Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 2, 276-283 doi:10.4236/msa.2011.24036 Published Online April 2011 (http://www.scirp.org/journal/msa) Copyright © 2011 SciRes. MSA Contribution to a Comparative Study of the Corrosion Inhibiting Effect of Some Azoles, to Protect the Cu70-30Ni Alloy in Aerated NaCl 3% in Presence of Ammonia Mohammed Benmessaoud1,3, Karima Es-salah1,2,, Ahmed Kabouri3, Najjat Hajjaji1, Hisai Takenouti2, Abdelah Srhiri1 1Laboratory of Electrochemistry, Materials and Environment (LEME), Faculty of Sciences, Ibn Tofail University, Kenitra, Morocco; 2CNRS UPR 15, LISE, UPMC University, Paris, France; 3University of Mohammed V Agdal, Energy Systems Laboratory, Materials and Environment, High School of Technology, Salé Medina, Morocco E-mail: benmessaoud_mma@yahoo.fr Received November 20th, 2010; revised January 17th, 2011; accepted March 11th, 2011. ABSTRACT Some azoles were tested such as 3-amino-1,2,4-triazole (ATA), 3-4’-bitriazole -1,2,4 (BiTA)and 2-Mercaptobenzimi- dazole (MBI) against Cu-30Ni alloy corrosion in 3%NaCl polluted by ammonia using potentiodynamic measurements and electrochemical impedance spectroscopy and non-electrochemical techniques (scanning Electron Microscopy (SEM)) studied surface morphology has been used to cha racterize electrode surface. This study permitted to follo w the evolution of the inhibitory effect of some azoles, on Cu-30Ni alloy corrosion in 3% NaCl polluted by ammonia and in- dicate that the tested inhibitors act as a good mixed-type inhibitor retarding the anodic and cathodic reactions. An in- crease of the inhibitors concentration lea ds to a decrease of corrosion rate and inhibition efficiency increase. Keywords: Cu-30Ni Alloy, Electrochemical Impedance Spectroscopy, Ammonia, Inhibition, Azole 1. Introduction Organic inhibitors are largely used with success to pro- tect copper and copper alloys against corrosion. Many papers reported that heterocyclic compounds, such as azole, reveal marked inhibition efficiency [1-12]. In a neutral chloride medium, Otmacic and Stupnišek-Lisac examined the inhibiting effect of non-toxic imidazoles derivatives, and showed that those containing phenyl groups showed a better performance [7]. El Issami et al. studied the inhibiting effect of triazoles (triazole, amino-triazole and diamino-triazole) in hydrochloric acid, and they found that the diamino-triazole has the best performance [8]. For substituted uracils with two azole radicals, the grafting of thiol groups increased substan- tially the inhibiting effect [9]. Huynh et al. examined the alkyl esters of carboxybenzotriazole in sulphate medium at different pH. In weakly acidic to neutral solutions, they stated the formation of polymeric complex that hin- ders corrosion of copper [10]. Laachach et al. confirmed also that 3-amino-1,2,4-triazole (ATA) exhibits an ex- cellent protective effect on the corrosion of Cu-30Ni in NaCl solution because of the complex film with copper oxide and triazoles formed at the metal surface increases its stability [11,12]. These experiments were performed in the absence of pollutants on copper electrode. Es-Salah et al. examined the inhibiting effect of ATA and Bitriazole (BiTA) on the corrosion of Cu-30Ni alloy in 3% NaCl in presence of ammoniac, and they found that when the ATA and BiTA were added in the solution, a remarkable inhibiting effect was observed when its concentration is higher than 1 mM [13,14]. 