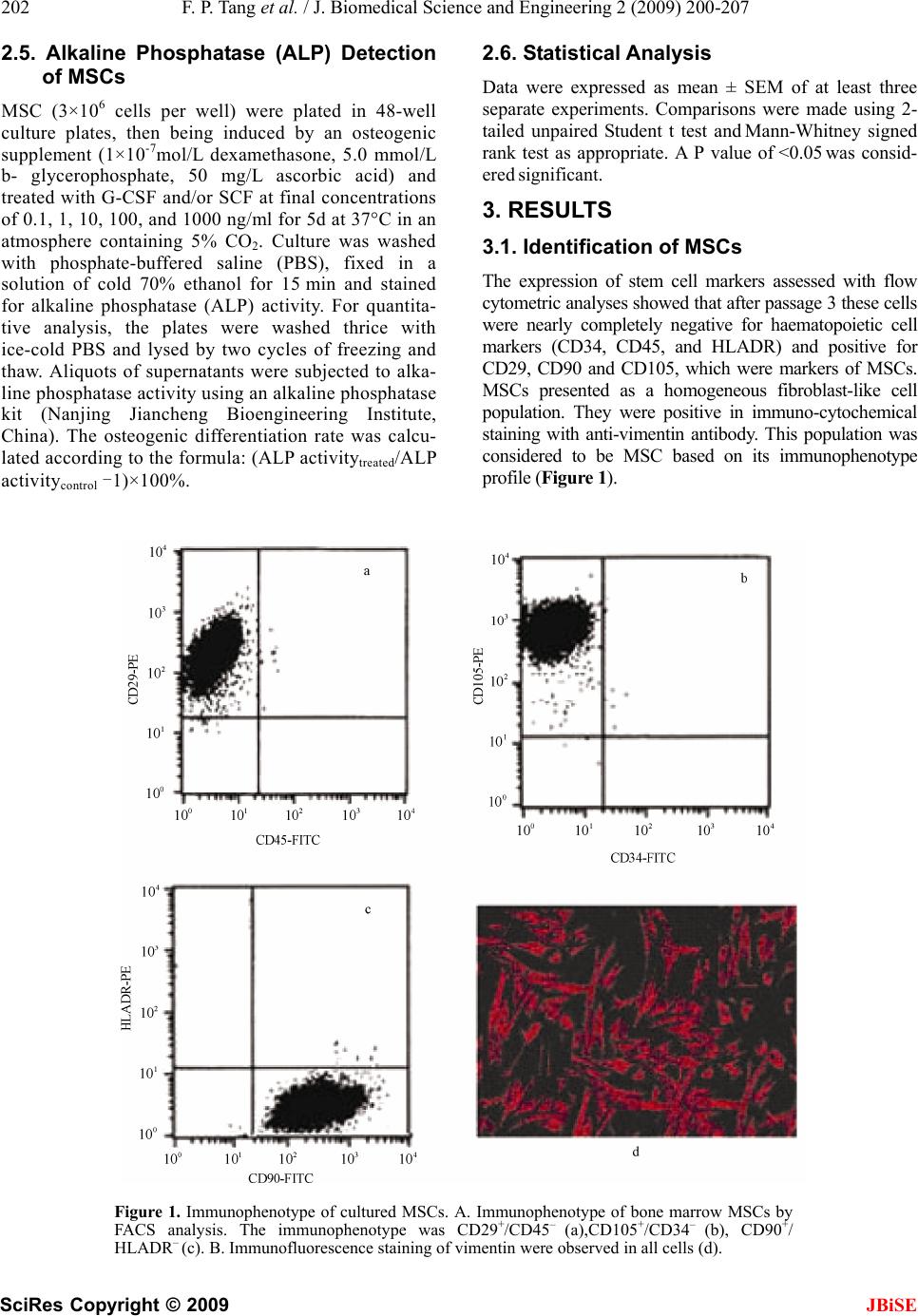 202 F. P. Tang et al. / J. Biomedical Science and Engineering 2 (2009) 200-207 SciRes Copyright © 2009 2.5. Alkaline Phosphatase (ALP) Detection of MSCs 2.6. Statistical Analysis Data were expressed as mean ± SEM of at least three separate experiments. Comparisons were made using 2- tailed unpaired Student t test and Mann-Whitney signed rank test as appropriate. A P value of <0.05 was consid- ered significant. MSC (3×106 cells per well) were plated in 48-well culture plates, then being induced by an osteogenic supplement (1×10-7mol/L dexamethasone, 5.0 mmol/L b- glycerophosphate, 50 mg/L ascorbic acid) and treated with G-CSF and/or SCF at final concentrations of 0.1, 1, 10, 100, and 1000 ng/ml for 5d at 37°C in an atmosphere containing 5% CO2. Culture was washed with phosphate-buffered saline (PBS), fixed in a solution of cold 70% ethanol for 15 min and stained for alkaline phosphatase (ALP) activity. For quantita- tive analysis, the plates were washed thrice with ice-cold PBS and lysed by two cycles of freezing and thaw. Aliquots of supernatants were subjected to alka- line phosphatase activity using an alkaline phosphatase kit (Nanjing Jiancheng Bioengineering Institute, China). The osteogenic differentiation rate was calcu- lated according to the formula: (ALP activitytreated/ALP activitycontrol -1)×100%. 3. RESULTS 3.1. Identification of MSCs The expression of stem cell markers assessed with flow cytometric ana lyses showed that after passag e 3 thes e cells were nearly completely negative for haematopoietic cell markers (CD34, CD45, and HLADR) and positive for CD29, CD90 and CD105, which were markers of MSCs. MSCs presented as a homogeneous fibroblast-like cell population. They were positive in immuno-cytochemical staining with anti-vimentin antibody. This population was considered to be MSC based on its immunophenotype profile (Figur e 1). Figure 1. Immunophenotype of cultured MSCs. A. Immunophenotype of bone marrow MSCs by FACS analysis. The immunophenotype was CD29+/CD45– (a),CD105+/CD34– (b), CD90+/ HLADR– (c). B. Immunofluorescence staining of vimentin were observed in all cells (d). JBiSE 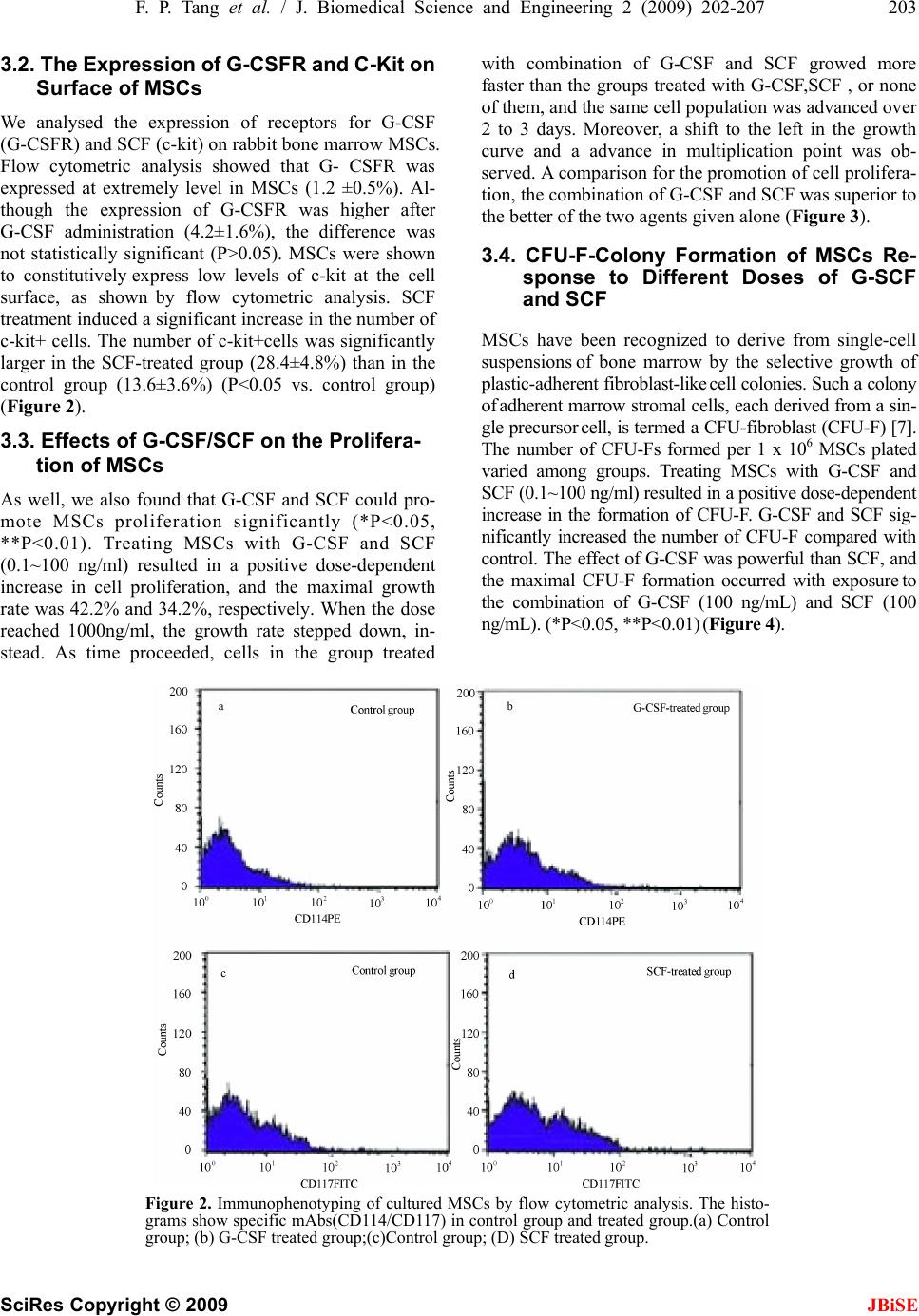 F. P. Tang et al. / J. Biomedical Science and Engineering 2 (2009) 202-207 203 SciRes Copyright © 2009 JBiSE 3.2. The Expression of G-CSFR a nd C-Kit on Surface of MSCs We analysed the expression of receptors for G-CSF (G-CSFR) and SCF (c-kit) on rabbit bone marrow MSCs. Flow cytometric analysis showed that G- CSFR