Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 114-124 doi:10.4236/jbnb.2011.22015 Published Online April 2011 (http://www.scirp.org/journal/jbnb) Copyright © 2011 SciRes. JBNB Surface Properties and Compatibility with Blood of New Quaternized Polysulfones Raluca Marinica Albu, Ecaterina Avram, Iuliana Stoica, Emil Ghiocel Ioanid, Dumitru Popovici, Silvia Ioan Institute of Macromolecular Chemistry, Iasi, Romania. E-mail: ioan_silvia@yahoo.com Received January 13th, 2011; revised January 16th, 2011; accepted January 21st, 2011. ABSTRACT The paper describes some properties of new quaternized polysulfones obtained by quaternization of chloromethylated polysulfone with different tertiary amines-N,N-dimethylethylamine and N,N-dimethyloctylamine. Hydrophilic/hydropho- bic properties, morphological aspects and interface properties with red blood cells and platelets are affected by the alkyl radicals and by history of the formed films from solutions in N,N-dimethylformamide/methanol and N,N-dimethyl- formamide/water solvent/nonsolvent mixtures. The results obtained are useful in biomedical applications, including evaluation of bacterial adhesion to the surfaces, or utilization of modified polysulfones as semipermeable membranes. Keywords: Quaternized Po lysulfones, Surface Properties, Blood Compatibility 1.Introduction In recent decades, considerable attention has been de- voted to the investigation of new applications of poly- sulfones, and also, of chloromethylated and quaternized polysulfones, which was mainly due to their specific properties. Literature showed that polysulfones and their derivatives were widely used as new functional materials in biochemical, industrial and medical fields, due to their structure and physical characteristics, such as good opti- cal properties, high thermal and chemical stability, me- chanical strength, resistance to extreme pH values and low creep [1-4]. Chain rigidity is derived from the rela- tively inflexible and immobile phenyl and SO2 groups, while toughness - from the connecting ether oxygen [4]. The polysulfone can be modified to improve its per- formance for specific applications [4,5], by chloro- methylation, a reaction of considerab le interest from both theoretical and practical points of view, such as obtaining of precursors for functional membranes, coatings, ion exchange resins, ion exchange fibers and selectively permeable membranes [6,7]. Also, quaternization with ammonium groups is an efficient method to obtain some properties recommended in various applications, e.g. as biomaterials and semipermeable membranes. These groups can modify hydrophilicity (of special interest for biomedical applications) [8], the antimicrobial action [9,10], solubility characteristics [11,12], allowing water permeability and separation [13,14]. In addition, the functional groups are an intrinsic requirement for affinity, ion exchange and other specialy membranes [15]. More- over, the bioapplication of polysulfones can be divided in two categories, namely blood contacting devices – for example, hemodialysis, hemodiafiltration and hemofil- tration as membrane, and cell or tissue contacting devices for example, bioreactor made by hollow fibre membrane, nerve generation through polysulfone semipermeable hollow membrane, et c [5]. In previous publications, the synthesis [16-18] and some solution properties [11,19-23] of modified polysul- fones have been presented. Studies have been carried out on the quaternization reaction of chloromethylated poly- sulfones, for obtaining water soluble polymers with various amounts of ionic chlorine. The conformational behavior in solution and the experimental and theoretical results on the preferential adsorption coefficients versus solvent composition have been discussed in correlation with the interaction parameters of quaternized polysul- fones/mixed solvents [10,20,23]. Surface wettability and hydrophilicity trends, as well as the morphological char- acteristics of some modified polysulfones were also ana- lyzed, for biomaterials and semipermeable membrane applications [10,12,22,24]. On the other hand, it is well- known that surface-induced blood coagulation is one of the main problems in the development of blood- contact- ing materials. From a clinical point of view, literature  Surface Properties and Compatibility with Blood of New Quaternized Polysulfones 115 shows that a biomaterial can be considered as hemo- compatible only when its interaction with blood does no t provoke da mage of blood cells or chang e in the structure of plasma proteins [25-27]. The surface free energy of biomaterials and the corresponding values of the work of spreading can be used as characterization parameters for predicting cell spreading onto their surfaces and hence, for establishing their blood compatibilit y. The objective of the present study was to investigate the morphological characteristics of quaternized poly- sulfones obtained from chloromethylated