2-Mercaptoben- zimidazole (MBI), as an inhibitor, has been studied on the inhibition of mild steel in sulphuric acid solution [15], steel in hydrochloric acid [16,17], copper and its alloys in hydrochloric acid [18], mild steel in phosphoric acid [19]. Cu-Ni alloys are frequently employed in marine ap- plications. Al-Hachem and Carew studied the influence of pollutants such as chlorine and ammonia at low con- centration (5 ppm) in natural seawater. Both ammonia 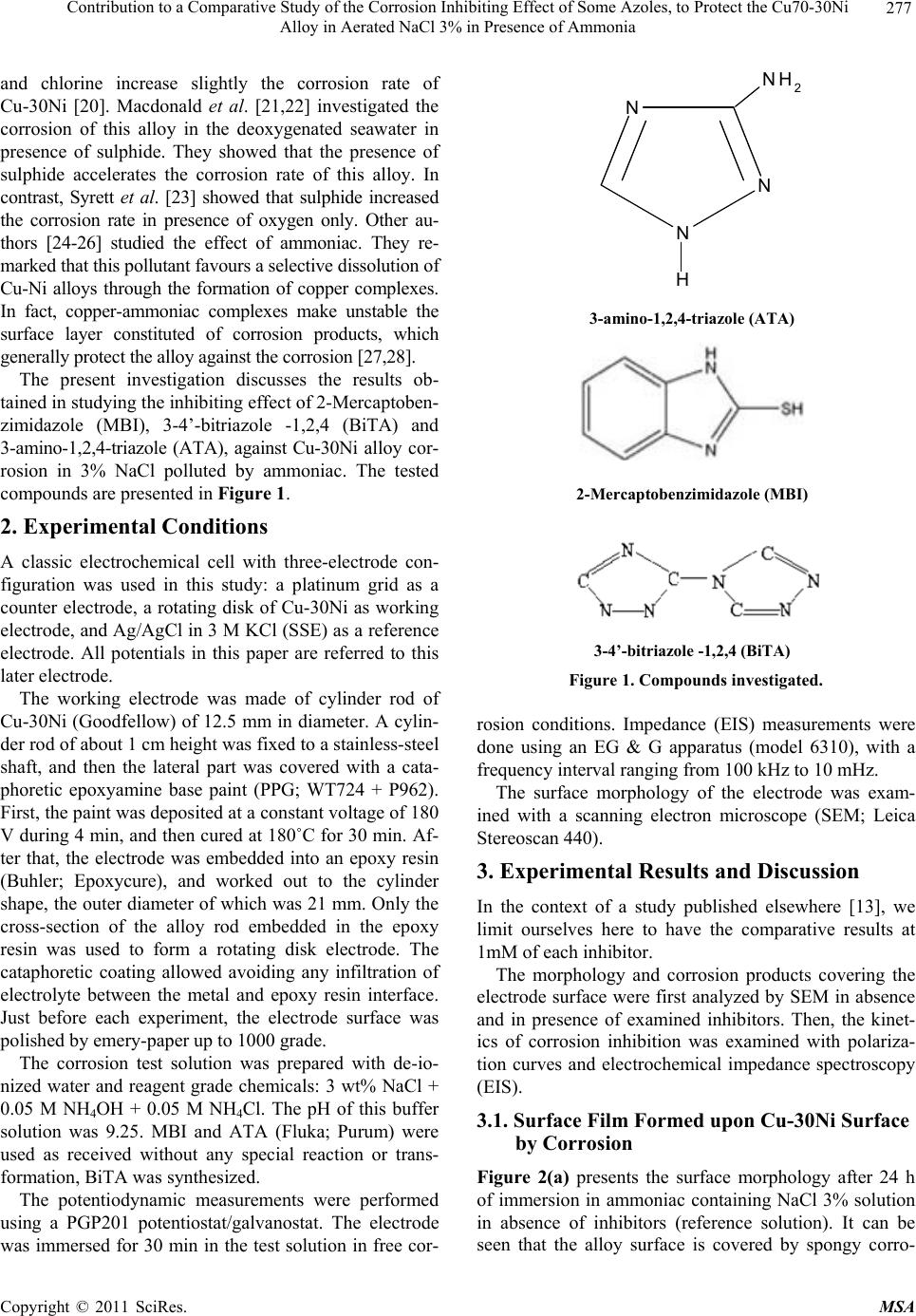 Contribution to a Comparative Study of the Corrosion Inhibiting Effect of Some Azoles, to Protect the Cu70-30Ni Alloy in Aerated NaCl 3% in Presence of Ammonia Copyright © 2011 SciRes. MSA 277 and chlorine increase slightly the corrosion rate of Cu-30Ni [20]. Macdonald et al. [21,22] investigated the corrosion of this alloy in the deoxygenated seawater in presence of sulphide. They showed that the presence of sulphide accelerates the corrosion rate of this alloy. In contrast, Syrett et al. [23] showed that sulphide increased the corrosion rate in presence of oxygen only. Other au- thors [24-26] studied the effect of ammoniac. They re- marked that this pollutant favours a selective dissolution of Cu-Ni alloys through the formation of copper complexes. In fact, copper-ammoniac complexes make unstable the surface layer constituted of corrosion products, which generally protect the alloy against the corrosion [27,28]. The present investigation discusses the results ob- tained in studying the inhibiting effect of 2-Mercaptoben- zimidazole (MBI), 3-4’-bitriazole -1,2,4 (BiTA) and 3-amino-1,2,4-triazole (ATA), against Cu-30Ni alloy cor- rosion in 3% NaCl polluted by ammoniac. The tested compounds are presented in Figure 1. 2. Experimental Conditions A classic electrochemical cell with three-electrode con- figuration was used in this study: a platinum grid as a counter electrode, a rotating disk of Cu-30Ni as working electrode, and Ag/AgCl in 3 M KCl (SSE) as a reference electrode. All potentials in this paper are referred to this later electrode. The working electrode was made of cylinder rod of Cu-30Ni (Goodfellow) of 12.5 mm in diameter. A cylin- der rod of about 1 cm height was fixed to a stainless-steel shaft, and then the lateral part was covered with a cata- phoretic epoxyamine base paint (PPG; WT724 + P962). First, the paint was deposited at a constant voltage of 180 V during 4 min, and then cured at 180˚C for 30 min. Af- ter that, the electrode was embedded into an epoxy resin (Buhler; Epoxycure), and worked out to the cylinder shape, the outer diameter of which was 21 mm. Only the cross-section of the alloy rod embedded in the epoxy resin was used to form a rotating disk electrode. The cataphoretic coating allowed avoiding any infiltration of electrolyte between the metal and epoxy resin interface. Just before each experiment, the electrode surface was polished by emery-paper up to 1000 grade. The corrosion test solution was prepared with de-io- nized water and reagent grade chemicals: 3 wt% NaCl + 0.05 M NH4OH + 0.05 M NH4Cl. The pH of this buffer solution was 9.25. MBI and ATA (Fluka; Purum) were used as received without any special reaction or trans- formation, BiTA was synthesized. The potentiodynamic measurements were performed using a PGP201 potentiostat/galvanostat. The electrode was immersed for 30 min in the test solution in free cor- N N N H NH2 3-amino-1,2,4-triazole (ATA) 2-Mercaptobenzimidazole (MBI) 3-4’-bitriazole -1 , 2 , 4 ( BiTA ) Figure 1. Compounds investigated. rosion conditions. Impedance (EIS) measurements were done using an EG & G apparatus (model 6310), with a frequency interval ranging from 100 kHz to 10 mHz. The surface morphology of the electrode was exam- ined with a scanning electron microscope (SEM; Leica Stereoscan 440). 