was expressed at extremely level in MSCs (1.2 ±0.5%). Al- though the expression of G-CSFR was higher after G-CSF administration (4.2±1.6%), the difference was not statistically significant (P>0.05). MSCs were shown to constitutively express low levels of c-kit at the cell surface, as shown by flow cytometric analysis. SCF treatment induced a significant increase in the number of c-kit+ cells. The number of c-kit+cells was significantly larger in the SCF-treated group (28.4±4.8%) than in the control group (13.6±3.6%) (P<0.05 vs. control group) (Figure 2). 3.3. Effects of G-CSF/SCF on the Prolifera- tion of MSCs As well, we also found that G-CSF and SCF could pro- mote MSCs proliferation significantly (*P<0.05, **P<0.01). Treating MSCs with G-CSF and SCF (0.1~100 ng/ml) resulted in a positive dose-dependent increase in cell proliferation, and the maximal growth rate was 42.2% and 34.2%, respectively. When the dose reached 1000ng/ml, the growth rate stepped down, in- stead. As time proceeded, cells in the group treated with combination of G-CSF and SCF growed more faster than the groups treated with G-CSF,SCF , or none of them, and the same cell population was advanced over 2 to 3 days. Moreover, a shift to the left in the growth curve and a advance in multiplication point was ob- served. A comparison for the promotion of cell prolifera- tion, the combination of G-CSF and SCF was superior to the better of the two agents given alone (Figure 3). 3.4. CFU-F-Colony Formation of MSCs Re- sponse to Different Doses of G-SCF and SCF MSCs have been recognized to derive from single-cell suspensions of bone marrow by the selective growth of plastic-adherent fibroblast-like cell colonies. Such a colony of adherent marrow stromal cells, each derived from a sin- gle precursor cell, is termed a CFU-fibroblast (CFU-F) [7]. The number of CFU-Fs formed per 1 x 106 MSCs plated varied among groups. Treating MSCs with G-CSF and SCF (0.1~100 ng/ml) resulted in a positive dose-dependent increase in the formation of CFU-F. G-CSF and SCF sig- nificantly increased the number of CFU-F compared with control. The effect of G-CSF was powerful than SCF, and the maximal CFU-F formation occurred with exposure to the combination of G-CSF (100 ng/mL) and SCF (100 ng/mL). (*P<0.05, **P<0.01) (Figure 4). Figure 2. Immunophenotyping of cultured MSCs by flow cytometric analysis. The histo- grams show specific mAbs(CD114/CD117) in control group and treated group.(a) Control group; (b) G-CSF treated group;(c)Control group; (D) SCF treated group. 