polysulfones with tertiary amines, N,N-dimethylethylamine and N, N-dimethyl-octylamine. The corresponding films, ob- tained from solutions in N,N-dimethylformamide (DMF) /methanol (MeOH) and DMF/water mixtures, were ana- lyzed by atomic force microscopy, to emphasize the in- fluence of casting solutions on the morphological proper- ties. The results were correlated with the hydrophilic/ hydrophobic properties and red blood cells and platelets compatibility. Influence of the alkyl radical sizes from the side groups was evidenced comparatively with the modified polysulfones with N-dimethyloc-tylammonium chloride pendant groups [27]. In this context, such inves- tigations were discussed in correlation with the comput- erized chemical structure, which provides a generalized view on the chemical conformations of the repeating units, realized by the HyperChem professional program (Demo version). This representation helps to identify aspects of molecular structure which may be relevant to the structure-property problem here under consideration, blood compatibility inclu ded. 2. Materials and Methods 2.1. Materials UDEL-3500 polysulfone (PSF) (Union Carbide, Mn = 39000; Mw/Mn = 1.625), a commercial p roduct, was puri- fied by repeated reprecipitations from chloroform and dried for 24 h in vacuum, at 40˚C, before being used in the synthesis of chloromethylated polysulfon e. A mixture of commercial paraformaldehyde with an equimolar amount of chlorotrimethylsilane (Me3SiCl) as a chloro- methylation agent, and stannic tetrachloride (SnCl4) as a catalyst, was used for the chloromethylation reaction of polysulfone, at 50˚C. The reaction time necessary to ob- tain chloromethylated polysulfones with 6.58% chlorine content (CMPSF) was 72 h [7]. Finally, the samples were dried under vacuum at 40˚C. Polysulfones with different alkyl side groups, PSF- DMEA and PSF-DMOA, were synthesized by reacting chloromethylated polysulfone with different tertiary amines-N,N-dimethylethylamine (DMEA) and N, N- dimethyloctylamine (DMOA), respectively. The quarter- nization reaction was performed in DMF, using a CMPSF/ tertiary amine molar ratio of 1:1.5, for 24 h. The quater- nary polymers were isolated from the reaction medium by precipitation in diethylether, washed three times with diethylether, and dried for 48 h under vacuum, at room temperature. The contents of ionic chlorine of 2.89 and 3.23, and total chlorine of 3.10 and 3.29 for PSF- DMEA and PSF-DMOA, respectively, were determined b y S cho n- inger’s method followed by potentiometric titration with AgNO3, using an automatic TitraLab Radiometer 840. The ratios between ionic chlorine and the total chlo- rine contents show that the quaternization reaction of CMPSF occurs at a transformation degree close to 98%. Thus, one may consider that almost all chloromethylenic groups were quaternized. Scheme 1 presents the general chemical structures and conformational structures - ob- tained by a computerized method using the HyperChem professional program (Demo version) considering four structural units, of PSF-DMEA and PSF-DMOA. 2.2. Contact Angle Contact angle analysis for surface tension investigations and atomic force microscopy (AFM) measurements were realized on quaternized polysulfones films. PSF-DMEA and PSF-DMOA were dissolved in DMF, DMF/MeOH (over the 75/25% - 25/75% v/v and 75/25% - 45/55% v/v composition range, respectively), and DMF/Water (over the 75/25% - 40/60% v/v and 75/25% - 50/50% v/v composition range, respectively), to reach the concentra- tions of approximatively 7 g/dL. The solutions were cast on a glass plate and initially solidified by slow drying in saturated atmosphere with the used solvent, and finally by drying at 50˚C under vacuum. Uniform drops of the 2L test liquid were deposited on the film surface and the contact angles were measured after 30 s, with a vid- eo-based optical contact angle measuring device, in a temperature of 25˚C. The used test liquids are water, methylene iodide (CH2I2), and 1-brom-naphtalin (1-Bn). 2.3. Atomic Force Microscopy Atomic force microscopy (AFM) measurements were performed in air, at room temperature (23˚C), in the tap- ping mode using a Scanning Probe Microscope (Solver PRO-M, NT-MDT, Russia) with commercially available NSGI0 cantilevers. The manufactu re’s value for the p rob e tip radius was l0 nm and for the typical force constant was 11.5 N/m. In the tapping mode, the cantilever was oscillated at a frequency of 286 kHz, over a 20 × 20 µm2 scan area for each sample. 3. Results and Discussion 3.1. Surface Tension Parameters The geometric mean method (GM ) (Equati ons (1) and (2)) C opyright © 2011 SciRes. JBNB  Surface Properties and Compatibility with Blood of New Quaternized Polysulfones Copyright © 2011 SciRes. JBNB 116 PSF-DMEA PSF-DMOA Scheme 1. Chemical structures and conformational structures with minimized energies, considering four repeating units of polysulfones with quaternary groups. [28,29] and the acid/base method (LW/AB) (equation (3) and (4) [30,31] were utilized for calculating the surface tension parameters of PSF-DMEA and PSF-DMOA, with surface properties of test liquids [32] from Table 1, and the contact angles measured between these solvents and quaternized polysulfone films from Table 2. The contact angles between these solvents and PSF-DMOA films are presented in previous paper [27] . 