Paper Menu >>
Journal Menu >>
 Advances in Bioscience and Biotechnology, 2011, 2, 75-84 ABB doi:10.4236/abb.2011.22012 Published Online April 2011 (http://www.SciRP.org/journal/abb/). Published Online April 2011 in SciRes. http://www.scirp.org/journal/ABB Increased serotonin concentration and tryptophan hydroxylase activity in reproductive organs of copulator males: a case of adaptive plasticity Ana Ingrid Pichardo1, José L.Tlachi-L ó pe z 2, Francisco Jiménez-Trejo3, Alma L. Fuentes-Farías4, Armida Báez-Saldaña1, María L. Molina-Cerón1, Gabriel Manjarréz-Gutiérrez5, Gabriel Gutiérrez-Ospina1*, Rosa Angélica Lucio2* 1Departamento de Biología Celular y Fisiología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, Ciudad Universitaria 04510, México, D.F., México; 2Centro Tlaxcala de Biología de la Conducta, Universidad Autónoma de Tlaxcala, Carretera Tlaxcala-Puebla km 1.5 s/n, Loma Xicotencatl, 90062, Tlaxcala, México; 3Departamento de Farmacología, Facultad de Medicina, Universidad Nacional Autónoma de México, Ciudad Universitaria 04510, México, D.F., México; 4Laboratorio de Invertebrados y Ecofisiología, Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo, Ciudad Universitaria 58030, Michoacán, México; 5Unidad de Investigación Biomolecular, Hospital de Cardiología, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social 06720, México, D.F., México. Email: lucioral@yahoo.com.mx; gabo@correo.biomedicas.unam.mx Received 27 December 2010; revised 19 February 2011; accepted 27 February 2011. ABSTRACT Individual male rats may systematically display or not copulatory behavior when paired with receptive females. Although these phenotypes are associated with differences in brain organization and function, they might also do so at the level of the reproductive organs. We then used high performance liquid chro- matography to quantify serotonin concentration and the activity of tryptophan hydroxylase in the repro- ductive organs of copulator and non-copulator males. Sexual behavior display was compared between groups and parameters of fertility and reproductive fitness were determined for copulator males. Copu- lator males had higher concentrations of serotonin in the epididymis, testicle and ventral prostate than their non-copulator counterparts, as it was found for epididymal and testicular tryptophan hydroxylase activity. However, preliminary data shows that sero- tonin elevation occurs in copulator males only until they