3. Experimental Results and Discussion In the context of a study published elsewhere [13], we limit ourselves here to have the comparative results at 1mM of each inhibitor. The morphology and corrosion products covering the electrode surface were first analyzed by SEM in absence and in presence of examined inhibitors. Then, the kinet- ics of corrosion inhibition was examined with polariza- tion curves and electrochemical impedance spectroscopy (EIS). 3.1. Surface Film Formed upon Cu-30Ni Surface by Corrosion Figure 2(a) presents the surface morphology after 24 h of immersion in ammoniac containing NaCl 3% solution in absence of inhibitors (reference solution). It can be seen that the alloy surface is covered by spongy corro- 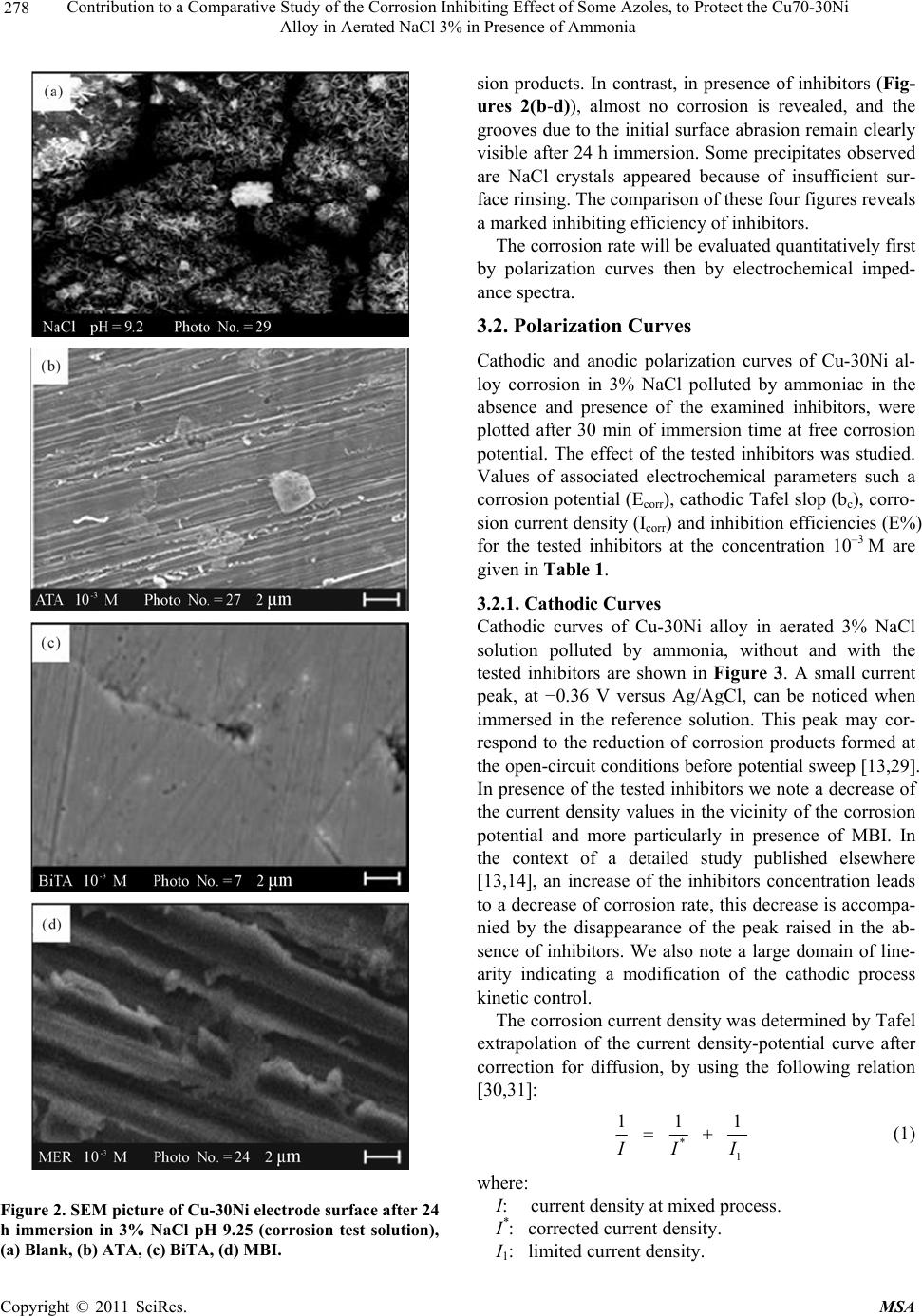 Contribution to a Comparative Study of the Corrosion Inhibiting Effect of Some Azoles, to Protect the Cu70-30Ni Alloy in Aerated NaCl 3% in Presence of Ammonia Copyright © 2011 SciRes. MSA 278 Figure 2. SEM picture of Cu-30Ni electrode surface after 24 h immersion in 3% NaCl pH 9.25 (corrosion test solution), (a) Blank, (b) ATA, (c) BiTA, (d) MBI. sion products. In contrast, in presence of inhibitors (Fig- ures 2(b-d)), almost no corrosion is revealed, and the grooves due to the initial surface abrasion remain clearly visible after 24 h immersion. Some precipitates observed are NaCl crystals appeared because of insufficient sur- face rinsing. The comparison of these four figures reveals a marked inhibiting efficiency of inhibitors. The corrosion rate will be evaluated quantitatively first by polarization curves then by electrochemical imped- ance spectra. 3.2. Polarization Curves Cathodic and anodic polarization curves of Cu-30Ni al- loy corrosion in 3% NaCl polluted by ammoniac in the absence and presence of the examined inhibitors, were plotted after 30 min of immersion time at free corrosion potential. The effect of the tested inhibitors was studied. Values of associated electrochemical parameters such a corrosion potential (Ecorr), cathodic Tafel slop (bc), corro- sion current density (Icorr) and inhibition efficiencies (E%) for the tested inhibitors at the concentration 10–3 M are given in Table 1. 3.2.1. Cathodic Curves Cathodic curves of Cu-30Ni alloy in aerated 3% NaCl solution polluted by ammonia, without and with the tested inhibitors are shown in Figure 3. A small current peak, at −0.36 V versus Ag/AgCl, can be noticed when immersed in the reference solution. This peak may cor- respond to the reduction of corrosion products formed at the open-circuit conditions before potential sweep [13,29]. In presence of the tested inhibitors we note a decrease of the current density values in the vicinity of the corrosion potential and more particularly in presence of MBI. In the context of a detailed study published elsewhere [13,14], an increase of the inhibitors concentration leads to a decrease of corrosion rate, this decrease is accompa- nied by the disappearance of the peak raised in the ab- sence of inhibitors. We also note a large domain of line- arity indicating a modification of the cathodic process kinetic control. The corrosion current density was determined by Tafel extrapolation of the current density-potential curve after correction for diffusion, by using the following relation [30,31]: * 1 111 I I I (1) where: I: current density at mixed process. I*: corrected current density. I1: limited current density. 