1cos 2 p p d lv lv s v d dlv lv sv (1) dp s vsvsv (2) where is the contact angle determined for test liquids, subscripts “lv” and “sv” denote the liquid-vapor and sur- face-vapor interfacial tension, respectively, while super- scripts “p” and “d” denote the polar and disperse com- ponents, respectively, of total surface tension, s v . 2 1cos LW LW s v lvsvlvsvlv lv (3) L WABLW AB s vsvsv (4) where 2 AB s vsvsv , superscript “LW/AB” indi- cates the total surface tension, and also , superscript “AB” and “LW” represent the polar component obtained from the electron-donor, s v , and the electron-acceptor, s v , interactions, and the disperse component, respectively. Table 3 shows the results for the surface tension com- ponents, evaluated with both methods. The surface ten- sion parameters are influenced by the solvent/nonsolvent composition from which the films had been prepared. Some studies have reported that the chain shape of a po- lymer in solution could affect the morphology of the po- lymer in bulk. In this context, the conformations of both PSF-DMEA and PSF-DMOA are affected by the charged groups from different alkyl radicals of the studied qua- ternized samples, and also by the compositition of the solvent mixtures. Thus, Figure 1 depicts the variation of intrinsic viscosity with the volume fraction of DMF, in DMF/MeOH and DMF/water mixtures, for PSF-DMEA Table 1. Surface tension parameters (mN/m) of the liquids used for contact angle measurements, red blood cells and platelets. Liquid lv d lv p lv lv lv Water [32] 72.80 21.80 51.00 25.5025.50 Methylene iodide (CH2I2) [32] 50.80 50.80 0 0.720 1-Brom-naphtalin (1-Bn) [32] 44.40 44.40 0 0 0 Red blood cell [35]36.56 35.20 1.36 0.0146.2 Platelet [35] 118.2499.14 19.10 12.267.44 Table 2. Contact angle (°) of different liquids on films pre- pared from solutions of PSF-DMEA in DMF/MeOH and DMF/water (% v/v) (column 1). Contact angle Solvent mixtures W MI 1-Bn 100/0 DMF/MeOH 71 28 17 75/25 DMF/MeOH 70 31 22 50/50 DMF/MeOH 61 30 20 25/75 DMF/MeOH 63 31 24 75/25 DMF/water 59 35 21 50/50 DMF/water 60 33 18 40/60 DMF/water 56 33 16 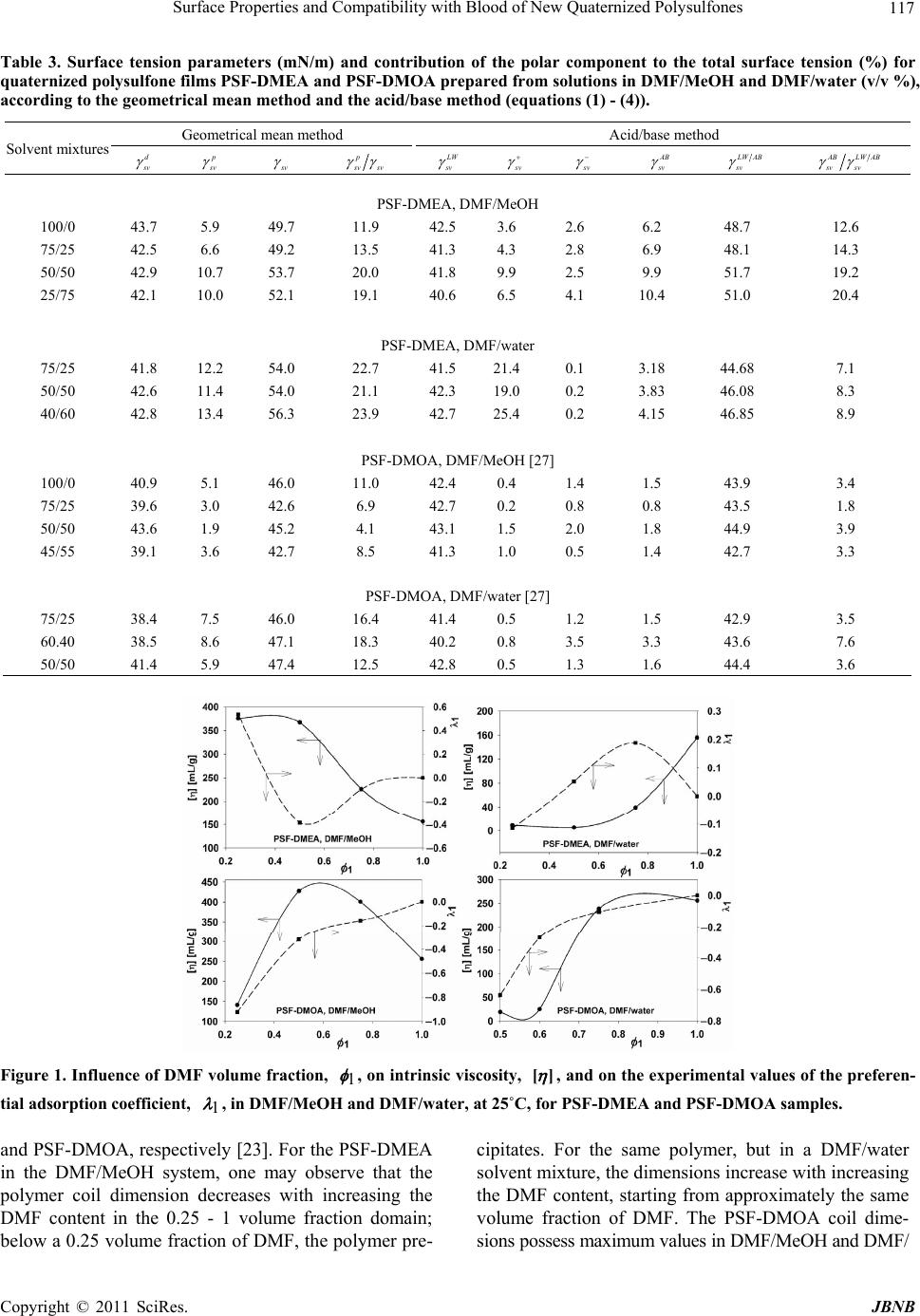 Surface Properties and Compatibility with Blood of New Quaternized Polysulfones 117 Table 3. Surface tension parameters (mN/m) and contribution of the polar component to the total surface tension (%) for quaternized polysulfone films PSF-DMEA and PSF-DMOA prepared from solutions in DMF/MeOH and DMF/water (v/v %), according to the geometrical mean method and the acid/base method (equations (1) - (4)). Geometrical mean method Acid/base method Solvent mixtures d s v p s v s v p s vsv L W s v s v s v AB s v L WAB sv ABLW AB sv sv PSF-DMEA, DMF/MeOH 100/0 43.7 5.9 49.7 11.9 42.5 3.6 2.6 6.2 48.7 12.6 75/25 42.5 6.6 49.2 13.5 41.3 4.3 2.8 6.9 48.1 14.3 50/50 42.9 10.7 53.7 20.0 41.8 9.9 2.5 9.9 51.7 19.2 25/75 42.1 10.0 52.1 19.1 40.6 6.5 4.1 10.4 51.0 20.4 PSF-DMEA, DMF/water 75/25 41.8 12.2 54.0 22.7 41.5 21.4 0.1 3.18 44.68 7.1 50/50 42.6 11.4 54.0 21.1 42.3 19.0 0.2 3.83 46.08 8.3 40/60 42.8 13.4 56.3 23.9 42.7 25.4 0.2 4.15 46.85 8.9 PSF-DMOA, DMF/MeOH [27] 100/0 40.9 5.1 46.0 11.0 42.4 0.4 1.4 1.5 43.9 3.4 75/25 39.6 3.0 42.6 6.9 42.7 0.2 0.8 0.8 43.5 1.8 50/50 43.6 1.9 45.2 4.1 43.1 1.5 2.0 1.8 44.9 3.9 45/55 39.1 3.6 42.7 8.5 41.3 1.0 0.5 1.4 42.7 3.3 PSF-DMOA, DMF/water [27] 75/25 38.4 7.5 46.0 16.4 41.4 0.5 1.2 1.5 42.9 3.5 60.40 38.5 8.6 47.1 18.3 40.2 0.8 3.5 3.3 43.6 7.6 50/50 41.4 5.9 47.4 12.5 42.8 0.5 1.3 1.6 44.4 3.6 Figure 1. Influence of DMF volume fraction, , on intrinsic viscosity, 1 ][ , and on the experimental values of the preferen- tial adsorption coefficient, , in DMF/MeOH and DMF/water, at 25˚C, for PSF-DMEA and PSF-DMOA samples. 1 and PSF-DMOA, respectively [23]. For the PSF-DMEA in the DMF/MeOH system, one may observe that the polymer coil dimension decreases with increasing the DMF content in the 0.25 - 1 volume fraction domain; below a 0.25 volume fraction of D MF, the polymer pre- cipitates. For the same polymer, but in a DMF/water solvent mixture, the dimension s increase with in creasing the DMF content, starting from approximately the same volume fraction of DMF. The PSF-DMOA coil dime- sions possess maximum values in DMF/MeOH and DMF/ C opyright © 2011 SciRes. JBNB  Surface Properties and Compatibility with Blood of New Quaternized Polysulfones 118 water, around 0.6 and 0.8 DMF volume fractions, resp e c- tively. For volume fractions of DMF below 0.25 in DMF/MeOH and 0.5 in DMF/water, the PSF-DMOA precipitates due to the nature of the alkyl radicals and content of