have accumulated several sexual encounters, so it might be a response to genital gratification or sex- ual rewarding. Interestingly, only epididymal sero- tonin concentration correlated with reproductive fit- ness, offspring number, mating success and seminal plug volume in copulator males. Our results support that copulator and non-copulator male rats feature a phenotype-specific serotoninergic tone in the epidi- dymis, testicle and ventral prostate gland. The ob- servation documenting that epididymal serotonin concentration correlated with parameters that moni- tor male fertility and reproductive fitness in copula- tor males predicts that epididymal factors increase their chances of parenting offspring. Keywords: Sexual Accessory Glands; Copulation; Indolamines; Seminal Fluid; Seminal Plug; Successful Mating; Reproductive Fitness 1. INTRODUCTION Copulatory or consummatory behavior in male rats is displayed as a stereotyped motor pattern that comprises mounts, intromissions and ejaculation [1]. Although this pattern suits well the majority of male rats, a small per- centage of them exhibit either none or incomplete copu- latory behavior when repeatedly paired with sexually receptive females [2]. Even though the origin of the rat’s copulator and non-copulator phenotypes is unclear, evi- dence showing differences in motivational and precopu- latory behaviors, detection of sexually significant odors, number of hypothalamic and amygdaline neurons con- taining androgen or estrogen receptors and levels of hy- pothalamic aromatase activity between both rat groups [3-6] suggests that these are innate phenotypes. The display of successful sexual behavioral responses ,These authors contributed equally to the present work.  A. I. Pichardo et al. / Advances in Bioscience and Biotechnology 2 (2011) 75-84 Copyright © 2011 SciRes. ABB 76 depends upon the ability of each male to accurately as- sess his internal physiological state and precisely match it with his sexual/reproductive expectations/output under a given context [1]. It is then likely that copulator and non-copulator rat males also feature distinct traits in their genital organs. Accordingly, the present work evaluated possible differences of the serotoninergic tone in reproductive organs of males displaying or not normal copulatory behavior. Studying the serotoninergic tone seems an adequate election because this parameter shifts across genital/reproductive organs as sexual