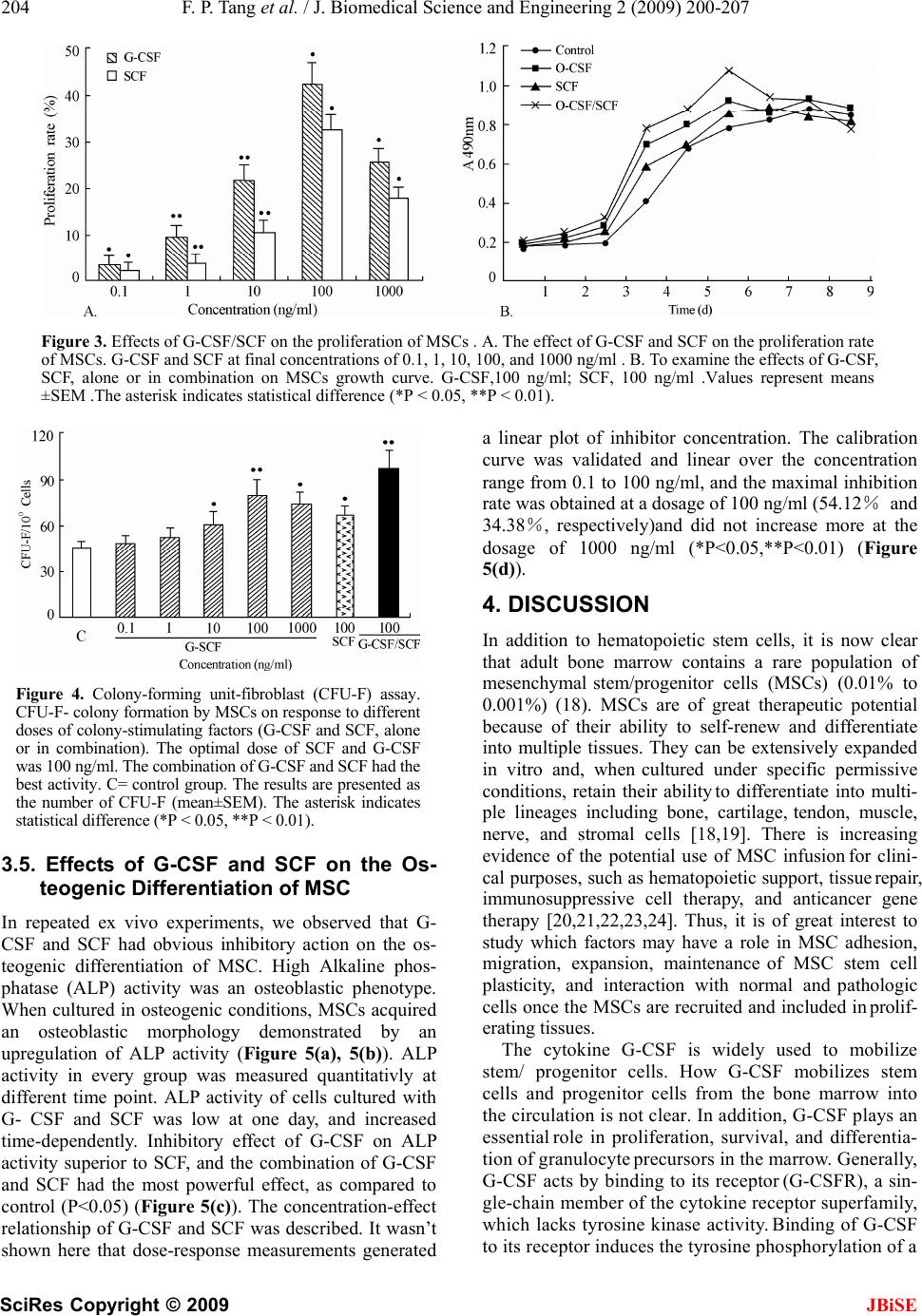 204 F. P. Tang et al. / J. Biomedical Science and Engineering 2 (2009) 200-207 SciRes Copyright © 2009 JBiSE Figure 3. Effects of G-CSF/SCF on the proliferation of MSCs . A. The effect of G-CSF and SCF on the proliferation rate of MSCs. G-CSF and SCF at final concentrations of 0.1, 1, 10, 100, and 1000 ng/ml . B. To examine the effects of G-CSF, SCF, alone or in combination on MSCs growth curve. G-CSF,100 ng/ml; SCF, 100 ng/ml .Values represent means ±SEM .The asterisk indicates statistical difference (*P < 0.05, **P < 0.01). Figure 4. Colony-forming unit-fibroblast (CFU-F) assay. CFU-F- colony formation by MSCs on re sponse t o differen t doses of colony-stimulatin g factors (G-CSF and SCF, alone or in combination). The optimal dose of SCF and G-CSF was 100 ng/ml. The combination of G-CSF and SCF had the best activity. C= control group. The results are presented a s the number of CFU-F (mean±SEM). The asterisk indicates statistical difference (*P < 0.05, **P < 0.01). 3.5. Effects of G-CSF and SCF on the Os- teogenic Differentiation of MSC In repeated ex vivo experiments, we observed that G- CSF and SCF had obvious inhibitory action on the os- teogenic differentiation of MSC. High Alkaline phos- phatase (ALP) activity was an osteoblastic phenotype. When cultured in osteogenic conditions, MSCs acquired an osteoblastic morphology demonstrated by an upregulation of ALP activity (Figure 5(a), 5(b)). ALP activity in every group was measured quantitativly at different time point. ALP activity of cells cultured with G- CSF and SCF was low at one day, and increased time-dependently. Inhibitory effect of G-CSF on ALP activity superior to SCF, and the combination of G-CSF and SCF had the most powerful effect, as compared to control (P<0.05) (Figure 5(c)). The concentration-effect relationship of G-CSF and SCF was described. It wasn’t shown here that dose-response measurements generated a linear plot of inhibitor concentration. The calibration curve was validated and linear over the concentration range from 0.1 to 100 ng/ml, and the maximal inhibitio n rate was obtained at a dosage of 100 ng /ml (54.12% and 34.38%, respectively)and did not increase more at the dosage of 1000 ng/ml (*P<0.05,**P<0.01) (Figure 5(d)). 