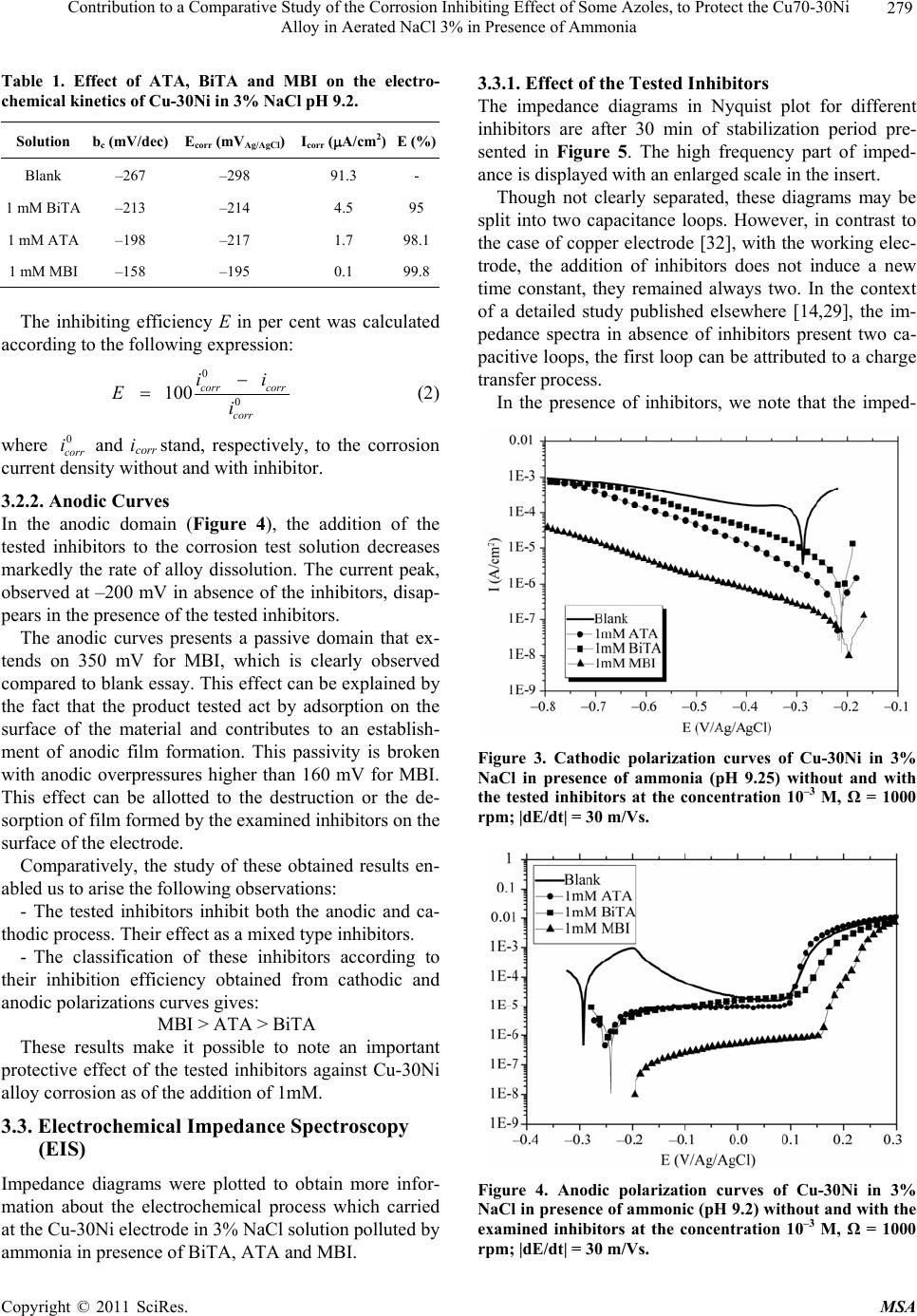 Contribution to a Comparative Study of the Corrosion Inhibiting Effect of Some Azoles, to Protect the Cu70-30Ni Alloy in Aerated NaCl 3% in Presence of Ammonia Copyright © 2011 SciRes. MSA 279 Table 1. Effect of ATA, BiTA and MBI on the electro- chemical kinetics of Cu-30Ni in 3% NaCl pH 9.2. Solution bc (mV/dec) Ecorr (mVAg/AgCl) Icorr (A/cm2)E (%) Blank –267 –298 91.3 - 1 mM BiTA –213 –214 4.5 95 1 mM ATA –198 –217 1.7 98.1 1 mM MBI –158 –195 0.1 99.8 The inhibiting efficiency E in per cent was calculated according to the following expression: 0 0 100 corr corr corr ii Ei (2) where 0 corr i and icorr stand, respectively, to the corrosion current density without and with inhibitor. 3.2.2. Anodic Curves In the anodic domain (Figure 4), the addition of the tested inhibitors to the corrosion test solution decreases markedly the rate of alloy dissolution. The current peak, observed at –200 mV in absence of the inhibitors, disap- pears in the presence of the tested inhibitors. The anodic curves presents a passive domain that ex- tends on 350 mV for MBI, which is clearly observed compared to blank essay. This effect can be explained by the fact that the product tested act by adsorption on the surface of the material and contributes to an establish- ment of anodic film formation. This passivity is broken with anodic overpressures higher than 160 mV for MBI. This effect can be allotted to the destruction or the de- sorption of film formed by the examined inhibitors on the surface of the electrode. Comparatively, the study of these obtained results en- abled us to arise the following observations: - The tested inhibitors inhibit both the anodic and ca- thodic process. Their effect as a mixed type inhibitors. - The classification of these inhibitors according to their inhibition efficiency obtained from cathodic and anodic polarizations curves gives: MBI > ATA > BiTA These results make it possible to note an important protective effect of the tested inhibitors against Cu-30Ni alloy corrosion as of the addition of 1mM. 3.3. Electrochemical Impedance Spectroscopy (EIS) Impedance diagrams were plotted to obtain more infor- mation about the electrochemical process which carried at the Cu-30Ni electrode in 3% NaCl solution polluted by ammonia in presence of BiTA, ATA and MBI. 3.3.1. Effect of the Tested Inhibitors The impedance diagrams in Nyquist plot for different inhibitors are after 30 min of stabilization period pre- sented in Figure 5. The high frequency part of imped- ance is displayed with an enlarged scale in the insert. Though not clearly separated, these diagrams may be split into two capacitance loops. However, in contrast to the case of copper electrode [32], with the working elec- trode, the addition of inhibitors does not induce a new time constant, they remained always two. In the context of a detailed study published elsewhere [14,29], the im- pedance spectra in absence of inhibitors present two ca- pacitive loops, the first loop can be attributed to a charge transfer process. In the presence of inhibitors, we note that the imped- Figure 3. Cathodic polarization curves of Cu-30Ni in 3% NaCl in presence of ammonia (pH 9.25) without and with the tested inhibitors at the concentration 10–3 M, Ω = 1000 rpm; |dE/dt| = 30 m/Vs. Figure 4. Anodic polarization curves of Cu-30Ni in 3% NaCl in presence of ammonic (pH 9.2) without and with the examined inhibitors at the concentration 10–3 M, Ω = 1000 rpm; |dE/dt| = 30 m/Vs.  