Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2009, 2, 158-165 Published Online June 2009 in SciRes. http://www.scirp.org/journal/jbise JBiSE A muscle spindle model and study the effects of static and dynamic γ stimulations on primary and secondary ending outputs Glayol Nazari Golpayegani1, Amir Homayoun Jafari1 1Biomedical Engineering Department Islamic Azad University, Science and Research Branch Tehran, Iran Email: Gelayol777@yahoo.com, Amir_j73@yahoo.com Received 31 October 2008; revised 6 January 2009; accepted 19 January 2009. ABSTRACT A linear lumped-parameter mechanical model of the muscle spindle is presented. It is shown that the model simulation exhibits the spindle be- havior in most aspects of transient ramp-stretch performance. The requirements that such a model places on the mechanisms of fusimotor control are discussed. Then the efficacy of static γ and dynamic γ stimulation on primary ending output and secondary ending output is studied. The results of simulations show that primary ending of muscle spindle is affected either by static and dynamic stimulation and Secondary ending of muscle spindle is affected only by static fiber stimulation. The bias of pri- mary ending output is increased by an increase in static γ stimulation. The Ramp of primary ending response is increased by an increase in dynamic γ stimulation. An increase in static γ stimulation in secondary ending of muscle spindle increases the dc level of secondary ending output so the bias of output is increased too. Keywords: Modeling; Muscle Spindle; Postural Control Systems 1. INTRODUCTION The mammalian muscle spindle, as recent anatomical studies have shown [1,2,3,4,5], is a highly complex sen- sory organ connected in parallel with skeletal muscles for detecting change and rate of change in length in those muscles. Each spindle consists of several “inter- fusal” fibers (as distinct from “extrafusal” fibers of which the bulk of skeletal muscle consists) which appear to fall into two groups, with respect to their anatomical and their physiological characteristics. A simple example is illustrated in Figure 1. The larger type of fiber con- sists of striated contractile tissue with a non contractile equatorial region which possesses nuclei but lacks myo- filaments. The distribution of nuclei within the equato- rial region has led to naming this type the “nuclear-bag fiber.” The smaller fibers have striations and nuclei throughout their entire length and are called “nuclear- chain fibers.” The efferent innervation to the fibers is by way of the gamma-motor (γ-motor) or fusimotor system. It is agreed that there are two anatomically distinct kinds of fusimotor innervation, distinguishable by the nature of the nerve endings. There are “γ-plate” fibers, ending in small end plates similar to those at extrafusal myoneural junctions and “γ-trail” fibers whose endings are more diffuse. (There is actually a third kind of innervation, the β-plate ending, which we will not discuss here). It is not agreed as to whether both types of endings are found on both bag and chain fibers, as Barker [1] holds. Accord- ing to Boyd [2], plates are only found on bag fibers and trail only on chain fibers. Activation of skeletal muscle has definite influence in increasing the stiffness and viscosity of the muscle [7]. In all likelihood, this happens to intrafusal muscles too, although it has not been verified