4. DISCUSSION In addition to hematopoietic stem cells, it is now clear that adult bone marrow contains a rare population of mesenchymal stem/progenitor cells (MSCs) (0.01% to 0.001%) (18). MSCs are of great therapeutic potential because of their ability to self-renew and differentiate into multiple tissues. They can be extensively expanded in vitro and, when cultured under specific permissive conditions, retain their ability to differentiate into multi- ple lineages including bone, cartilage, tendon, muscle, nerve, and stromal cells [18,19]. There is increasing evidence of the potential use of MSC infusion for clini- cal purposes, such as hematopoietic support, tissue repair, immunosuppressive cell therapy, and anticancer gene therapy [20,21,22,23,24]. Thus, it is of great interest to study which factors may have a role in MSC adhesion, migration, expansion, maintenance of MSC stem cell plasticity, and interaction with normal and pathologic cells once the MSCs are recruited and included in prolif- erating tissues. The cytokine G-CSF is widely used to mobilize stem/ progenitor cells. How G-CSF mobilizes stem cells and progenitor cells from the bone marrow into the circulation is not clear. In addition, G-CSF plays an essential role in proliferation, survival, and differentia- tion of granulocyte precursors in the marrow. Generally, G-CSF acts by binding to its receptor (G-CSFR), a sin- gle-chain member of the cytokine receptor superfamily, which lacks tyrosine kinase activity. Binding of G-CSF to its receptor induces the tyrosine phosphorylation of a 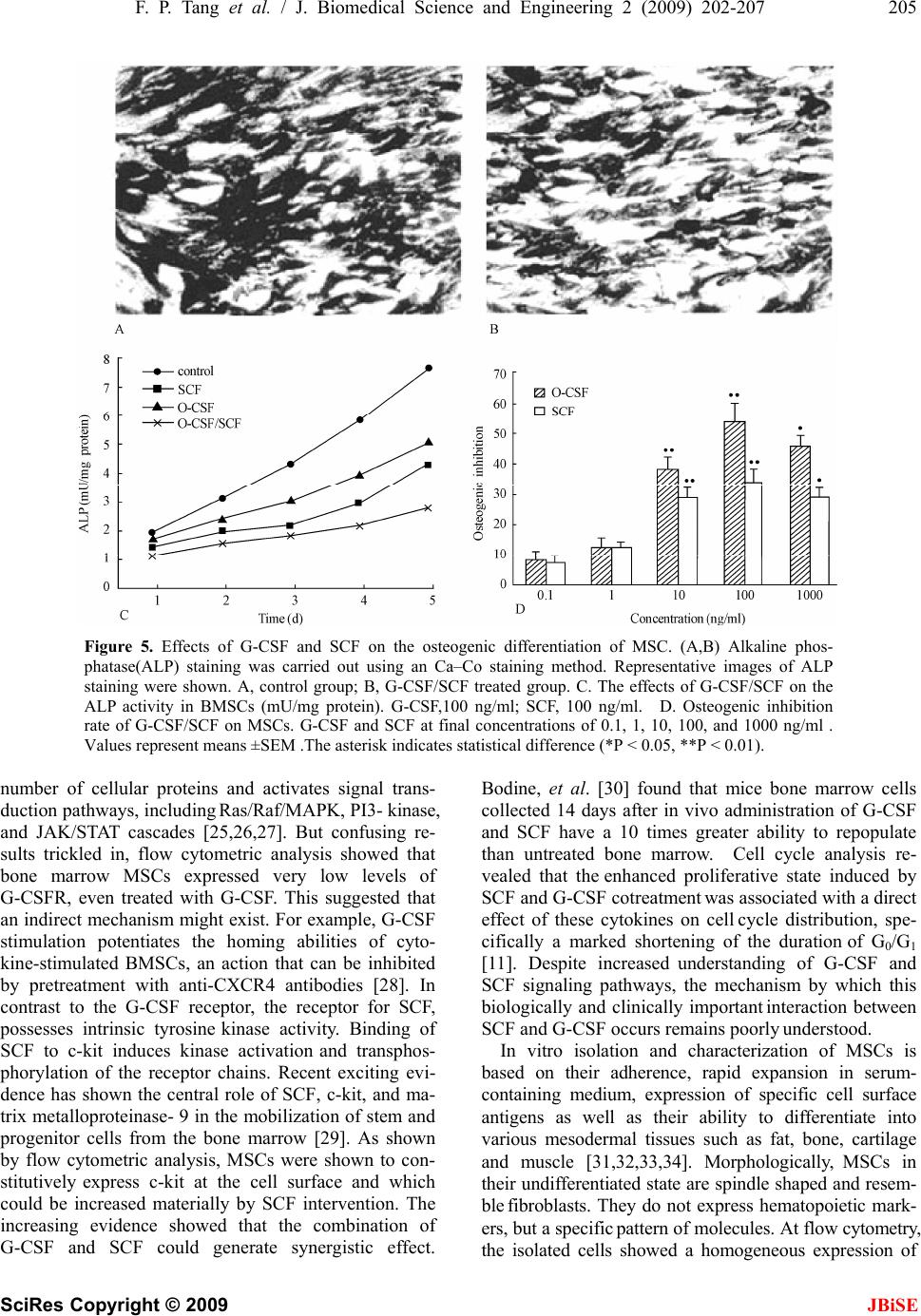 F. P. Tang et al. / J. Biomedical Science and Engineering 2 (2009) 202-207 205 SciRes Copyright © 2009 JBiSE Figure 5. Effects of G-CSF and SCF on the osteogenic differentiation of MSC. (A,B) Alkaline phos- phatase(ALP) staining was carried out using an Ca–Co staining method. Representative images of ALP staining were shown. A, control group; B, G-CSF/SCF treated group. C. The effects of G-CSF/SCF on the ALP activity in BMSCs (mU/mg protein). G-CSF,100 ng/ml; SCF, 100 ng/ml. D. Osteogenic inhibition rate of G-CSF/SCF on MSCs. G-CSF and SCF at final concentrations of 0.1, 1, 10, 100, and 1000 ng/ml . Values represent means ±SEM .The asterisk indicates statistical difference (*P < 0.05, **P < 0.01). number of cellular proteins and activates signal trans- duction pathways, including Ras/Raf/MAPK, PI3- kinase, and JAK/STAT cascades [25,26,27]. But confusing re- sults trickled in, flow cytometric analysis showed that bone marrow MSCs expressed very low levels of G-CSFR, even treated with G-CSF. This suggested that an indirect mechanism might exist. For example, G-CSF stimulation potentiates the homing abilities of cyto- kine-stimulated BMSCs, an action