Contribution to a Comparative Study of the Corrosion Inhibiting Effect of Some Azoles, to Protect the Cu70-30Ni Alloy in Aerated NaCl 3% in Presence of Ammonia Copyright © 2011 SciRes. MSA 280 ance display of the electrode in inhibitors containing the solution changes in shape and size, other it can be no- ticed that the impedance modulus increased dramatically in presence of tested inhibitors. The presence of two ca- pacitive loops seems to indicate a diffusion contribution to the beginning of the experiment. At high frequency loop, it is found that the charge transfer resistance Rt value increased in presence of the tested inhibitors, whereas the double layer capacity value found to be de- creased (Table 2). The decrease in capacity value was due to the adsorption of inhibitor molecules on the metal surface and act as barrier to the oxygen diffusion process. 3.3.2. Effect of the Rotation Speed Figure 6 presents the impedance spectra in presence of BiTA, ATA and MBI, when the electrode rotation speed is limited to 200 rpm and 1000 rpm after 30 min of immer- sion. No marked effect of rotation speed. This confirms the appearance of a broad tafelien field in the cathodic domain, when the tested inhibitors were added, on the one hand, and makes it possible to allot the first loop has a charge transfer process. As expected from the effect of rotation speed [13,14,29], the diffusion does not modify the electrode ki- netics at low cathodic or at the corrosion potentials. 3.3.3. Influence of Time Immersion Figure 7 shows the impedance diagrams obtained with- out and with 10–3 M of BiTA, ATA and MBI at the cor- Table 2. The results of non-linear values regression for the impedance spectra presented in Figure 5. Solution Rt (k·cm2) Cd (F/cm2) E’ (%) Blank 0.450 145 - 1 mM BiTA 7.25 87.2 94 1 mM ATA 11.16 17 96 1 mM MBI 34.5 6.6 97 Table 3. Rt and Cd changes with respect to immersion pe- riod in the test solution added of 1 mM of BiTA, ATA and MBI for = 1000 rpm. Solution Time (h)Rt k·cm2 Cd F/cm2 E (%) 0.5 0.450 145 - 2 0.372 171 - 4 0.196 123 - Blank 24 0.177 90.1 - 0.5 4.6 138 90 2 11.5 43 96.7 4 12 46 98.3 1 mM BiTA 24 14 10 98.7 0.5 11.2 17 96 2 17.3 3.3 97.8 4 21.1 5.3 99 1 mM ATA 24 33 1.1 99.4 0.5 34.5 6.6 97.1 2 45.6 3.5 99.2 4 57.6 4.8 99.6 1 mM MBI 24 125 1.3 99.7 Figure 5. Impedance diagrams of Cu-30Ni in the corrosion test solution in absence and presence of BiTA, ATA and MBI af- ter 30 min of stabilization period. Ω = 1000 rpm; |dE/dt| = 30 m/Vs.  Contribution to a Comparative Study of the Corrosion Inhibiting Effect of Some Azoles, to Protect the Cu70-30Ni Alloy in Aerated NaCl 3% in Presence of Ammonia Copyright © 2011 SciRes. MSA 281 Figure 6. Impedance diagrams of Cu-30Ni in the corrosion test solution in presence of BiTA, ATA and MBI after 30 min of immersion at different rotation speed. rosion potential after the Cu-30Ni was exposed to the solution for different times. The evolution of the characteristic parameters associ- ated with the capacitive loop with time is summarized in Table 3. The value of the associated capacity was calcu- lated from the relation C = 1/(2fRt), where f is the characteristic frequency in the maximum of the loop and Rt is the diameter of the first capacitive loop. The various diagrams of Figure 6 show a capacitive behavior of the interface in the field of