experimentally. Such changes in parameter value would modify spindle re- sponse to stretch. The amount of force produced by intrafusal contrac- tion is known only roughly by measurement [8,9] or computation [10,11], and those measurements are of whole spindles without differentiation between bag or chain fiber contraction. Boyd has stated [12,13] that con- traction is more pronounced and faster in chain fibers than in bag fibers but much remains to be learned about this aspect of spindle behavior. The very tentative con- clusion that has been proposed [14] is that dynamic stimulation controls output mainly through modification of parameter values by trail endings, while static stimu- lation works through force generated in the chain fibers through the plate endings. The afferent innervation from the spindles is also of two types. The primary afferent has its endings both on the nuclear bag and on the nuclear chain. The secondary  G. N. Golpayegani et al. / J. Biomedical Science and Engineering 2 (2009) 158-165 159 SciRes Copyright © 2009 JBiSE afferent usually has endings only on the nuclear chain. When the muscle is stretched or the y-motor nerves stimu- lated, action potentials are recorded in the primary and secondary afferent nerves. Corresponding to the anatomi- cal observations described above, there is an equally bi- modal response with respect to both stretch and stimulation as recorded from the two types of nerves. The purpose of this paper is to describe some simula- tions of a linear lumped-parameter mechanical model of the muscle spindle as a neurally controlled transducer of stretch and discuss the requirements such a model places on the mechanisms of control. To do this, we shall first summarize the various types of responses to the various types of inputs that have been described by numerous previous investigators [15,16,17,18]. In order to clarify our terminology yet remain rea- sonably consistent with the physiological literature, let us define three terms: dynamic sensitivity, static sensi- tivity, and bias. These refer to steady-state behavior after transients have died out. The dynamic sensitivity is a measure of that component of the spindle afferent output which is proportional to the rate of spindle stretch. It has units of pps/mm/s. The static sensitivity is a measure of that component of the spindle afferent output which is proportional to the amplitude of spindle stretch. It has units of pps/mm. The bias is the tonic component of the spindle output that is independent of stretch. It has units of pps. Note that the bias corresponds to they intercept on the length-firing rate curve and, as such, it is sensitive to changes in the slope of that curve. Thus changes in the static sensitivity may be reflected, in part, by propor- tional changes in bias. The γ-motor system can be divided into two groups, dynamic and static fusimotor fibers, on the basis of their differential effects on the primary and secondary afferents. These effects are summarized in Table І. The primary afferent is influenced by stimulation of both dynamic an static fibers, the former having their most pronounced effect on the dynamic sensitivity and the latter enhancing the bias and static sensitivity. The secondary afferent is influenced almost solely by the static fusimotor fibers. This paper is organized as following sections: we de- scribe our model in Section 2. We show our simulation results in Section 3. In Section 4 we study the effects of static γ and dynamic γ stimulation on primary ending output and secondary ending output and we have discus- sion in last section. 