that can be inhibited by pretreatment with anti-CXCR4 antibodies [28]. In contrast to the G-CSF receptor, the receptor for SCF, possesses intrinsic tyrosine kinase activity. Binding of SCF to c-kit induces kinase activation and transphos- phorylation of the receptor chains. Recent exciting evi- dence has shown the central role of SCF, c-kit, and ma- trix metalloproteinase- 9 in the mobilization of stem and progenitor cells from the bone marrow [29]. As shown by flow cytometric analysis, MSCs were shown to con- stitutively express c-kit at the cell surface and which could be increased materially by SCF intervention. The increasing evidence showed that the combination of G-CSF and SCF could generate synergistic effect. Bodine, et al. [30] found that mice bone marrow cells collected 14 days after in vivo administration of G-CSF and SCF have a 10 times greater ability to repopulate than untreated bone marrow. Cell cycle analysis re- vealed that the enhanced proliferative state induced by SCF and G-CSF cotreatment was associated with a direct effect of these cytokines on cell cycle distribution, spe- cifically a marked shortening of the duration of G0/G1 [11]. Despite increased understanding of G-CSF and SCF signaling pathways, the mechanism by which this biologically and clinically important interaction between SCF and G-CSF occurs remains poorly understood. In vitro isolation and characterization of MSCs is based on their adherence, rapid expansion in serum- containing medium, expression of specific cell surface antigens as well as their ability to differentiate into various mesodermal tissues such as fat, bone, cartilage and muscle [31,32,33,34]. Morphologically, MSCs in their undifferentiated state are spindle shaped and resem- ble fibroblasts. They do not express hematopoietic mark- ers, but a specific pattern of molecules. At flow cytometry, the isolated cells showed a homogeneous expression of  206 F. P. Tang et al. / J. Biomedical Science and Engineering 2 (2009) 200-207 SciRes Copyright © 2009 JBiSE markers commonly used to identify hMSCs, i.e., CD29, CD90, CD105 positivities, and CD34, CD45, HLADR negativities, consistent with that reported for bone mar- row-derived MSCs [20,34]. Although these markers have been used by various groups, there is still no general consensus on th e optimal marker combination for MSCs. At present we did not know how long MSCs will main- tain innate characteristics, so we used early MSCs not exceeding 4 passages based on the assumption that early passage cells