nonsolvent in the system. Also, the values of intrinsic viscosity are h igh er for PSF-DMOA-with bulky carbon atoms in the alkyl side chain, compared with PSF-DMEA, where the alkyl side chain possesses two carbon atoms. Therefore, for a given composition of the DMF/MeOH and DMF/water solvent mixtures, one of the components is preferentially adsorbed by the quater- nized polysulfone molecules in the direction of a ther- modynamically most effective mixture. These aspects influence the surface properties of the polymer. Moreover, according to previous data [27], it was found out that PSF evidences the lowest hydro- philicity, induced by the aromatic rings connected by on e carbon and two methyl groups, oxygen elements, and sulfonic groups, while chlorometh ylation of PSF with the functional group -CH2Cl increases hydrophilicity (see the values of surface tension for PSF and CMPSF in Table 3 from reference [27]). On the other hand, PSF-DMEA films possess low polar surface tension parameters, but slightly higher than those for PSF-DMOA. The hydro- phobic character is given by the ethyl radical from the N-dimethylethylammonium chloride pendant group and by the octyl radical from the N-dimethyloctylammonium chloride pendant group, respectively, as visualized in the conformational structures from Scheme 1. Furthermore, the electron donor interactions, s v , are smaller than the electron acceptor ones, s v , for PSF-DMEA, and elec- tron donor in teractions, s v , exceed the electron acceptor interactions, s v , for PSF-DMOA, caused by the induc- tive phenomena from alkyl radical. The results reflect the capacity of the N-dimethylethylammonium chloride or N-dimethyloctylammonium chloride pendant groups to determine the acceptor or donor character of the polar terms, generated by these inductive phenomena. 3.2. Surface and Interfacial Free Energy The effect of alkyl radicals of quaternized polysulfones and of the history of the films formed from solutions on surface properties was analyzed by surface free energy, w - expressing the balance between surface hydro- phobicity and hydrophilicity (equation (5)) [32], by in- terfacial free energy between two particles of quaternized polysulfones in water phase, G GM s ws G (equations (6) and (7)), and by the work of spreading of water, s W (equa- tion (8)). 1cos wlv water G (5) where lv and water are given in Tables 1 and 2, re- spectively. 2 GM s ws sl G (6) 22 pp dd sllv svlv sv (7) 12 12 12 22 sac LW d s v lvsvlvsvlvlv WWW (8) According to literature [33,34] which specifies that 113 w G GmJ/ m2 for more hydrophobic materials, w evidences a high hydrophobicity for both samples, depending on the conditions of films preparation (Table 4). Moreover, the interfacial free energy, GM s ws G evalu- ated from solid-liquid interfacial tension, s l , (equation (7)) has negative values (Table 4). Therefore, an attrac- tion occurs between the two polymer surfaces, s, im- mersed in water, w, confirming the hydrophobic charac- teristics of both polymers, with higher hydrophobicity for PSF-DMOA films. Also, the hydrophobicity of these polymers was described by the work of spreading of wa- ter, s W, over the surface, which represents the differ- ence between the work of water adhesn, a W, and the work of water cohen, c W. According to the negative values of the interfacial free energy of PSF-DMEA and PSF-DMOA, the work of spreading of water, , io sio s w, (Figure 2) takes negative values, caused by the hydro- phobic surfaces, where the wo rk of water adhesion is low, comparatively with the work of cohesion; at the same time, it is observed that W ,, s wP WSF DMEA > ,, s w PSF WDMOA . 3.3. Blood-Quaternized Polysulfone Interactions Blood compatibility is dictated by the manner in which their surfaces interact with blood constituents, like red blood cells and platelets. To analyze the possibilities of using the polysulfone with N-dimethylethylammonium and N-dimethyloctylammonium chloride groups in bio- medical applications, and for establishing its compatibil- ity with blood, equation (8) was used, where , s rbc and , W s p describe the work of spreading of red blood cells and platelets [35]; when blood is exposed to a biomate- rial surface, adhesion of cells occurs and the extent of adhesion decides the life of the implanted biomaterials; thus, cellular adhesion to biomaterial surfaces could ac- tivate coagulation and the immunological cascades. Therefore, cellular adhesion has a direct bearing on the thrombogenicity and immunogenicity of a biomaterial, and thus dictates its blood compatibility. In this paper, we used the work of adhesion of the red blood cells as a parameter for characterizing biomaterials versus cell ad- hesion. The materials which exhibit a lower work of ad- hesion would lead to a lower extent of cell adhesion than hose with a higher w ork of adhesion. W t C opyright © 2011 SciRes. JBNB 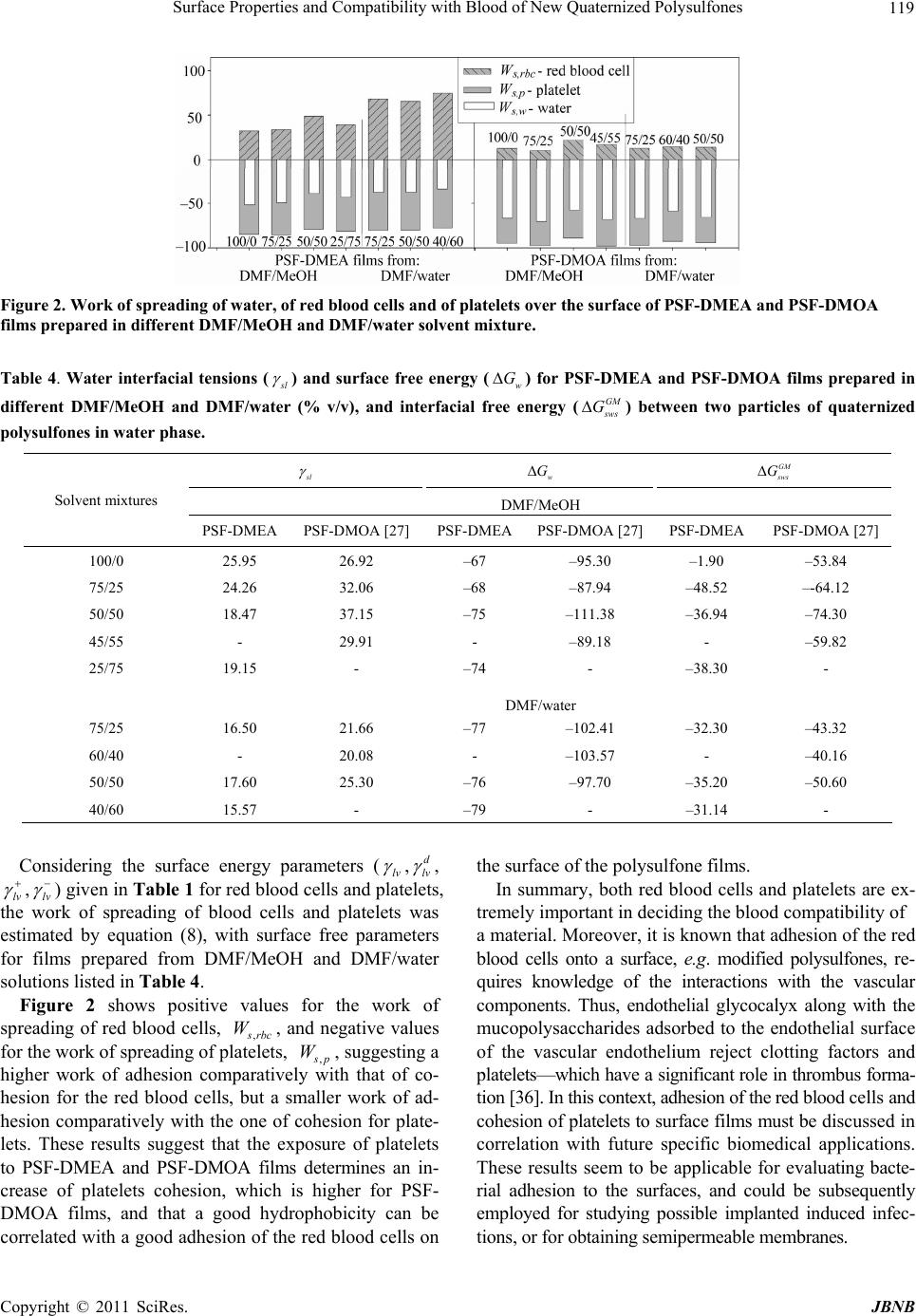 Surface Properties and Compatibility with