2. MODEL The model we have chosen for our simulation is shown in Figure 2. It is an extremely general viscoelastic sys- tem for which we wish to establish, not merely a transfer function of the spindle, but an anatomical-topological equivalence such that displacements of the model nodes give a direct quantitative measure of deformations of the spindle’s sensory regions. The upper half of Figure 2 is the nuclear-bag fiber which consists of a lumped tendon and series elasticity, two identical contractile sections representing the larger intrafusal muscle fibers, and a non contractile nuclear bag from which part of the primary output is taken. The nuclear chain fiber is the lower half of the figure is at- Figure 1. Simple two-fiber mammalian muscle spindle with two types of fibers and efferent and afferent innervation. 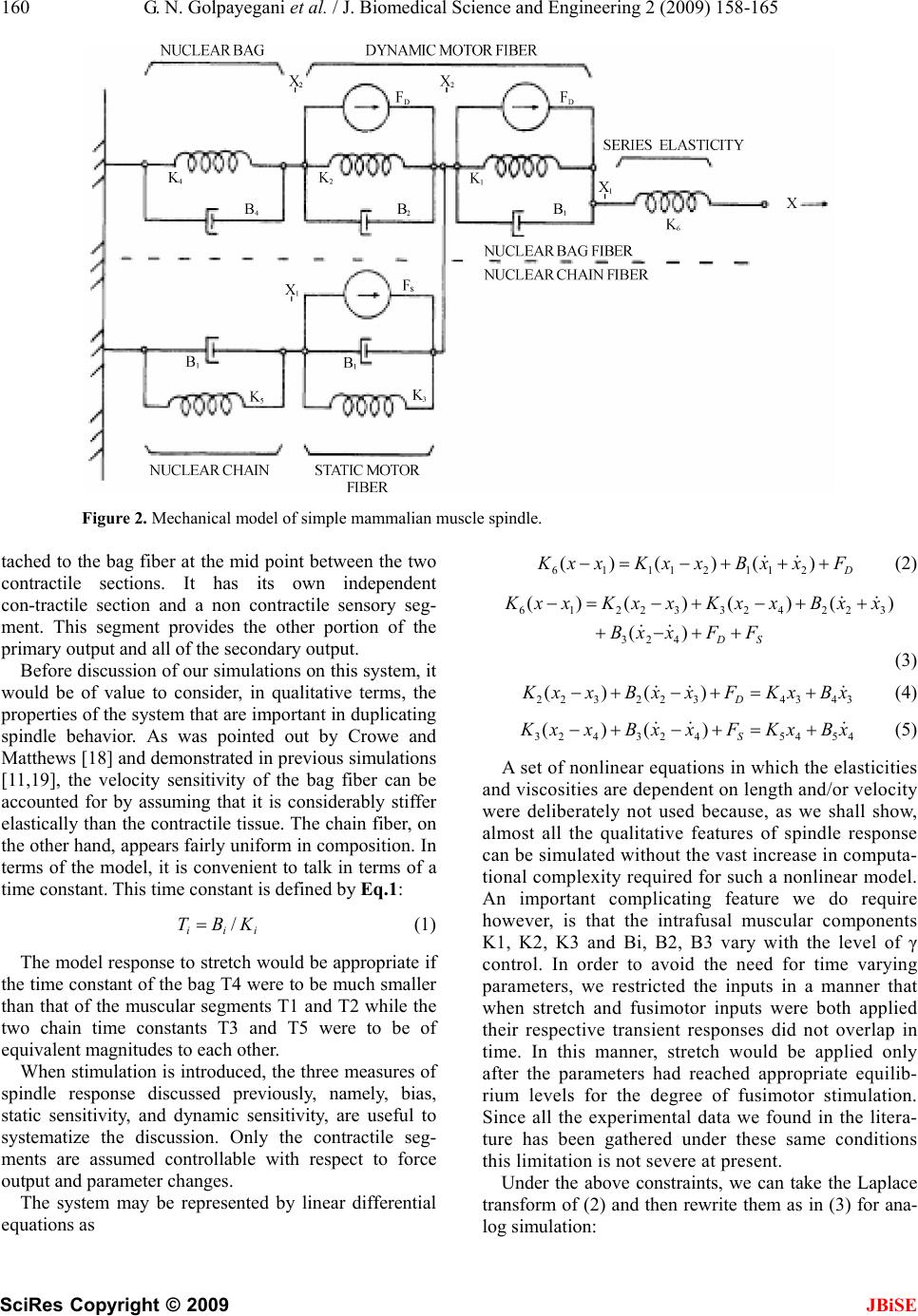 160 G. N. Golpayegani et al. / J. Biomedical Science and Engineering 2 (2009) 158-165 SciRes Copyright © 2009 JBiSE Figure 2. Mechanical model of simple mammalian muscle spindle. tached to the bag fiber at the mid point between the two contractile sections. It has its own independent con-tractile section and a non contractile sensory seg- ment. This segment provides the other portion of the primary output and all of the secondary output. Before discussion of our simulations on this system, it would be of value to consider, in qualitative terms, the properties of the system that are important in duplicating spindle behavior. As was pointed out by Crowe and Matthews [18] and demonstrated in previous simulations [11,19], the velocity sensitivity of the bag fiber can be accounted for by assuming that it is considerably stiffer elastically than the contractile tissue. The chain fiber, on the other hand, appears fairly uniform in composition. In terms of the model, it is convenient to talk in terms of a time constant. This time constant is defined by Eq.1: iii KBT / (1) The model response to stretch would be appropriate if the time constant of the bag T4 were to be much smaller than that of the muscular segments T1 and T2 while the two chain time constants T3 and T5 were to be of equivalent magnitudes to each other. When stimulation is introduced, the three measures of spindle response discussed previously, namely, bias, static sensitivity, and dynamic sensitivity, are useful to systematize the discussion. Only the contractile seg- ments are assumed controllable with respect to force output and parameter changes. The system may be represented by linear differential equations as D FxxBxxKxxK )()()( 21121116 (2) SD FFxxB xxBxxKxxKxxK )( )()()()( 423 32242332216 (3) 3434322322 )()( xBxKFxxBxxK D (4) 4545423423 )()( xBxKFxxBxxK S (5) A set of nonlinear equations in which the elasticities and viscosities are dependent on length and/or velocity were deliberately not used because, as we shall show, almost all the qualitative features of spindle response can be simulated without the vast increase in computa- tional complexity required for such a nonlinear model. An important complicating feature we do require however, is that the intrafusal muscular components K1, K2, K3 and Bi, B2, B3 vary with the level of γ control. In