maturation progresses, with reproductive status or photoperiod length and along the breeding season in various different species [7-9]. Also genital serotonin modulates, among other important reproductive processes, ejaculation, se- miniferous tubule fluid transit, production and clea- rance, sexual accessory glands contraction, epididymal fluid composition, testicular steroidogenesis and sper- matogenesis [10-15]. 2. MATERIALS AND METHODS 2.1. Animals Sexually inexperienced adult male Wistar rats (Rattus norvegicus; 90 days of age/300 grams of body weight; n 22) were chosen randomly from the regular stock of the colony raised at the animal facility of the Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México. Males were screened for their copulatory abilities and grouped as copulators or non- copulators (see below). Male rats were then caged in clusters of three or four individuals of the same class. The animals were kept in rooms with constant tempera- ture (22°C ± 1°C) and humidity under an inverted light cycle (12:12 hours, lights off at 7:00 hrs). Food (2018S Pellets, Harlan) and water were available ad libitum. Animal handling and experimentation followed the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health. Local Animal Right’s Committees approved all of the animal protocols described in this work. 2.2. Male Selection Male rats were classified as copulators (n 10) or non-copulators (n 13) after analyzing their sexual per- formance along four consecutive copulatory encounters with receptive, ovariectomized female rats [16]. Copu- lator males displayed fully the copulatory pattern in every encounter within a 15 minute period of having being initiated the testing session. In contrast, non-copu- lator males executed incomplete copulatory behavior in every encounter within a 45 minute period of having being started the testing trial. Mounting, intromission and ejaculation latencies and the number of mounts and intromissions associated with pelvic thrusting were re- corded for each male during each encounter. The hit rate, an index taken to reflect erectile potential, was also es- timated [17]. 2.3. Semen and Seminal Plug Evaluation in Copulator Males Semen and seminal plug quality was evaluated only in copulator males (n 7) because non-copulators never reached the ejaculatory threshold. These analyses were carried out in ejaculates withdrawn from the uterine horns and in seminal plugs released from the vagina of the inseminated females used during the third and fourth copulatory encounters, as described elsewhere [16]. The parameters obtained for copulator males were compara- ble to those reported previously for sexually experienced rat males of the same strain (see Tables 1 and 2 and ref- erence [16]). 