Blood of New Quaternized Polysulfones Copyright © 2011 SciRes. JBNB 119 Figure 2. Work of spreading of water, of red blood cells and of platelets over the surface of PSF-DMEA and PSF-DMOA films prepared in different DMF/MeOH and DMF/water solvent mixture. Table 4. Water interfacial tensions ( s l ) and surface free energy (w G ) for PSF-DMEA and PSF-DMOA films prepared in different DMF/MeOH and DMF/water (% v/v), and interfacial free energy (GM s ws G) between two particles of quaternized polysulfones in water phase. s l w G GM s ws G DMF/MeOH Solvent mixtures PSF-DMEA PSF-DMOA [27] PSF-DMEAPSF-DMOA [27] PSF-DMEA PSF-DMOA [27] 100/0 25.95 26.92 –67 –95.30 –1.90 –53.84 75/25 24.26 32.06 –68 –87.94 –48.52 –-64.12 50/50 18.47 37.15 –75 –111.38 –36.94 –74.30 45/55 - 29.91 - –89.18 - –59.82 25/75 19.15 - –74 - –38.30 - DMF/water 75/25 16.50 21.66 –77 –102.41 –32.30 –43.32 60/40 - 20.08 - –103.57 - –40.16 50/50 17.60 25.30 –76 –97.70 –35.20 –50.60 40/60 15.57 - –79 - –31.14 - Considering the surface energy parameters (lv ,d lv , lv ,lv ) given in Table 1 for red blood cells and platelets, the work of spreading of blood cells and platelets was estimated by equation (8), with surface free parameters for films prepared from DMF/MeOH and DMF/water solutions listed in Table 4. Figure 2 shows positive values for the work of spreading of red blood cells, , s rbc , and negative values for the work of spreading of platelets, , W s p, suggesting a higher work of adhesion comparatively with that of co- hesion for the red blood cells, but a smaller work of ad- hesion comparatively with the one of cohesion for plate- lets. These results suggest that the exposure of platelets to PSF-DMEA and PSF-DMOA films determines an in- crease of platelets cohesion, which is higher for PSF- DMOA films, and that a good hydrophobicity can be correlated with a good adhesion of the red blood cells on the surface of the polysulfone films. W In summary, both red blood cells and platelets are ex- tremely important in deciding the blood compatibility of a material. Moreover, it is known that adhesion of the red blood cells onto a surface, e.g. modified polysulfones, re- quires knowledge of the interactions with the vascular components. Thus, endothelial glycocalyx along with the mucopolysaccharides adsorbed to the endothelial surface of the vascular endothelium reject clotting factors and platel ets—which have a sign ifican t rol e in th rombu s forma- tion [3 6] . I n t h i s c o nt e xt , adhes i on o f the re d bl o od cells and cohesion of platelets to surface films must be disc uss ed in correlation with future specific biomedical applications. These results seem to be applicable for evaluating bacte- rial adhesion to the surfaces, and could be subsequently employed for studying possible implanted induced infec- ions, or for obtaining sem iperm eable mem b r a n es . t 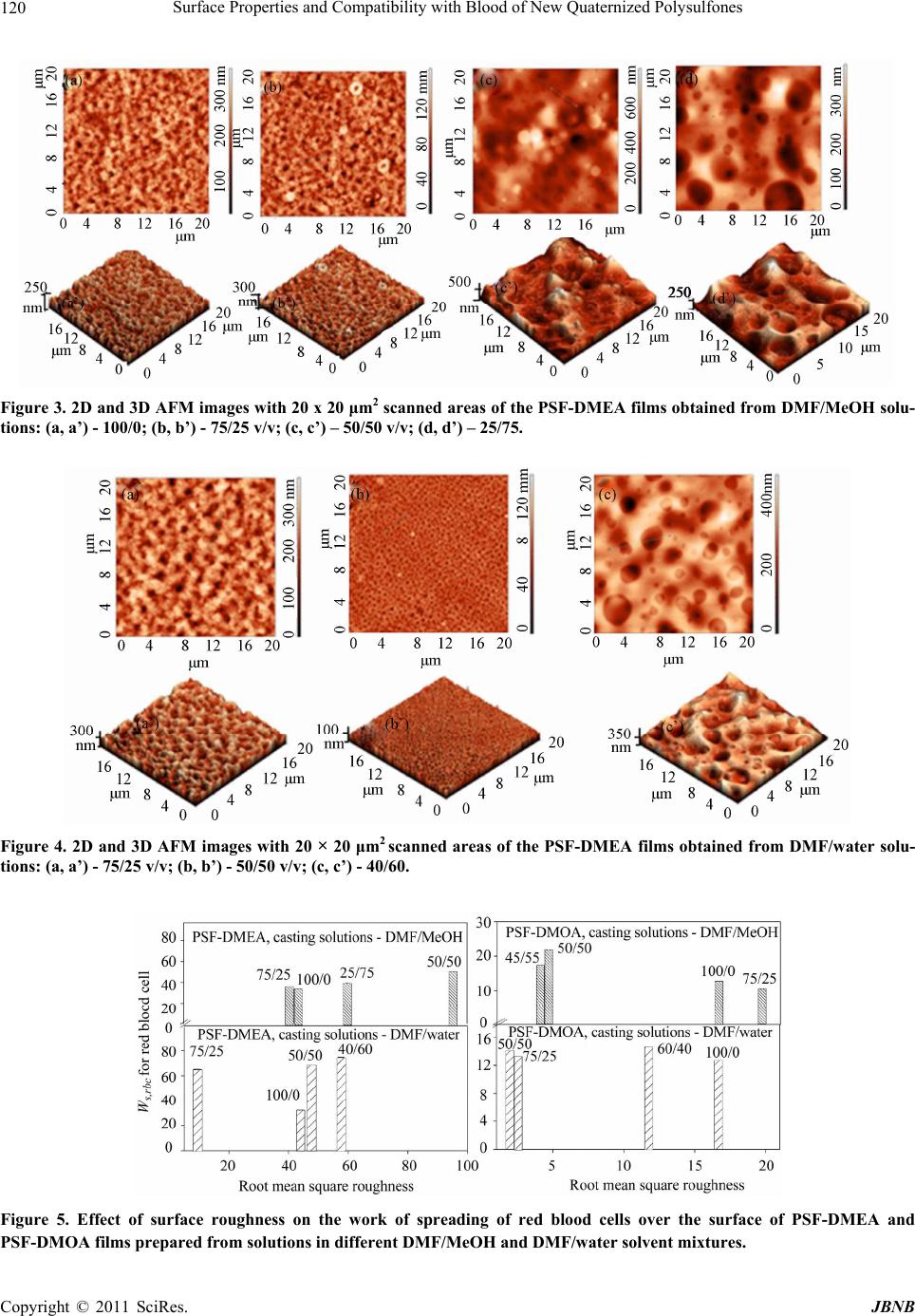 Surface Properties and Compatibility with Blood of New Quaternized Polysulfones 120 Figure 3. 2D and 3D AFM images with 20 x 20 μm2 scanned areas of the PSF-DMEA films obtained from DMF/MeOH solu- tions: (a, a’) - 100/0; (b, b’) - 75/25 v/v; (c, c’) – 50/50 v/v; (d, d’) – 25/75. Figure 4. 2D and 3D AFM images with 20 × 20 μm2 scanned areas of the PSF-DMEA films obtained from DMF/water solu- tions: (a, a’) - 75/25 v/v; (b, b’) - 50/50 v/v; (c, c’) - 40/60. Figure 5. Effect of surface roughness on the work of spreading of red blood cells over the surface of PSF-DMEA and SF-DMOA films prepared from solutions in different DMF/MeOH and DMF/water solvent mixtures. P C opyright © 2011 SciRes. JBNB 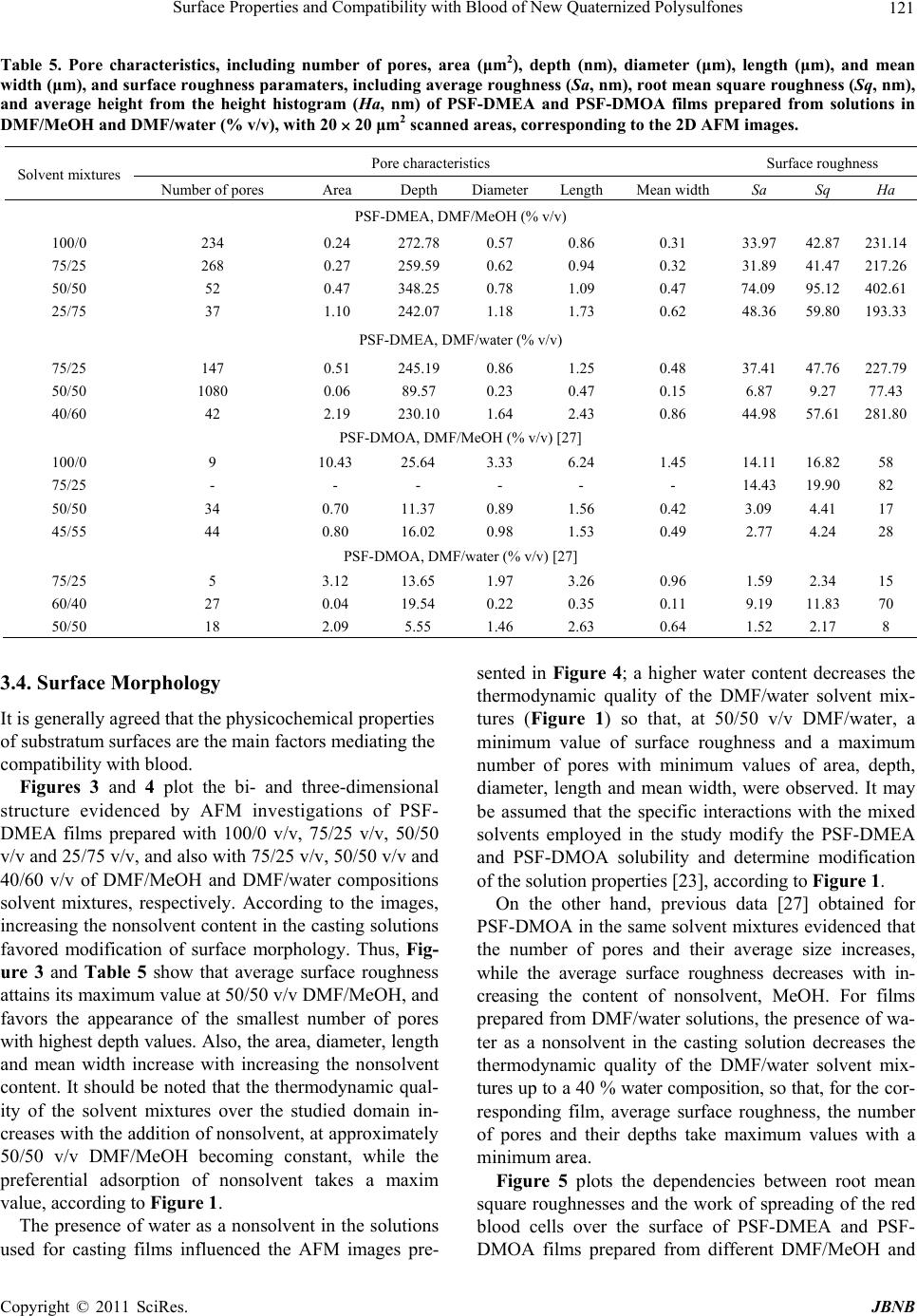 Surface Properties and Compatibility with Blood of New Quaternized Polysulfones 121 Table 5. Pore characteristics, including number of pores, area (μm2), depth (nm), diameter (μm), length (μm), and mean width (μm), and surface roughness paramaters, including average roughness (Sa, nm), root mean square roughness (Sq, nm), and average height from the height histogram (Ha, nm) of PSF-DMEA and PSF-DMOA films prepared from solutions in DMF/MeOH and DMF/water (% v/v), with 20 20 μm2 scanned areas, corresponding to the 2D AFM images. Pore characteristics Surface roughness Solvent mixtures Number of pores Area Depth Diameter LengthMean width Sa Sq Ha PSF-DMEA, DMF/MeOH (% v/v) 100/0 234 0.24 272.78 0.57 0.86 0.31 33.97 42.87231.14 75/25 268 0.27 259.59 0.62 0.94 0.32 31.89 41.47217.26 50/50 52 0.47 348.25 0.78 1.09 0.47 74.09 95.12402.61 25/75 37 1.10 242.07 1.18 1.73 0.62 48.36 59.80193.33 PSF-DMEA, DMF/water (% v/v) 75/25 147 0.51 245.19 0.86 1.25 0.48 37.41 47.76227.79 50/50 1080 0.06 89.57 0.23 0.47 0.15 6.87 9.27 77.43 40/60 42 2.19 230.10 1.64 2.43 0.86 44.98 57.61281.80 PSF-DMOA, DMF/MeOH (% v/v) [27] 100/0 9 10.43 25.64 3.33 6.24 1.45 14.11 16.8258 75/25 - - - - - - 14.43 19.9082 50/50 34 0.70 11.37 0.89 1.56 0.42 3.09 4.41 17 45/55 44 0.80 16.02 0.98 1.53 0.49 2.77 4.24 28 PSF-DMOA, DMF/water (% v/v) [27] 