frequency exam- Figure 7. Impedance diagrams of Cu-30Ni in the corrosion test solution in presence of 1 mM of tested inhibitors ex- posed for different times. = 1000 rpm. Real part (kOhm·cm2) Imaginary part (kOhm·cm2)  Contribution to a Comparative Study of the Corrosion Inhibiting Effect of Some Azoles, to Protect the Cu70-30Ni Alloy in Aerated NaCl 3% in Presence of Ammonia Copyright © 2011 SciRes. MSA 282 values of polarization resistances can be classified ac ined. As can be seen in this figure, the impedance dia- grams in the Nyquist plot become larger with time. The increase of the polarization resistance with the immersion period is often reported for the inhibiting action of het- erocyclic on copper corrosion [5-7,9,10]. However the cording to an order ascending: p MBIp ATAp BiTA RRR The capacitive behavior seems is not affected with immersion period. The Cd values observed in presence of examined inhibitors correspond to those usually allotted to the double layer. If we interpret the action of the inhibitors by the varia- tion of charge transfer resistance Rt,, the inhibition effi- cien cies evaluated using the following relation: 0 100 i tt i t RR ER (3) where Rti and Rt0 are respectively charge transfer resis- tance with and without inhibitor, corroborate those ob- tained with the stationary curves of polarization. In the whole cases, the protective effectiveness is higher than 99%. Furthermore, the inhibiting efficiency tends to increase with immersion period. Compared with the solution without ammoniac [11], it was observed that protective effect of inhibitors is reinforced, and the cor- rosion current becomes smaller in spite of Cu-NH3 com- plex formation. 4. Conclusions The comparative analysis of the results obtained in this work with the examined inhibitors shows that: The ATA, BiTA and MBI act at the same time on the reactions anodic and cathodic of the process of corrosion. Indeed the cathodic domain is characterized for the ex- amined inhibitors, by decrease of the current density values in the vicinity of the corrosion potential and more particularly in presence of MBI and disappearance of the cathodic peak, recorded in the absence of inhibitors. The anodic polarization curves show a decrease of the current density values with disappearance the current peak re- corded in the absence of the examined inhibitors. The values of charge transfer resistances raised of the im- pedance diagrams show the same evolution in time for all tested inhibitors, but with different values. The calculated inhibiting effectiveness remains important for all inhibi- tors. The kinetic effect of the tested inhibitors is two-fold: in one hand, their presence suppresses completely the oxygen evolution reaction, and merely the hydrogen evolution reaction is taking place at the electrode surface. On the other hand, the latter is reduced at least by one order of magnitude when 1 mM was added into the test solution. What shows that the use of only one compound is very sufficient to ensure an important protection of alloy in the corrosive medium used, that shows that the use of only one compound can be satisfactory to ensure an important protection of alloy in the studied corrosive medium? REFERENCES [1] C. Fiaud, “Inhibition of Copper Corrosion. The Comple- mentary Role of Oxides and Corrosion Inhibitors,” Pro- ceedings of the Eighth European Symposium on Corro- sion Inhibitors, Anna University, Ferrara, Vol. 2, 1995, pp. 929-949. [2] E. M. M. Sutter, F. Ammeloot, M. J. Pouet, C. Fiaud