order to avoid the need for time varying parameters, we restricted the inputs in a manner that when stretch and fusimotor inputs were both applied their respective transient responses did not overlap in time. In this manner, stretch would be applied only after the parameters had reached appropriate equilib- rium levels for the degree of fusimotor stimulation. Since all the experimental data we found in the litera- ture has been gathered under these same conditions this limitation is not severe at present. Under the above constraints, we can take the Laplace transform of (2) and then rewrite them as in (3) for ana- log simulation:  G. N. Golpayegani et al. / J. Biomedical Science and Engineering 2 (2009) 158-165 161 SciRes Copyright © 2009 JBiSE 243 2 2 1 11 x s F a s x a s x a s x ax D (6) 49387 65 4 4 3 3 2 2 1 12 xbxb s Fs b s F b s x b s x b s x b s x b s x bx D (7) 243 3 2 2 13 xc s F c s x c s x cx D (8) 243 4 2 2 14 xd s Fs d s x d s x dx (9) We chose to rewrite (7) by combining it with (8) and (9) and get (10) (equations for ai, bi, ci, di, and ei in terms of the model parameters are listed in Appen- dix І): s F e s F e s x e s x e s x e s x e s x ex S D 765 4 4 3 3 2 2 1 12 (10) The block simulation of these four Eqs.6, 8, 9, and 10 is shown in Figure 3. The output of Figure 3(c) is nuclear bag stretch and output of 3(d) is nuclear chain stretch. The primary output is given by Eq.11 and the secondary output by Eq.12: 431xxf (11) 42 xf (12) 3. RESULTS OF SIMULATIONS Figure 4 shows some results of our simulations for pri- mary and secondary outputs. The param eters used in the si of 4. SECON- imulation on primary and secon- 4.1. timu- increased, 2) the dc level is increased and 3) final with mulation are listed in Appendix ІІ. In Figure 4(a) the primary output in response to stretch and γ stimulation is shown. In the lower curve, the spindle is relaxed and unstretched for 0.5 second and then stretched 3 mm at 6 mm/s. The middle curve shows the effects of adding static γ-motor stimulation of 100 pps starting at t=0 while the upper curve shows the ef- fects of dynamic γ-motor stimulation of 100 pps. Quite clearly, dynamic stimulation effects dynamic and static sensitivity as well as bias by stretching the nuclear bag and by increasing the viscosity of the bag fiber’s contrac- tile components. The effects of static stimulation are sim- ply increased bias and static sensitivity from the chain branches of the ending. Figure 4(b) shows the output of the secondary afferent for the same inputs. The absence of dynamic sensitivity and the ineffectiveness of dynamic stimulation are apparent. These curves show that our model does mimic spindle behavior in most aspects transient ramp-stretch performance as listed in Table 1. STUDY THE EFFECTS OF STATIC AND DYNAMIC GAMMA STIMULA- TION ON PRIMARY AND DARY ENDING OUTPUTS After we simulated our model, we studied the effects of static and dynamic γ st dary ending outputs. Effects of Static and Dynamic γ S lation on Primary Ending Output As we can see from Figure 4(a), with applying the dy- namic γ stimulation on primary ending, we have follow- ing results: 1) the ramp of primary ending response is Figure 3. Analog computer simulation of model of Figure 2.  