2.4. Fertility and Reproductive Fitness in Copulator Males To evaluate individual reproductive fitness in copulator males (n 7), each male was paired in a regular enclo- sure with a naturally receptive, intact female during 48 hours, fifteen days after the last copulatory encounter. Reproductive encounters were repeated four times for each copulator male every 15 days. By the end of each reproductive encounter, the couple was separated and the dam caged individually and visually inspected every day first to corroborate pregnancy and then to assure normal pregnancy progression. After delivery, the offspring re- mained with the mother until weaning by postnatal day 28 when living pups were counted. Individual reproductive fitness was then estimated by calculating what we called the reproductive fitness index. This parameter resulted from summing up the number of opportunities to mate, the number of pregnancies achieved and the number of living pups by the end of weaning divided by 47. The constant in the divisor represents the sum of the total number of mating opportunities, pregnancies and living pups of the male with the greatest reproductive efficacy. This constant then defines the conditions that each male would have must achieved to assure maximal reproduc- tive outcome under the experimental paradigm tested. 2.5. Serotonin Concentration and Tryptophan Hydroxylase Activity in Copulator Males Copulator (n 7) and non-copulator males (n 7) were sacrificed immediately after the last reproductive or copulatory encounter, respectively. The rats were sacri- ficed by overdosing pentobarbital administered intrap- eritoneally (26 mg/Kg of body weight). Then, the right reproductive organs (testicle and epididymis) and ac- cessory sexual glands (ventral prostate, seminal vesicle, 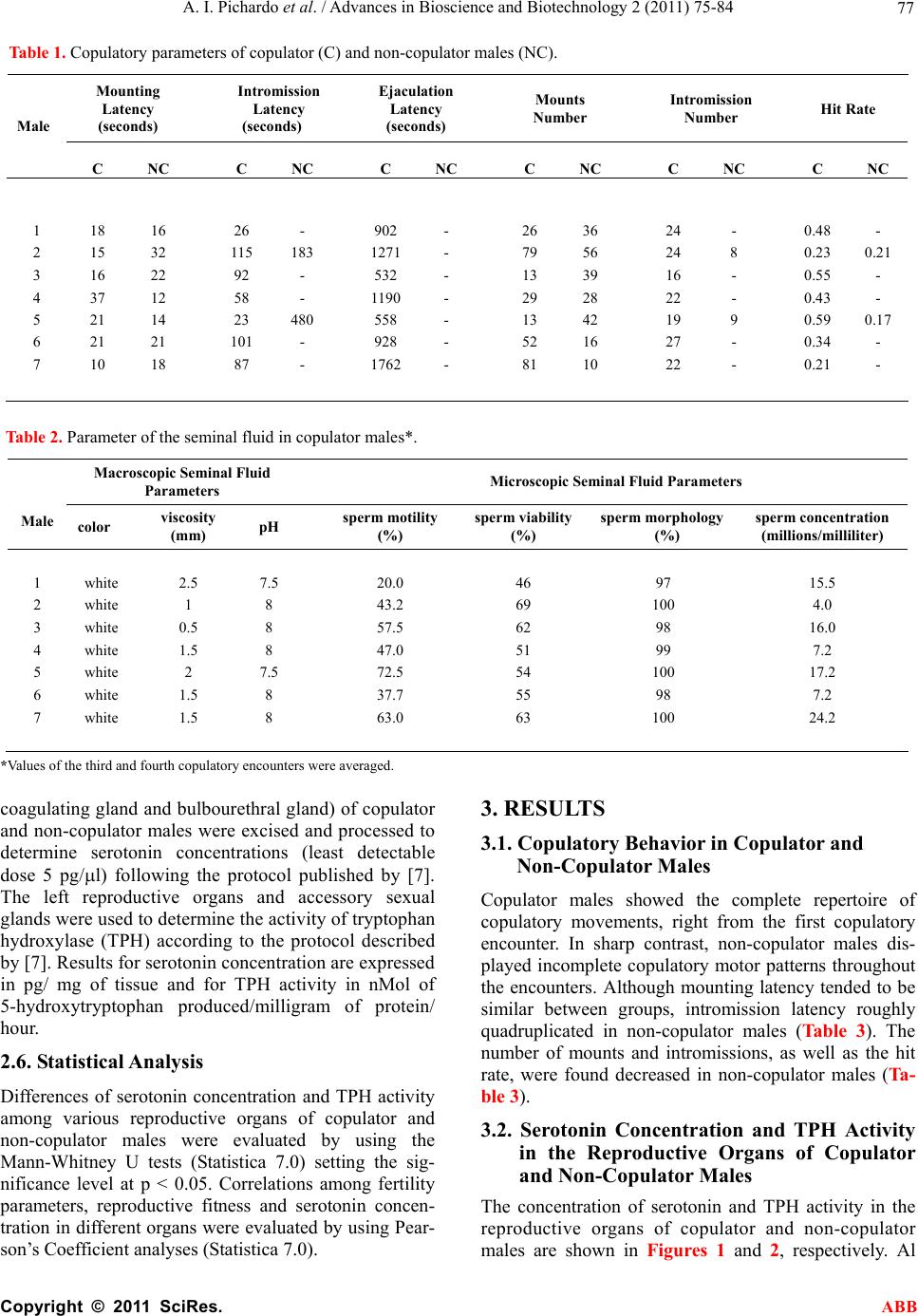 A. I. Pichardo et al. / Advances in Bioscience and Biotechnology 2 (2011) 75-84 Copyright © 2011 SciRes. ABB 77 Table 1. Copulatory parameters of copulator (C) and non-copulator males (NC). Mounting Latency (seconds) Intromission Latency (seconds) Ejaculation Latency (seconds) Mounts Number Intromission Number Hit Rate