and R. Couffignal, “Heterocyclic Compounds Used as Corro- sion Inhibitors: Correlation between 13C and 1H NMR Spectroscopy and Inhibition Efficiency,” Corrosion Sci- ence, Vol. 41, No. 1, 1999, pp. 105-115. doi:10.1016/S0010-938X(98)00099-7 [3] D. Tromans and R. Sun, “The Anodic Polarization Be- havior of Copper in Aqueous Chloride/Benzotriazole So- lutions,” Journal of the Electrochemical Society, Vol. 138, 1991, pp. 3235-3244. doi:10.1149/1.2085397 [4] S. Ferina, M. Loncar and M. Metikos-Hocovic, Proceed- ings of the Eighth European Symposium on Corrosion In- hibitors, Anna University, Ferrara, Vol. 1, 1995, p. 1065. [5] B. Trachli, “Etude sur la Corrosion du Cuivre en Milieu NaCl 0.5 M et sa Protection par des Inhibiteurs Organiques et des Films Polyméres Obtenus par Électropolymerisation,” Cotutelle Thesis, P&M Curie University–Kénitra Uni- versity, Paris–Kénitra, 2001. [6] B. Trachli, M. Keddam, A. Srhiri and H. Takenouti, “Pro- tective Effect of Electropolymerized 3-Amino 1,2,4-Triazole towards Corrosion of Copper in 0.5 M NaCl,” Corrosion Science, Vol. 44, No. 5, 2002, pp. 997-1008. doi:10.1016/S0010-938X(01)00124-X [7] H. Otmačić and E. Stupnišek-Lisac, “Copper Corrosion Inhibitors in near Neutral Media,” Electrochimica Acta, Vol. 48, No. 8, 2003, pp. 985-991. [8] S. El Issami, L. Bazzi, M. Hilal, R. Saloghi, S. Kertit, “Inhibition of Copper Corrosion in HCl 0.5 M Medium by some Triazolic Compounds,” Annales de Chimie Sci- ence des Matériaux, Vol. 27, 2002, pp. 63-72. doi:10.1016/S0151-9107(02)80019-8 [9] A. Dafali, B. Hammouti, R. Makhlisse and S. Kertit,” Substituted Uracils as Corrosion Inhibitors for Copper in 3% NaCl Solution,” Corrosion Science, Vol. 45, No. 8, 2003, pp. 1619-1630. doi:10.1016/S0010-938X(02)00255-X [10] N. Huynh, S. E. Bottle, T. Notoya, A. Trueman, B. Hin- ton and D. P. Schweinsberg, “Studies on Alkyl Esters of Carboxybenzotriazole as Inhibitors for Copper Corro-  Contribution to a Comparative Study of the Corrosion Inhibiting Effect of Some Azoles, to Protect the Cu70-30Ni Alloy in Aerated NaCl 3% in Presence of Ammonia Copyright © 2011 SciRes. MSA 283 sion,” Corrosion Science, Vol. 44, No. 6, 2002, pp. 1257- 1276. doi:10.1016/S0010-938X(01)00109-3 [11] A. Laachach, “Study of the Inhibition of Corrosion of Cu-30Ni Alloy in 3% NaCl Medium of Triazole Deriva- tives,” Thesis, Mohamed V University, Rabat, 1991. [12] A. Laachach, M. Srhiri, A. Aouial and A. Benbachir, “Corriosion Inhibition of a 70/30 Cupronickel in a 3% Sodium Chloride Medium by Various Azoles,” Journal of Chemical Physics, Vol. 89, No. 10, 1992, pp. 2011-2017. [13] K. Es-Salah, M. Keddam, K. Rahmouni, A. Srhiri and H. Takenouti, “Aminotriazole as Corrosion Inhibitor of Cu-30Ni Alloy in 3% NaCl in Presence of Ammoniac,” Electrochimica Acta, Vol. 49, No. 17-18, 2004, pp. 2771-2778. doi:10.1016/j.electacta.2004.01.038 [14] K. Es-Salah, F. Said, M. Benmessoud, N. Hajjaji, M. Aouial, H. Takenouti and A. Srhiri, “Corrosion of Cop- per-Nickel (Cu-30Ni) Alloy in 3% NaCl Solution, in Presence of Ammoniac to pH 9.25: The Inhibition Effect of the Bitriazole (BITA),” Physical and Chemical News, Vol. 23, May 2005, pp. 108-118. [15] M. Th. Makhhuf, S. A. El-Shatory and A. El-Said, “The Synergistic Effect of Halide Ions and some Selected Thiols as a Combined Corrosion Inhibitor for Pickling of Mild Steel in Sulphuric Acid Solution,” Materials Chem- istry and Physics, Vol. 43, No. 1, 1996, pp. 76-82. doi:10.1016/0254-0584(95)01593-J [16] A. N. Starchak, A. N. Krasovskii, V. A. Anishchenko and L. D. Kosnkhina, “Anticorrosion Activity of Some 2-Mercaptobenzimidazole Derivatives,” Zashchita Met- allov, Vol. 30, No. 4, 1994, pp. 405-409. (In Russian) [17] G. L. Makovei, V. G. Ushakov, V. K. Bagin and V. P. Shemshei, “Inhibition of Acid Corrosion of Iron by Gamma Irradiated 2-Mercaptobenzimidazole,” Zashchita Metallov, Vol. 22, No. 3, 1986, pp. 470-472. (In Russian) [18] S. Thibault and J. Talbot, “Application of Multiple Re- flection Infrared Spectrometry to the Study of Several Corrosion Problems,” Metaux Corrosion-Industrie, Vol. 50, 1975, p. 51. (In French) [19] L. Wang, “Evaluation of 2-Mercaptobenzimidazole as Corrosion Inhibitor for Mild Steel in Phosphoric Acid,” Corrosion Science, Vol. 43, No. 12, 2001, pp. 2281-2289. doi:10.1016/S0010-938X(01)00036-1 [20] A. Al-Hachemi and J. Carew, “The Use of Electrochemical Impedance Spectroscopy to Study the Effect of Chlorine and Ammonia Residuals on the Corrosion of Copper-Based and Nickel-Based Alloys in Seawater,” Desalination, Vol. 150, No. 3, 2002, pp. 255-262. [21] D. D. Macdonald, B. C. Syrett and S. S. Wing, “Corro- sion of Cu-Ni Alloys 706 and 715 in Flowing Seawater. II,” Corrosion, Vol. 35, 1979, p. 367. [22] L. E. Eiselstein, R. D. Caligiuri, S. S. Wing and B. C. Syrett, “Annual Report to Office of Naval Research,” ONR Contract (No. 00014-77-0046, NR 36-116), 1979. [23] B. C. Syrett and D. D. Macdonald, “The Validity of Elec- trochemical Methods for Measuring Corrosion Rates of Copper-Nickel Alloys in Sea Water,” Corrosion, Vol. 35, 1979, p. 505. [24] R. Francis, “Effect of Pollutants on Corrosion of Copper Alloys in Sea Water,” British Corrosion Journal, Vol. 20, No. 4, 1985, pp. 167-173. [25] D. A. Jones, “Principles and Prevention of Corrosion, Maxwell MacMillan Pub., New York, 1992, Chapter 11. [26] M. R. Gennero, De Chialvo, S. L. Marchiano and A. J. Arvia, “The Mechanism of Oxidation of Copper in Alka- line Solutions,” Journal of Applied Electrochemistry, Vol. 14, No. 2, 1984, pp. 165-175. [27] H. D. Speckmann, M. M. Lohrengel, J. W. Schutze and H. H. Strehblow, “The Growth and Reduction of Duplex Oxide Films on Copper,” Berichte der Bunsengesellschaft für Physikalische Chemie, Vol. 89, No. 4, 1985, pp. 392- 402. [28] N. Bellakhal, K. Draou and J. L. Brisset, “Electrochemi- cal Investigation of Copper Oxide Films Formed by Oxy- gen Plasma Treatment,” Jou rn al of Applied Electrochemistry, Vol. 27, No. 4, 1997, pp. 414-421. doi:10.1023/A:1018409620079 [29] K. Es-Salah, “Study of the Inhibition of Corrosion of Cu-30Ni Alloy in 3% NaCl Medium Polluted by Ammo- nia Effect of Triazole Derivatives,” Cotutelle Thesis, Ibn Toufail University, Kenitra, 2006. [30] M. Dupart, F. Dabosi, F. Moran and S. Rocher, “Inhibi- tion of Corrosion of a Carbon Steel by the Aliphatic Fatty Polyamines in Association with Organic Phosphorous Compounds in 3% Sodium Chloride Solutions,” Corro- sion-Nace, Vol. 37, 1981, pp. 262-266. [31] A. Bonnel, F. Dabosi, C. Deslouis, M. Duprat, M. Ked- dam and B. Tribollet, “Corrosion Study of a Carbon Steel in Neutral Chloride Solutions by Impedance Techniques,” Journal of the Electrochemical Society, Vol. 130, No. 4, 1983, pp. 753-761. doi:10.1149/1.2119798 [32] M. Sfaira, A. Srhiri, H. Takenouti, M. M. de Focquel- mon-Loizos, A. Ben Bachir and M. Khalkhil, “Corrosion of Mild Steel in Low Conductive Media Simulating Natu- ral Waters,” Journal of Applied Electrochemistry, Vol. 31, No. 5, 2001, pp. 537-546. doi:10.1023/A:1017540522687 |