162 G. N. Golpayegani et al. / J. Biomedical Science and Engineering 2 (2009) 158-165 SciRes Copyright © 2009 JBiSE nstimula ending ou 4.2. Effects of Static and Dynamic γ Stimu- As wic γ ndary end- in uted situation. With applying static γ stimulation on primary ending output, dc level is increased and the ramp of primary ending output dose not change. Briefly, we have following results for primary tput with applying dynamic γ stimulation: 1) the bias can be increased by either an increase in k1, k2, or out- put from the force generators FD. 2) Increased static sensitivity requires an increase in k1, k2. 3) Increased dynamic sensitivity requires an increase in T1/T4 and T2/T4. lation on Secondary Ending Output e can see from Figure 4(b), applying dynam stimulation, almost dose not have any clear effect on secondary ending output and it just increases the static sensitivity a little. With applying static γ stimulation we have following results: 1) the dc level is increased, 2) the ramp of secondary ending response is not changed therefore the dynamic sensitivity is not increased and 3) the static sensitivity is highly increased. Briefly, we have following results for seco g output with applying static γ stimulation: 1) The bias is increased by an increase in k3 or output from the force generator Fs. 2) The static sensitivity is increased by an increase in k3. 3) The dynamic sensitivity is not signifi- cantly changed. Figure 4. Stimulated response of a mammalian muscle spin- b le 1 . Effects of dynamic and static stimulation on bias and d imulation dle during stretch. (a) Primary ending. (b) Secondary ending. ynamic and static stimulation of spindle afferents. Ta Unstimulated Dynamic Fiber Stimulation Static Fiber St Primary ending Bias ity gnificant t in crease rease l ecrease sensitivity ight t o effect o effect Dynamic sensitiv sensitivityStatic variable si significan crease in slight inc arge increase d increase Secondary ending Bias Dynamic sensitivity Static variable sl significan no effect n no effect increase n increase dd mulabias n lis namic γ ut crease in Static γ Stimu- stimur curve The effects of ynamic anstatic stition on ad dynamic and static sensitivity of spindle afferent is ted in Table І. As we can see from Table І, primary ending is affected either by static and dynamic stimula- tion. Secondary ending is affected only by static fiber stimulation. .3. Effects of an Increase in Dy4 Stimulation on Primary Ending Outp Figure 5 shows the effect of an increase in dynamic γ stimulation on primary ending output. Upper curve in Figure 5 (curve 2) shows the effect of an increase in dynamic γ in compare with mid curve (curve 1) with no increase in dynamic γ stimulation. As we can see from Figure 5, with applying an increase in dynamic γ stimu- lation we observe that: 1) Ramp of primary ending re- sponse is increased, therefore dynamic sensitivity is in- creased too, 2) If we consider primary ending output as a step jump, the height of this step is increased with an increase in dynamic γ, 3) dc level is not changed so we have no change in bias. 4.4. Effects of an In lation on Primary Ending Output Figure 6 shows the effect of an increase in static γ lation on primary ending output. Uppe (curve 2) in Figure 6 shows the primary ending output Figure 5. the effect of an increase in γd stimulation on primary ending output. Primary ending output without any increase in γd stimulation (curve 1) and primary ending output with an increase in γd stimulation (curve 2). 