would be more likely to have the innate characteristics of MSCs. Cell proliferation was determined using MTT assay, which showed the combination of G-CSF and SCF was superior to the better of the two agents given alone. Usually, MSCs were typically defined by their capacity to adhere on plastic and form a fibroblastic colony (CFU-F). The colony forming unit fibroblast (CFU-F) assay was a well-established method for the quantifica- tion of marrow stromal cells (MSCs). We observed that G-CSF/SCF enhanced ex vivo MSC proliferation, and treating MSCs with G-CSF and SCF (0.1~100 ng/ml) resulted in a positive dose-dependent increase in the formation of CFU-F. G-CSF/SCF proliferative effect on MSCs was direct, dose dependent, long lasting. In addi- tion, ALP activity of cells cultured with G-CSF and SCF was low at one day, and increased time-dependently. MSC differentiation potential was not affected obviously by the enhancement of self-renewal, as the proliferative effect was not associated with induced differentiation. On the whole, these studies demonstrated that MSCs responsed to G-CSF, SCF, and to G-CSF plus SCF in a manner that suppressed differentiation, and promoted proliferation and self-renewal, and supported the view that these factors could act synergistically. And the ef- fects of G-CSF/SCF on MSCs give the cu e to understand better the biology and the role of MSCs. However, the biochemical mechanism underlying this activity remains to be resolved . 5. ACKNOWLEDGEMENTS The authors appreciate the help of the members of the Center Labora- tory and the Osteonecrosis research team at the Department of Ortho- pedic Surgery of Union Hospital, Tongji Medical College. REFERENCES [1] P. Menasche Cell transplantation in myocardium. Ann Thorac Surg 2003, 75: S20-28. [2] E. M. Horwitz, (2003) Bone marrow transplantation: It’s not just about blood anymore! Pediatr Transplant, 7, 56-58. [3] N. Koike, D. Fukumura, O. Gralla, et al., (2004) Tissue engineering: Creation of long-lasting blood vessels, Na- ture, 428, 138-139. [4] K. D. Lee, T. K. Kuo, J. Whang-Peng, et al., (2004) In vitro hepatic differentiation of human mesenchymal stem cells, Hepatology, 40, 1275-1284. [5] D. Baksh, L. Song, and R. S. Tuan, (2004) Adult mesen- chymal stem cells: characterization, differentiation, and application in cell and gene therapy, J Cell Mol Med, 8, 301-316. [6] A. Peister, J. A. Mellad, B. L. Larson, B. M. Hall, L. F. Gibson, and D. J. Prockop, (2004) Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of