Male C NC C NC C NC C NC C NC C NC 1 18 16 26 - 902 - 26 36 24 - 0.48 - 2 15 32 115 183 1271 - 79 56 24 8 0.23 0.21 3 16 22 92 - 532 - 13 39 16 - 0.55 - 4 37 12 58 - 1190 - 29 28 22 - 0.43 - 5 21 14 23 480 558 - 13 42 19 9 0.59 0.17 6 21 21 101 - 928 - 52 16 27 - 0.34 - 7 10 18 87 - 1762 - 81 10 22 - 0.21 - Table 2. Parameter of the seminal fluid in copulator males*. Macroscopic Seminal Fluid Parameters Microscopic Seminal Fluid Parameters Male color viscosity (mm) pH sperm motility (%) sperm viability (%) sperm morphology (%) sperm concentration (millions/milliliter) 1 white 2.5 7.5 20.0 46 97 15.5 2 white 1 8 43.2 69 100 4.0 3 white 0.5 8 57.5 62 98 16.0 4 white 1.5 8 47.0 51 99 7.2 5 white 2 7.5 72.5 54 100 17.2 6 white 1.5 8 37.7 55 98 7.2 7 white 1.5 8 63.0 63 100 24.2 *Values of the third and fourth copulatory encounters were averaged. coagulating gland and bulbourethral gland) of copulator and non-copulator males were excised and processed to determine serotonin concentrations (least detectable dose 5 pg/l) following the protocol published by [7]. The left reproductive organs and accessory sexual glands were used to determine the activity of tryptophan hydroxylase (TPH) according to the protocol described by [7]. Results for serotonin concentration are expressed in pg/ mg of tissue and for TPH activity in nMol of 5-hydroxytryptophan produced/milligram of protein/ hour. 2.6. Statistical Analysis Differences of serotonin concentration and TPH activity among various reproductive organs of copulator and non-copulator males were evaluated by using the Mann-Whitney U tests (Statistica 7.0) setting the sig- nificance level at p < 0.05. Correlations among fertility parameters, reproductive fitness and serotonin concen- tration in different organs were evaluated by using Pear- son’s Coefficient analyses (Statistica 7.0). 3. RESULTS 3.1. Copulatory Behavior in Copulator and Non-Copulator Males Copulator males showed the complete repertoire of copulatory movements, right from the first copulatory encounter. In sharp contrast, non-copulator males dis- played incomplete copulatory motor patterns throughout the encounters. Although mounting latency tended to be similar between groups, intromission latency roughly quadruplicated in non-copulator males (Table 3). The number of mounts and intromissions, as well as the hit rate, were found decreased in non-copulator males (Ta- ble 3). 