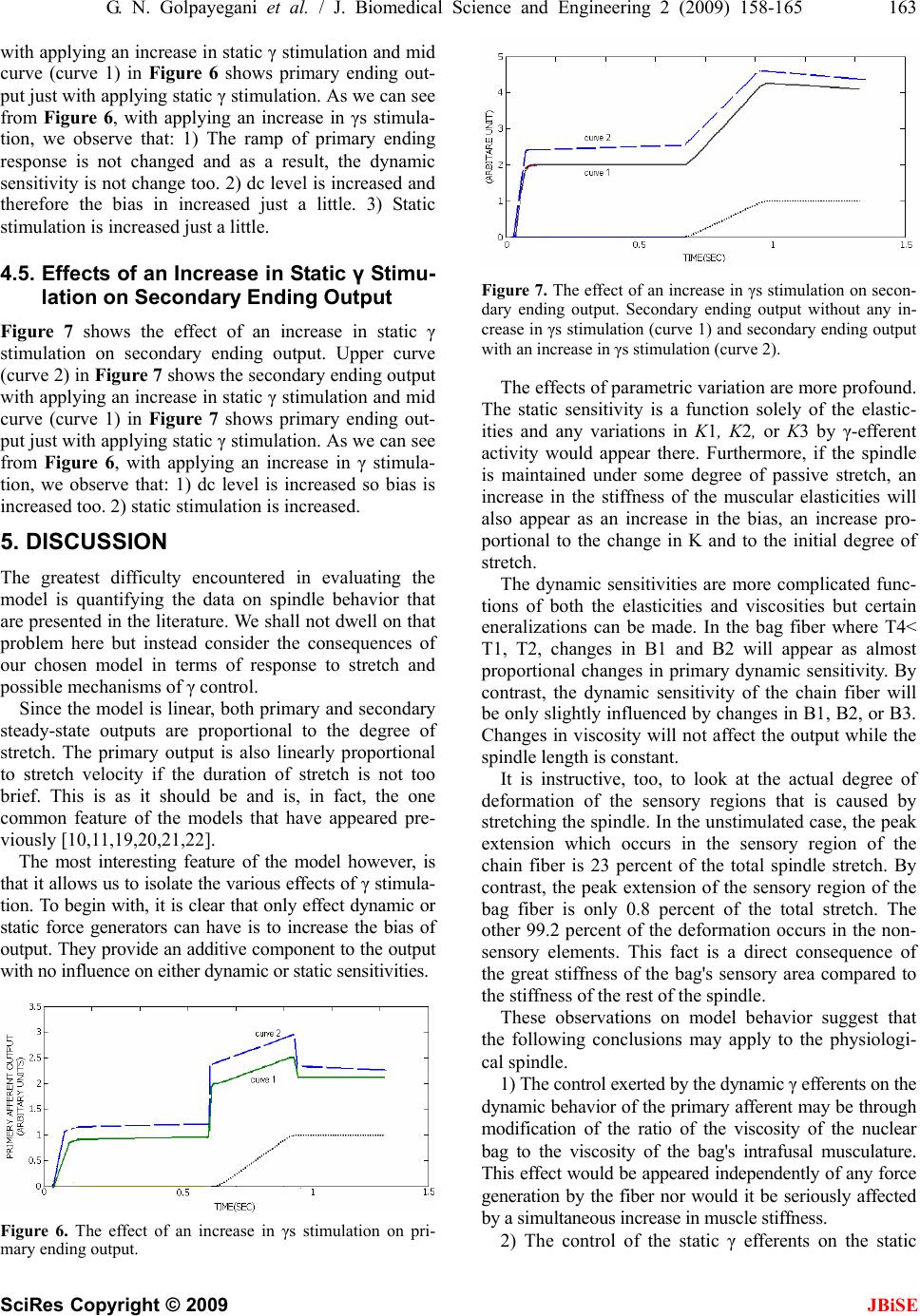 G. N. Golpayegani et al. / J. Biomedical Science and Engineering 2 (2009) 158-165 163 SciRes Copyright © 2009 JBiSE Static γ Stimu- Figu ic γ encountered in evaluating the with applying an increase in static γ stimulation and mid curve (curve 1) in Figure 6 shows primary ending out- put just with applying static γ stimulation. As we can see from Figure 6, with applying an increase in γs stimula- tion, we observe that: 1) The ramp of primary ending response is not changed and as a result, the dynamic sensitivity is not change too. 2) dc level is increased and therefore the bias in increased just a little. 3) Static stimulation is increased just a little. .5. Effects of an Increase in4 lation on Secondary Ending Output re 7 shows the effect of an increase in stat stimulation on secondary ending output. Upper curve (curve 2) in Figure 7 shows the secondary ending output with applying an increase in static γ stimulation and mid curve (curve 1) in Figure 7 shows primary ending out- put just with applying static γ stimulation. As we can see from Figure 6, with applying an increase in γ stimula- tion, we observe that: 1) dc level is increased so bias is increased too. 2) static stimulation is increased. 5. DISCUSSION The greatest difficulty model is quantifying the data on spindle behavior that are presented in the literature. We shall not dwell on that problem here but instead consider the consequences of our chosen model in terms of response to stretch and possible mechanisms of γ control. Since the model is linear, both primary and secondary steady-state outputs are proportional to the degree of stretch. The primary output is also linearly proportional to stretch velocity if the duration of stretch is not too brief. This is as it should be and is, in fact, the one common feature of the models that have appeared pre- viously [10,11,19,20,21,22]. The most interesting feature of the model however, is that it allows us to isolate the various effects of γ stimula- tion. To begin with, it is clear that only effect dynamic or static force generators can have is to increase the bias of output. They provide an additive component to the output with no influence on either dynamic or static sensitivities. Figure 7. The effect of an increase in γs stimulation on secon- y ending output without any in- ore profound. h dary ending output. Secondar crease in γs stimulation (curve 1) and secondary ending output with an increase in γs stimulation (curve 2). The effects of parametric variation are m Te static sensitivity is a function solely of the elastic- ities and any variations in K1, K2, or K3 by γ-efferent activity would appear there. Furthermore, if the spindle is maintained under some degree of passive stretch, an increase in the stiffness of the muscular elasticities will also appear as an increase in the bias, an increase pro- portional to the change in K and to the initial degree of stretch. The dynamic sensitivities are more complicated func- tions of both the elasticities and viscosities but certain eneralizations can be made. In the bag fiber where T4< T1, T2, changes in B1 and B2 will appear as almost proportional changes in primary dynamic sensitivity. By contrast, the dynamic sensitivity of the chain fiber will be only slightly influenced by changes in B1, B2, or B3. Changes in viscosity will not affect the output while the spindle length is constant. It is instructive, too, to look at the