75/25 5 3.12 13.65 1.97 3.26 0.96 1.59 2.34 15 60/40 27 0.04 19.54 0.22 0.35 0.11 9.19 11.8370 50/50 18 2.09 5.55 1.46 2.63 0.64 1.52 2.17 8 3.4. Surface Morphology It is generally agreed that the physicochem ical properties of substratum surfaces are the main factors mediating the compatibility with blood. Figures 3 and 4 plot the bi- and three-dimensional structure evidenced by AFM investigations of PSF- DMEA films prepared with 100/0 v/v, 75/25 v/v, 50/50 v/v and 25/75 v/v, an d also with 75/25 v/v, 50 /50 v/v and 40/60 v/v of DMF/MeOH and DMF/water compositions solvent mixtures, respectively. According to the images, increasing the nonsolv ent content in the casting solutions favored modification of surface morphology. Thus, Fig- ure 3 and Table 5 show that average surface roughness attains its maximum value at 50/50 v/v DMF/MeOH, and favors the appearance of the smallest number of pores with highest depth values. Also, the area, diameter, leng th and mean width increase with increasing the nonsolvent content. It shou ld be noted that the thermodynamic qua l- ity of the solvent mixtures over the studied domain in- creases with the addition of nonsolvent, at approximately 50/50 v/v DMF/MeOH becoming constant, while the preferential adsorption of nonsolvent takes a maxim value, according to Figure 1. The presence of water as a nonsolvent in the solutions used for casting films influenced the AFM images pre- sented in Figure 4; a higher water content decreases the thermodynamic quality of the DMF/water solvent mix- tures (Figure 1) so that, at 50/50 v/v DMF/water, a minimum value of surface roughness and a maximum number of pores with minimum values of area, depth, diameter, length and mean width, were observed. It may be assumed that the specific interactions with the mixed solvents employed in the study modify the PSF-DMEA and PSF-DMOA solubility and determine modification of the solution properties [23], according to Figure 1. On the other hand, previous data [27] obtained for PSF-DMOA in the same solvent mixtures evidenced that the number of pores and their average size increases, while the average surface roughness decreases with in- creasing the content of nonsolvent, MeOH. For films prepa red from DMF/water solu tions, the presence of w a- ter as a nonsolvent in the casting solution decreases the thermodynamic quality of the DMF/water solvent mix- tures up to a 40 % water co mposition, so that, for the cor - responding film, average surface roughness, the number of pores and their depths take maximum values with a minimum area. Figure 5 plots the dependencies between root mean square roughnesses and the work of spreading of the red blood cells over the surface of PSF-DMEA and PSF- DMOA films prepared from different DMF/MeOH and C opyright © 2011 SciRes. JBNB  Surface Properties and Compatibility with Blood of New Quaternized Polysulfones 122 DMF/water solvent mixtures. These results show that surface morphology depends on the history of the formed films, including the characteristics of quaternized poly- sulfones and the thermodynamic quality of solvents. Moreover, the results suggest that surface free energy (surface hydrophobicity) and surface roughness are the key parameters controlling the compatibility with the red blood cells, known as a complicated process that depends on many factors, including surface chemistry, hydropho- bicity, and surface roughness. The contribution of each of these factors i s difficult to establish , however, it is c lear ly seen that PSF-DMOA is characterized by a lower com- patibility with the red blood cells than PSF-DMEA. On the other hand, the compatibility values are higher for PSF- DMEA and lower for PSF-DMOA f ilms prepared in DMF/ water, compared with films prepared in DMF/ MeOH. 4. Conclusions New quaternized polysulfones, prepared by quaternization of chloromethylated polysulfone with N,N-dimethy- lethylamine and N,N-dimethyloctylamine were investi- gated to obtain information on their hydrophilic/hydrop- hobic properties and blood compatibility. The history of the formed films, prepared by a dry-cast process in DMF/ MeOH and DMF/water solvent/nonsolvent mixtures, influenced the surface tension parameters, surface and interfacial free energy and the work of spreading of water, maintaining the surfaces hydrophobic characteristics of both polysulfones. On the other hand, the results reflect the capacity of N-dimethylethylammonium or N-dime- thyloctylammonium chloride pendant groups to deter- mine the acceptor or donor character of the polar terms, caused by the inductive phenomena of alkyl radicals. The AFM images showed that surface morphology is characterized by roughness and nodules formations, de- pending on the composition of solvent/nonsolvent mix- tures, including the characteristics of quaternized poly- sulfones and the thermodynamic quality of the solvents. Moreover, the results suggest that: surface hydrophobicity and surface roughness are the