prolifera- tion, and differentiation potential, Blood, 103, 1662-1668. [7] B. Short, N. Brouard, T. Occhiodoro-Scott, A. Rama- krishnan, and P. J. Simmons, (2003) Mesenchymal stem cells, Arch. Med. Res, 34, 565-571. [8] V. Gangji, J. P. Hauzeur, C. Matos, V. De Maertelaer, M. Toungouz, and M. Lambermont, (2004) Treatment of os- teonecrosis of the femoral head with implantation of autologous bone-marrow cells: A pilot study, J Bone Joint Surg (Am), 86, 1153-1160. [9] V. Gangji, J. P. Hauzeur, A. Schoutens, M. Hinsenkamp, T. Appelboom, and D. Egrise, (2003) Abnormalities in the replicative capacity of Osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head, J Rheumatol, 30, 348-351. [10] D. Orlic, J. M. Hill, and A. E. Arai, (2002) Stem cells for myocardial regeneration, Circ Res, 91, 1092-1102. [11] M. K. Majumdar, M. A. Thiede, S. E. Haynesworth, S. P. Bruder, and S. L. Gerson, (2000) Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages, J Hematother Stem Cell Res, 9, 841-848. [12] R. F. Duarte and D. A. Frank, (2000) SCF and G-CSF lead to the synergistic induction of proliferation and gene expression through complementary signaling pathways, Blood, 96, 3422-3430. [13] R. Cancedda, G. Bianchi, A. Derubeis, and R. Quarto, (2003) Cell therapy for bone disease: A review of current status, Stem Cells, 21, 610-619. [14] F. R. Rose and R. O. Oreffo, (2002) Bone tissue engi- neering: Hope vs hype, Biochem. Biophys. Res Com- mun., 292, 1-7. [15] X. B. Yang, H. I. Roach, N. M. Clarke, et al., (2001) Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modifi- cation, Bone, 29, 523-531. [16] D. C. Colter, I. Sekiya, D. J. Prockop, (2001) Identifica- tion of a subpopulation of rapidly self-renewing and mul- tipotential adult stem cells in colonies of human marrow stromal cells, Proc Natl Acad Sci U S A, 98, 7841-7845. [17] S. A. Wexler, C. Donaldson, P. Denning-Kendall, C. Rice, B. Bradley, and J. M. Hows, (2003) Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not, British Journal of Haematology, 121, 368-374. [18] M. F. Pittenger, A. M. Mackay, S. C. Beck, et al., (1999) Multilineage potential of adult human mesenchymal stem cells, Science, 284, 143-147.  