actual degree of deformation of the sensory regions that is caused by stretching the spindle. In the unstimulated case, the peak extension which occurs in the sensory region of the chain fiber is 23 percent of the total spindle stretch. By contrast, the peak extension of the sensory region of the bag fiber is only 0.8 percent of the total stretch. The other 99.2 percent of the deformation occurs in the non- sensory elements. This fact is a direct consequence of the great stiffness of the bag's sensory area compared to the stiffness of the rest of the spindle. These observations on model behavior suggest that the following conclusions may apply to the physiologi- cal spindle. 1) The control exerted by the dynamic γ efferents on the dynamic behavior of the primary afferent may be through modification of the ratio of the viscosity of the nuclear bag to the viscosity of the bag's intrafusal musculature. This effect would be appeared independently of any force generation by the fiber nor would it be seriously affected by a simultaneous increase in muscle stiffness. 2) The control of the static γ efferents on the static Figure 6. The effect of an increase in γs stimulation on pri- mary ending output.  164 G. N. Golpayegani et al. / J. Biomedical Science and Engineering 2 (2009) 158-165 SciRes Copyright © 2009 e, in part, m eration but the existence of this co is o re al fibers are accept- ab are no 2) The motor ininervation of the cle spindle, in Nobel Symposium postural control systems, part 1: torque disturbance input, [8] ff, (2001) Tension development by isolated n L. Gottlieb, and L. Stark, (March 2004) tlieb, G. C. Agarwal, and L. Stark, (March 2003) vior of isolated mammalian echanical properties of mammalian static u, Y. Laporte, and B. Pages, (2001) Similitude ) Action 001) fects of k, (2001) A model of n, G. Lennerstrand, and U. Thoden, Cat fuseau alian behavior of the two types of afferents may b6, 116-121. K. Diete-Spi odification of the elasticities of either the bag or the chain but may include a large degree of output from the forcegenerators. 3) The control of bias by the γ efferents may be by in- trafusal force genntrol changes in crayfish stretch receptors, J. Physiol. (London), 159. [10] G. C. Agarwal, G. muscle spindles of the cat, J. Physiol. (London), 193. [9] K. Krnjevk and N. M. Van Gelder, (2002) Tensio open to question because changes in static sensitivity can appear as change in the bias of a stretched spindle. 4) The question of whether the bag fiber contracts in response to dynamic γ stimulation will be difficult t Models of muscle proprioceptive receptors, presented at the University of Michigan-NASA Conf. Manual Control. [11] G. L. Got solve by direct visual observation because the bag is very stiff and deforms only slightly. 5) In light of the above, either of the theories of y-efferent innervation of the intrafus Stretch receptor models, part I single-efferent single-afferent innervation, IEEE Trans. Man-Machine Systems, MMS-10, 17-27. [12] A. Boyd, (2001) The beha le. Even if both plate and trail endings are to be found on both bag and chain fibers, the distinguishing response of the bag fiber would be to only one type (the paramet- ric modifier, presumably the trail ending) while the chain could respond to either or both types of innervation. 