parameters controlling the compatibility with the red blood cells and platelets: a good hydropho- bicity can be correlated with a good adhesion of the red blood cells and with a good cohesions of the platelets on the surface of the quaternized polysul- fone films ; high work of adhesion comparatively with work of cohesion for the red blood cells, but a smaller work of adhesion comparatively with the one of cohesion for platelets was obtained; the exposure of blood to both quaternized polysul- fones involves higher platelets cohesion for PSF- DMOA films, comparatively with PSF-DMEA films. These results are useful in investigations on specific biomedical applications, including evaluation of bac- terial adhesion to the surfaces, and utilization of modified polysulfones as sem ipermeable membranes. REFERENCES [1] M. Barikani and S. Mehdipour-Ataei, “Synthesis, Char- acterization and Thermal Properties of Novel Arylene Sulfone Ether Polyimides and Polyamides,” Journal of Polymers Science, Part A: Polymer Chemistry, Vol. 38, No. 9, May 2000, pp. 1487-1492. doi:10.1002/(SICI)1099-0518(20000501)38:9<1487:AID -POLA11>3.0.CO;2-U [2] B. K. Mann and J. L West, “Tissue Engineering in the Cardiovascular System: Progress toward a Tissue Engi- neered Heart,” The Anatomical Record, Vol. 263, No. 4, August 2001, pp. 367-371. doi: 10.1002/ar.1116 [3] A. Higuchi, N. Iwata, M. Tsubaki and T. Nakagawa, “Surface-Modified Polysulfone Hollow Fibers,” Journal of Applied Polymer Science, Vol. 36, No. 8, October 1988, pp. 1753-1767. doi: 10.1002/app.1988.070360804 [4] R. N. Johnson, “Polysulfones. Plastics, Resins, Rubbers, Fibers,” Encyclopedia of Polymer Science and Tech- nology, Vol. 11, F. M. Herman, G. G. Norman and M. N. Bikales Eds., John Wiley & Sons, New York, London, Sydney and Toronto, 1969, pp. 447-463. [5] G. Khang, H. B. Lee and J. B. Park, “Biocompatibility of Polysulfone. I. Surface Modifications and Characteriza- tions,” Bio-Medical Materials and Engineering, Vol. 5, No. 4, January 1995, pp. 245-258. [6] A. Higuchi, K. Shirano, M. Harashima, B. O. Yoon, M. Hara, M. Hattori and K. Imamura, “Chemically Modified Polysulfone Hollow Fibers with Vinylpyrrolidone Having Improved Blood Compatibility,” Biomaterials, Vol. 23, No.13, July 2002, pp. 2659-2666. doi: 10.1016/S0142-9612(01)00406-9 [7] M. Tomaszewska, A. Jarosiewicz and K. Karakulski, “Physical and Chemical Characteristics of Polymer Coat- ings in CRF Formulation,” Desalination, Vol. 146, No. 1, September 2002, pp. 319-323. doi: 10.1016/S0011-9164(02)00501-5 [8] R. Guan, H. Zou, D. Lu, C. Gong and Y. Liu, “Poly- etheresulfone Sulfonated by Chlorosulfonic Acid and its Membrane Characteristics,” European Polymer Journal, Vol. 41, No. 7, July 2005, pp. 1554-1560. doi: 10.1016/j.europolyml.2005.01.018 [9] H. Yu, Y. Huang, H. Ying and C. Xiao, “Preparation and Characterization of a Quaternary Ammonium Derivative of Konjac Glucomannan,” Carbohydrate Polymers, Vol. 69, No. 1, May 2007, pp. 29-40. doi: 10.1016/j.carbpol.2006.08.024 [10] A. Filimon, E. Avram, S. Dunca, I. Stoica and S. Ioan, “Surface Properties and Antibacterial Activity of Quater- nized Polysulfones,” Journal of Applied Polymer Science, Vol. 112, No. 3, May 2009, pp. 1808-1816. doi: 10.1002/app.29591 C opyright © 2011 SciRes. JBNB  Surface Properties and Compatibility with Blood of New Quaternized Polysulfones 123 [11] A. Filimon, E. Avram and S. Ioan, “Influence of Mixted Solvents and Temperature on the Solution Properties of Quaternized Polysulfones,” Journal of Macromolecular Science, Part B: Physics, Vol. 46, No. 3, May 2007, pp. 503-520. doi: 10.1080/00222340701257752 [12] S. Ioan, A. Filimon and E. Avram, “Influence of the De- gree of Substitution on the Solution Properties of Chloro- methylated Polysulfone,” Journal of Applied Poly- mer Science, Vol. 101, No. 1, April 2006, pp. 524-531. doi: 10.1002/app.23340 [13] A. Idrisa, N. M. Zaina and M. Y. Noordinb, “Synthesis, Characterization and Performance of Asymmetric Poly- ethersulfone (PES) Ultrafiltration Membranes with Poly- ethylene Glycol of Different Molecular Weights as Addi- tives,” Desalination, Vol. 207, No. 1-3, March 2007, pp. 324-339. doi: 10.1016/j.desal.2006.08.008 [14] V. Kochkodan, S. Tsarenko, N. Potapchenko, V. Kosi- nova and V. Goncharuk, “Adhesion of Microorganisms to Polymer Membranes: A Photobactericidal Effect of Sur- face Treatment with TiO2,” Desalination, Vol. 220, No. 1-3, March 2008, pp. 380-385. doi: 10.1016/j.desal.2007.01.042 [15] M. D. Guiver, P. Black, C. M. Tam and Y. Deslandes, “Functionalized Polysulfone Membranes by Heterogene- ous Lithiation,” Journal of Applied Polymer Science, Vol. 48, No. 9, June 1993, pp.1597-1606. doi: 10.1002/app.1993.070480912 [16] E. Avram, E. Butuc, C. Luca and I. Druta, “Polymers with Pendent Functional Groups. III. Polysulfone Con- taining Viologen Group,” Journal of Macromolecular Science, Part A: Pure Applied Chemistry, Vol. 34, No. 9, September 1997, pp. 1701-1714. doi: 