3.2. Serotonin Concentration and TPH Activity in the Reproductive Organs of Copulator and Non-Copulator Males The concentration of serotonin and TPH activity in the reproductive organs of copulator and non-copulator males are shown in Figures 1 and 2, respectively. Al 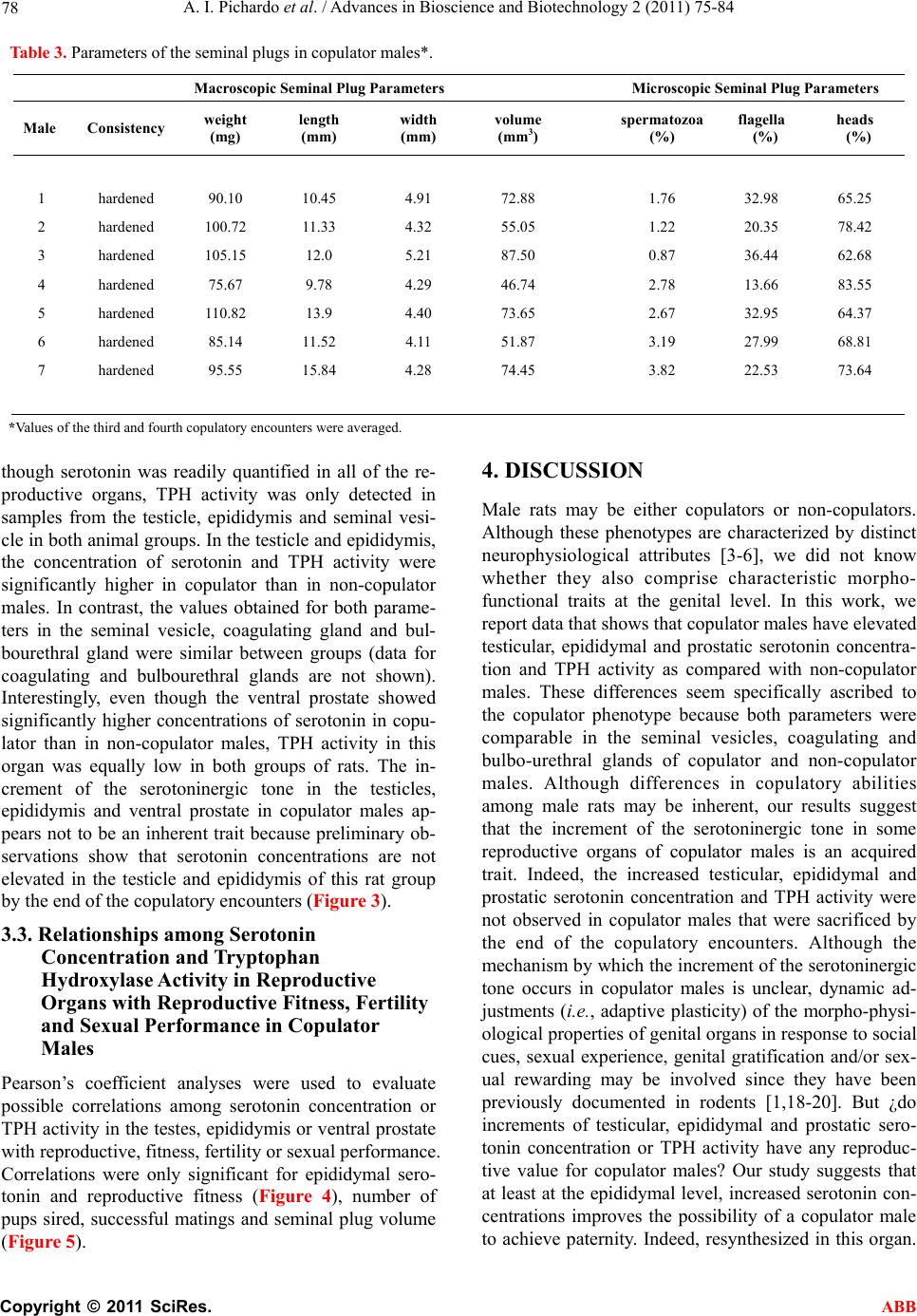 A. I. Pichardo et al. / Advances in Bioscience and Biotechnology 2 (2011) 75-84 Copyright © 2011 SciRes. ABB 78 Table 3. Parameters of the seminal plugs in copulator males*. Macroscopic Seminal Plug Parameters Microscopic Seminal Plug Parameters Male Consistency weight (mg) length (mm) width (mm) volume (mm3) spermatozoa (%) flagella (%) heads (%) 1 hardened 90.10 10.45 4.91 72.88 1.76 32.98 65.25 2 hardened 100.72 11.33 4.32 55.05 1.22 20.35 78.42 3 hardened 105.15 12.0 5.21 87.50 0.87 36.44 62.68 4 hardened 75.67 9.78 4.29 46.74 2.78 13.66 83.55 5 hardened 110.82 13.9 4.40 73.65 2.67 32.95 64.37 6 hardened 85.14 11.52 4.11 51.87 3.19 27.99 68.81 7 hardened 95.55 15.84 4.28 74.45 3.82 22.53 73.64 *Values of the third and fourth copulatory encounters were averaged. though serotonin was readily quantified in all of the re- productive organs, TPH activity was only detected in samples from the testicle, epididymis and seminal vesi- cle in both animal groups. In the testicle and epididymis, the concentration of serotonin and TPH activity were significantly higher in copulator than in non-copulator males. In contrast, the values obtained for both parame- ters in the seminal vesicle, coagulating gland and bul- bourethral gland were similar between groups (data for coagulating and bulbourethral glands are not shown). Interestingly, even though the ventral prostate showed significantly higher concentrations of serotonin in copu- lator than in non-copulator males, TPH activity in this organ was equally low in both groups of rats. The in- crement of the serotoninergic tone in the testicles, epididymis and ventral prostate in copulator males ap- pears not to be an inherent trait because preliminary ob- servations show that serotonin concentrations are not elevated in the testicle and epididymis of this rat group by the end of the copulatory encounters (Figure 3). 3.3. Relationships among Serotonin Concentration and Tryptophan Hydroxylase Activity in Reproductive Organs with Reproductive Fitness, Fertility and Sexual Performance in Copulator Males Pearson’s coefficient analyses were used to evaluate possible correlations among serotonin concentration or TPH activity in the testes, epididymis or ventral prostate with reproductive, fitness, fertility or sexual performance. Correlations were only significant for epididymal sero- tonin and reproductive fitness (Figure 4), number of pups sired, successful matings and seminal plug volume (Figure 5). 