6) We should also recognize the fact that the dynamics displayed by the spindle and mimicked by the model muscle spindles with intact innervation, J. Physiol. (London), 186. [13] (2001) The m intrafusal muscle fibers, J. Physiol. (London), 187. [14] P. Bessou and Y. Laporte, (2001) Observations on fusimotor fibres, in Nobel Synmposium-Muscular Afferents and Motor Control, R. Granit, Ed. New York: Wiley. [15] P. Besso t really sufficiently complex to require a fourth-order transfer function. The purpose of this elaborate model we have presented is to maintain an equivalence between the spindle anatomy and the model topology, but as a black box, simpler models presented previously [10,11], are quite adequate. REFERENCES des effets (statiques ou dynamiques) exerces par des fibres fusimotrices le chat, J. Physiol, Paris, 58. [16] B. Appelberg, P. Bessou, and Y. Laporte, (2001 of static and dynamic fusimotor fibres on secondary endings of cats’ spindles, J. Physiol. (London), 185. [17] E. Emonet-Denand, Y. Laporte, and B. Pages, (2 JBiSE [1] D. Barker, (200 mammalian mus stim Muscular Afterents and Motor Control, R. Granit, Ed. New York: Wiley. [2] A. Boyd, (1996) The motor innervation of mammalian muscle spindles, J. Physiol. (London), 159. [3] R. W. Banks, (2001) The tenuissiumus muscle of the cat, J. Physiol. (London), 133. [4] D. Barker (1999) The innervation of mammalian neuro-mulscular spindles, J. Physiol. (London), 140. [5] (2000) Simple and compound mammalian muscle spindles, J. Physiol. (London), 145. [6] D. Barker and I. A. Boyd, (2004) in Nobel Symposium Muscular Afferents and Motor Control, R. Granit, Ed. New York: Wiley. [7] G. C. Agarwal, B. M. Berman, and L. Stark, Studies in Fibres fusimotrices statiques et fibres fusimotrices dynamiques chez le lapin, Arch. Ital. Biol., 104. [18] Crowe and P. B. C. Matthews, (2003) The ef ulation of static and dynamic fusimotor fibres on the response to stretching of the primary endings of muscle spindles, J. Physiol. (London), 174. [19] J. C. Houk, R. Cornew, and L. Star adaptation in amphibian spindle receptors, J. Theoret. Biol., 12. [20] Anndersso muscle spindle model, Dig. 1999 Internatl. Conf. on Medical and Biological Engineering (Stockholm). [21] M. D. Angers, (2001) Model mecanique de neuromusculaire de-efferente: Terminaisons primaires et secondaires, Compt. Rend. Acad. Sci. (Paris), 261. [22] Crowe, (2003) A mechanical model of the mamm muscle spindle, J. Theoret. Biol., 21. 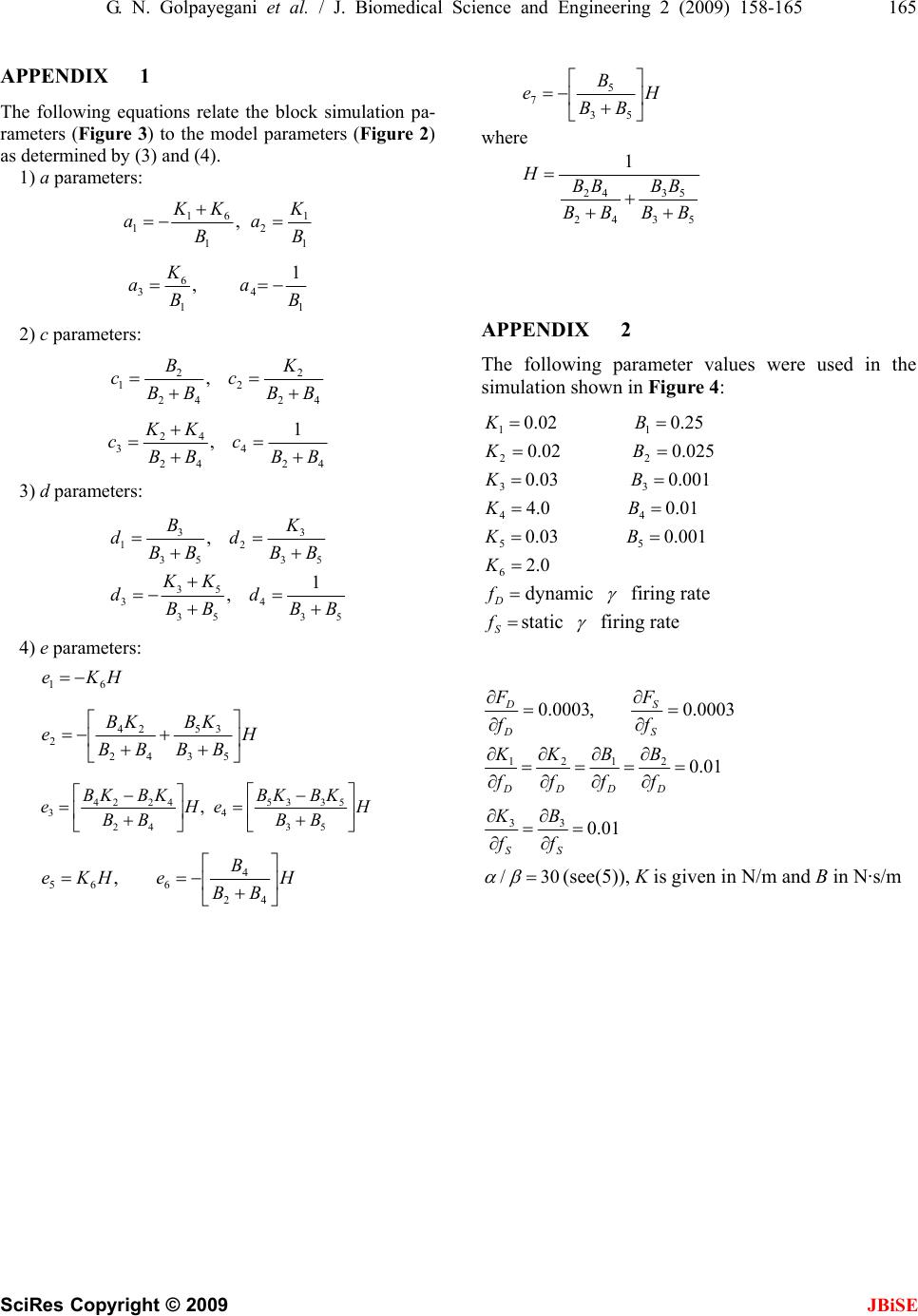 G. N. Golpayegani et al. / J. Biomedical Science and Engineering 2 (2009) 158-165 165 SciRes Copyright © 2009 JBiSE PPENDIX 1 ns relate the block simulation pa- A The following equatio rameters (Figure 3) to the model parameters (Figure 2) as determined by (3) and (4). 1) a parameters: 1 1 2 1 61 1,B K a B KK a 1 4 1 6 3 1 ,B a B K a 2) c parameters: 42 2 2 42 2 1,BB K c BB B c 42 4 42 42 3 1 ,BB c BB KK c 3) d parameters: 33 12 35 35 35 34 35 35 , 1 , BK dd BB BB KK dd BB BB 4) e parameters: HKe 61 H BB KB BB KB e 53 35 42 24 2 H BB KBKB eH BB KBKB e 53 5335 4 42 4224 3, H BB B eHKe 42 4 665 , H BB B e 53 5 7 where 53 53 42 42 1 BB BB BB BB H PPENDIX 2 meter values were used in the A The following para simulation shown in Figure 4: 25.002.0 11 BK 025.002.0 22 BK 001.003.0 33 BK 01.00.4 44 BK 001.003.0 55 BK 0.2 6 K dynamic firing rate D f S fstatic firing rate 0003.0,0003.0 S S D Df F f F 01.0 2121 DDDD f B f B f K f K 01.0 33 SS f B f K 30/ (see(5)), K is given in N/m and B in N·s/m |