F. P. Tang et al. / J. Biomedical Science and Engineering 2 (2009) 202-207 207 SciRes Copyright © 2009 93-204. JBiSE [19] A. P. Croff and S. A. Przyborski, (2004) Generation of neuroprogenitor-like cells from adult mammalian bone marrow stromal cells in vitro, Stem Cells Dev, 13, 409-420. [20] Y. Jiang, B. N. Jahagirdar, R. L. Reinhardt, et al., (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature, 418, 41-49. [21] M. Studeny, F. C. Marini, R. E. Champlin, et al., (2002) Bone marrow-derived mesenchymal stem cells as vehi- cles for interferon-β delivery into tumours, Cancer Res, 62, 3603-3608. [22] O. N. Koc, S. L. Gerson, B. W. Cooper, et al., (2000) Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded mar- row mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy, J Clin Oncol, 18, 307-316. [23] D. Orlic, J. Kajstura, S. Chimenti, et al., (2001) Bone marrow cells regenerate infarcted myocardium, Nature, 410, 701-705. [24] M. Krampera, S. Glennie, R. Laylor, et al., (2003) Bone marrow mesenchymal stem cells inhibit the response of naïve and memory antigen-specific T cells to their cog- nate peptide, Blood, 101, 3722-3729. [25] B. Avalos, (1996) Molecular analysis of the granulo- cyte-colony stimulating factor receptor, Blood, 88, 761-777. [26] M. L. McLemore, S. Grewal, F. Liu, et al., (2001) STAT-3 activation is required for normal G-CSF-dependent proliferation and granulocytic differ- entiation, Immunity, 14, 1 [27] J. R. Fu, W. L. Liu, J. F. Zhou, et al., (2006) Sonic hedgehog protein promotes bone marrow-derived endo- thelial progenitor cell proliferation, migration and VEGF production via PI 3- kinase/Akt signaling pathways, Acta Pharmacol Sin, 27, 685-693. [28] O. Kollet, A. Spiegel, A. Peled, et al., (2001) Rapid and efficient homing of human CD34 (+) CD38 (-/low) CXCR4 (+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice, Blood, 97, 3283-3291. [29] B. Heissig, K. Hattori, and S. Dias, (2002) Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand, Cell, 109, 625-637. [30] D. M. Bodine, N. E. Seidel, and D. Orlic, (1996) Bone marrow collected 14 days after in vivo administration of granulocyte colony- stimulating factor and stem cell factor to mice has 10-fold more repopulating ability than untreated bone marrow, Blood, 88, 89-97. [31] E. H. Javazon, K. J. Beggs, and A. W. Flake, (2004) Mesenchymal stem cells: Paradoxes of passaging, Exp Hematol, 32, 414-425. [32] P. A. Zuk, M. Zhu, H. Mizuno, et al., (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies, Tissue Eng, 7, 211-228. [33] M. F. Pittenger and B. J. Martin, (2004) Mesenchymal stem cells and their potential as cardiac therapeutics, Circ Res, 95, 9-20. [34] J. R. Smith, R. Pochampally, A. Perry, et al., (2004) Isolation of a highly clonogenic and multipotential sub- fraction of adult stem cells from bone marrow stroma, Stem Cells, 22, 823-831.
|