4. DISCUSSION Male rats may be either copulators or non-copulators. Although these phenotypes are characterized by distinct neurophysiological attributes [3-6], we did not know whether they also comprise characteristic morpho- functional traits at the genital level. In this work, we report data that shows that copulator males have elevated testicular, epididymal and prostatic serotonin concentra- tion and TPH activity as compared with non-copulator males. These differences seem specifically ascribed to the copulator phenotype because both parameters were comparable in the seminal vesicles, coagulating and bulbo-urethral glands of copulator and non-copulator males. Although differences in copulatory abilities among male rats may be inherent, our results suggest that the increment of the serotoninergic tone in some reproductive organs of copulator males is an acquired trait. Indeed, the increased testicular, epididymal and prostatic serotonin concentration and TPH activity were not observed in copulator males that were sacrificed by the end of the copulatory encounters. Although the mechanism by which the increment of the serotoninergic tone occurs in copulator males is unclear, dynamic ad- justments (i.e., adaptive plasticity) of the morpho-physi- ological properties of genital organs in response to social cues, sexual experience, genital gratification and/or sex- ual rewarding may be involved since they have been previously documented in rodents [1,18-20]. But ¿do increments of testicular, epididymal and prostatic sero- tonin concentration or TPH activity have any reproduc- tive value for copulator males? Our study suggests that at least at the epididymal level, increased serotonin con- centrations improves the possibility of a copulator male to achieve paternity. Indeed, resynthesized in this organ. 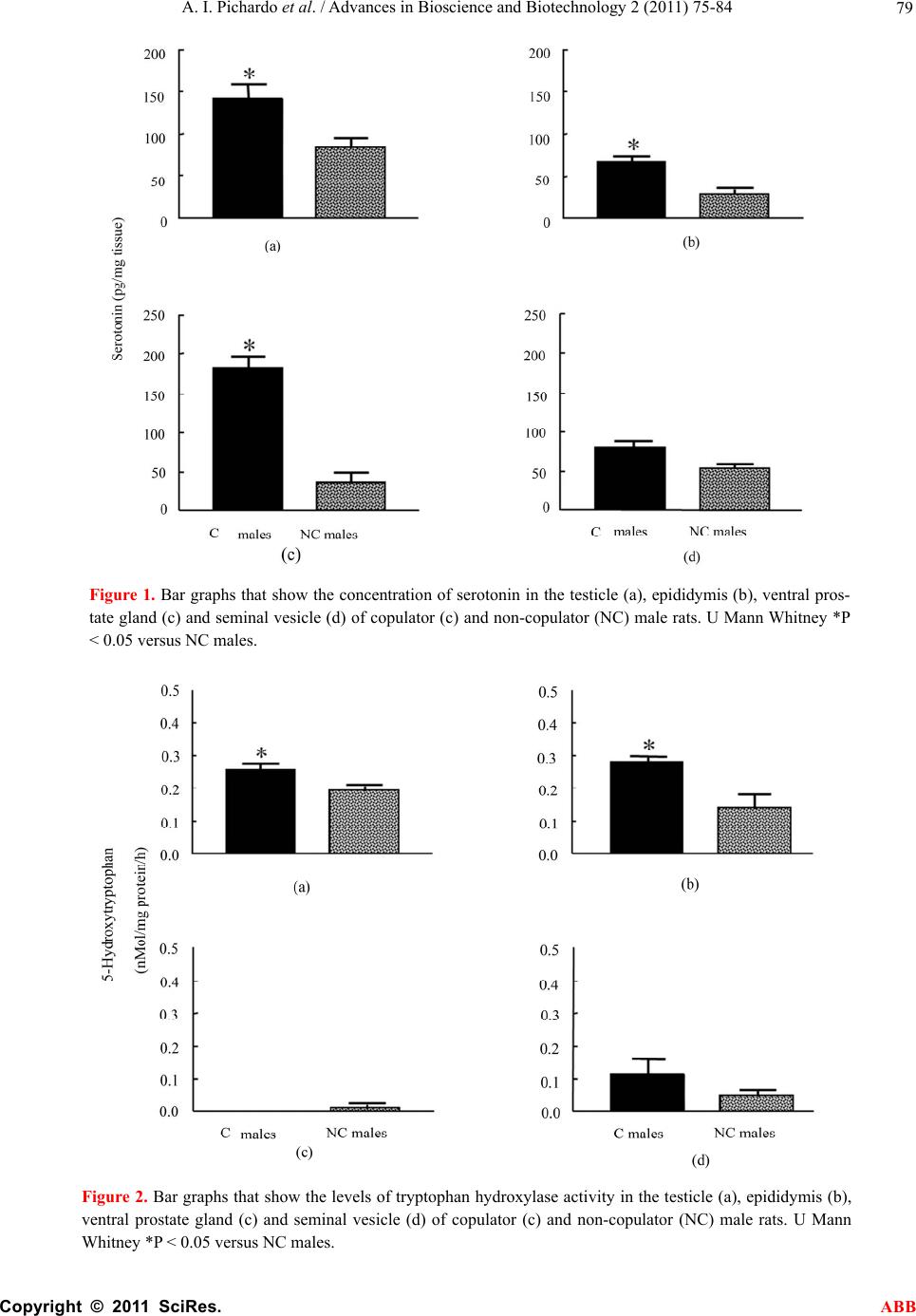 A. I. Pichardo et al. / Advances in Bioscience and Biotechnology 2 (2011) 75-84 Copyright © 2011 SciRes. ABB 79 Figure 1. Bar graphs that show the concentration of serotonin in the testicle (a), epididymis (b), ventral pros- tate gland (c) and seminal vesicle (d) of copulator (c) and non-copulator (NC) male rats. U Mann Whitney *P < 0.05 versus NC males. Figure 2. Bar graphs that show the levels of tryptophan hydroxylase activity in the testicle (a), epididymis (b), ventral prostate gland (c) and seminal vesicle (d) of copulator (c) and non-copulator (NC) male rats. U Mann Whitney *P < 0.05 versus NC males. 