10.1080/10601329708010036 [17] E. Avram, “Polymers with Pendent Functional Groups. VI. A Comparative Study on the Chloromethylation of Linear Polystyrene and Polysulfone with Paraphormal- dehy de/M e3SiCl,” Polymer-Plastics Technology and En- gineering, Vol. 40, No. 3, May 2001, pp. 275-281. doi: 10.1081/PPT-100000248 [18] C. Luca, E. Avram and I. Petrariu, “Quaternary Ammo- nium Polyelectrolytes. V. Amination Studies of Chloro- methylated Polystyrene with N,N-Dimethylalkylamines,” Journal of Macromolecular Science, Part A: Pure Ap- plied Chemistry, Vol. 25, No. 4, 1988, pp. 345-361. doi: 10.1080/00222338808053373 [19] L. Ghimici and E. Avram, “Viscosimetric Behavior of Quaternized Polysulfones,” Journal of Applied Polymer Science, Vol. 90, No. 2, October 2003, pp. 465-469. doi: 10.1002/app.12677 [20] S. Ioan, A. Filimon and E. Avram, “Conformation and Viscometric Behavior of Quaternized Polysulfone in Di- lute Solution,” Polymer Engineering and Science, Vol . 46, No. 7, July 2006, pp. 827-836. doi: 10.1002/pen.20526 [21] S. Ioan, A. Filimon and E. Avram, “Influence of Substitu- tion Degree to the Optical Properties of Chloromethylated Polysulfone,” Journal of Macromolecular Science, Part B: Physics, Vol. 44, No.1, February 2005, pp. 129-135. doi: 10.1081/MB-200044623 [22] S. Ioan, A. Filimon, E. Avram and G. Ioanid, “Effect of Chemical Structure and Plasma Treatment on the Surface Properties of Polysulfones,” e-Polymers, No. 031, March 2007, pp. 1-13 (ISSN 1618-7229). [23] A. Filimon, R. M. Albu, E. Avram and S. Ioan, “Effect of Alkyl Side Chain on the Conformational Properties of Polysulfones with Quaternary Groups,” Journal of Mac- romolecular Science, Part B: Physics, Vol. 49, No. 1, January 2010, pp. 207-217. doi: 10.1080/00222340903346494 [24] X. J. Huang, Z. K. Xu, L. S. Wan, Z. G. Wang and J. L. Wang, “Novel Acrylonitrile-Based Copolymers Con- taining Phospholipid Moities: Synthesis and Characteri- zation,” Macromolecular Bioscience, Vol. 5, No. 4, April 2005, pp. 322-330. doi: 10.1002/mabi.200400165 [25] M. H. Stenzel, C. Barner-Kowollik, T. P. Davis and H. M. Dalton, “Amphiphilic Block Copolymers Based on Poly (2-Acryloyloxyethyl Phosphorylcholine) Prepared via RAFT Polymerisa tion as Bioc ompatible Nanocontainers,” Macromolecular Bioscience, Vol. 4, No. 4, April 2004, pp. 445-453. doi: 10.1002/mabi.200300113 [26] L. Lewis, J. Berwick, M. C. Davies, C. J. Roberts, J. H. Wang, S. Small, A. Dunn, V. O’Byrne, R. P. Redman and S. A. Jones, “Synthesis and Characterisation of Cationi- cally Modified Phospholipid Polymers,” Biomaterials, Vol. 25, No. 15, July 2004, pp. 3099-3108. doi: 10.1016/j.biomaterials.2003.09.082 [27] S. Ioan, R. M. Albu, E. Avram, I. Stoica and E. G. Ioanid, “Surface Characterization of Quaternized Polysulfone Films and Biocompatiblity Studies,” Journal of Applied Polymer Science, Vol. 121, No. 1, July 2011, pp. 127- 137. doi: 10.1002/app.33380 [28] K. Owens and R. C. Wendt, “Estimation of the Surface Free Energy of Polymers,” Journal of Applied Polymer Science, Vol. 13, No. 8, August 1969, pp. 1741-1747. doi: 10.1002/app.1969.070130815 [29] H. Kälble, “Peel Adhesion: Influence of Surface Energies and Adhesive Rheology,” Journal Adheshion, Vol. 1, No. 2, April 1969, pp. 102-123. [30] C. J. van Oss, R. J. Good and M. K. Chaudhury, “Addi- tive and Nonadditive Surface Tension Components and the Interpretation of Contact Angles,” Langmuir, Vol. 4, No. 4, July 1988, pp. 884-891. doi: 10.1021/la00082a018 [31] C. J. van Oss, L. Ju, M. K. Chaudhury and R. J. Good, “Interfacial Lifshitz-van der Waals and Polar Interactions in Macroscopic Systems,” Chemical Reviews, Vol. 88, No. 6, September 1988, pp. 927-941. doi: 10.1021/cr00088a006 [32] M. Rankl, S. Laib and S. Seeger, “Surface Tension Proper- ties of Surface-Coatings for Application in Biodiagnostics Determined by Contact Angle Measurements,” Colloids and Surface B: Biointerfaces, Vol. 30, No. 3, July 2003, pp. 177-186. doi: 10.1016/S0927-7765(03)00085-7 [33] R. S. Faibish, W. Yoshida and Y. Cohen, “Contact Angle Study on Polymer-Grafted Silicon Wafers,” Journal of Colloid Interface Science, Vol. 256, No. 2, December C opyright © 2011 SciRes. JBNB  Surface Properties and Compatibility with Blood of New Quaternized Polysulfones Copyright © 2011 SciRes. JBNB 124 2002, pp. 341-350. doi: 10.1006/jcis.2002.8612 [34] C. J. van Oss, “Interfacial Forces in Aqueous Media,” Marcel Dekker, New York, 1994. [35] K. Vijayanand, K. Deepak, D. K. Pattanayak, T. R. Rama Mohan and R. Banerjee, “Interpenetring Blood-Bioma- terial Interactions from Surface Free Energy and Work of Adhesion,” Trends in Biomaterials and Artificial Organs, Vol. 18, No. 2, January 2005, pp. 73-83. [36] S. Reitsma, D. W. Slaaf, H. Vink, M. A. M. J. van Zand- voort and M. G. A. oude Egbrink, “The Endothelial Gly- cocalyx: Composition, Functions, and Visualization,” Pflügers Archiv—European Journal of Physiology, Vol. 454, No. 3, June 2007, pp. 345-359. doi: 10.1007/s00424-007-0212-8 |