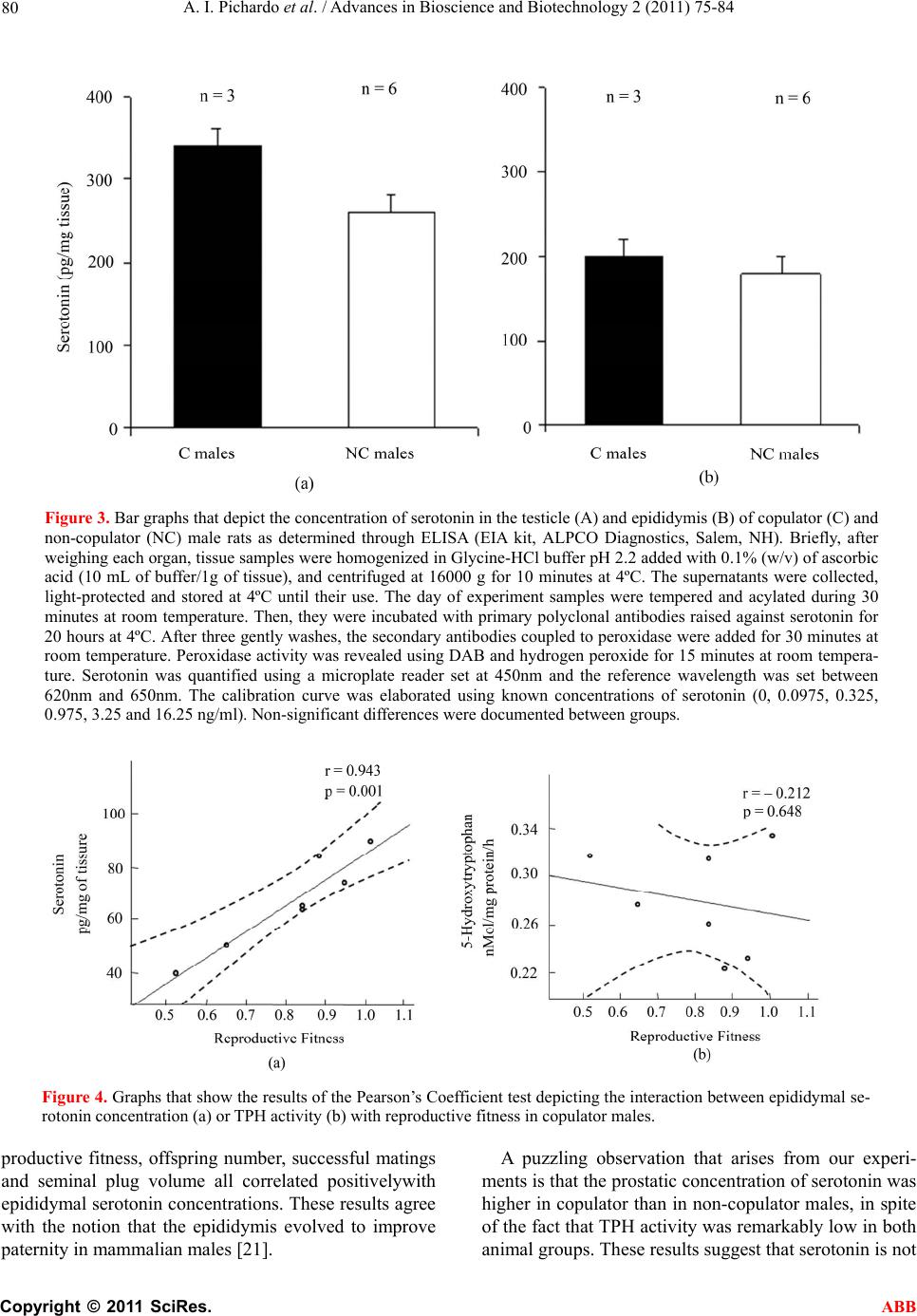 A. I. Pichardo et al. / Advances in Bioscience and Biotechnology 2 (2011) 75-84 Copyright © 2011 SciRes. ABB 80 Figure 3. Bar graphs that depict the concentration of serotonin in the testicle (A) and epididymis (B) of copulator (C) and non-copulator (NC) male rats as determined through ELISA (EIA kit, ALPCO Diagnostics, Salem, NH). Briefly, after weighing each organ, tissue samples were homogenized in Glycine-HCl buffer pH 2.2 added with 0.1% (w/v) of ascorbic acid (10 mL of buffer/1g of tissue), and centrifuged at 16000 g for 10 minutes at 4ºC. The supernatants were collected, light-protected and stored at 4ºC until their use. The day of experiment samples were tempered and acylated during 30 minutes at room temperature. Then, they were incubated with primary polyclonal antibodies raised against serotonin for 20 hours at 4ºC. After three gently washes, the secondary antibodies coupled to peroxidase were added for 30 minutes at room temperature. Peroxidase activity was revealed using DAB and hydrogen peroxide for 15 minutes at room tempera- ture. Serotonin was quantified using a microplate reader set at 450nm and the reference wavelength was set between 620nm and 650nm. The calibration curve was elaborated using known concentrations of serotonin (0, 0.0975, 0.325, 0.975, 3.25 and 16.25 ng/ml). Non-significant differences were documented between groups. Figure 4. Graphs that show the results of the Pearson’s Coefficient test depicting the interaction between epididymal se- rotonin concentration (a) or TPH activity (b) with reproductive fitness in copulator males. productive fitness, offspring number, successful matings and seminal plug volume all correlated positivelywith epididymal serotonin concentrations. These results agree with the notion that the epididymis evolved to improve paternity in mammalian males [21]. A puzzling observation that arises from our experi- ments is that the prostatic concentration of serotonin was higher in copulator than in non-copulator males, in spite of the fact that TPH activity was remarkably low in both animal groups. These results suggest that serotonin is not 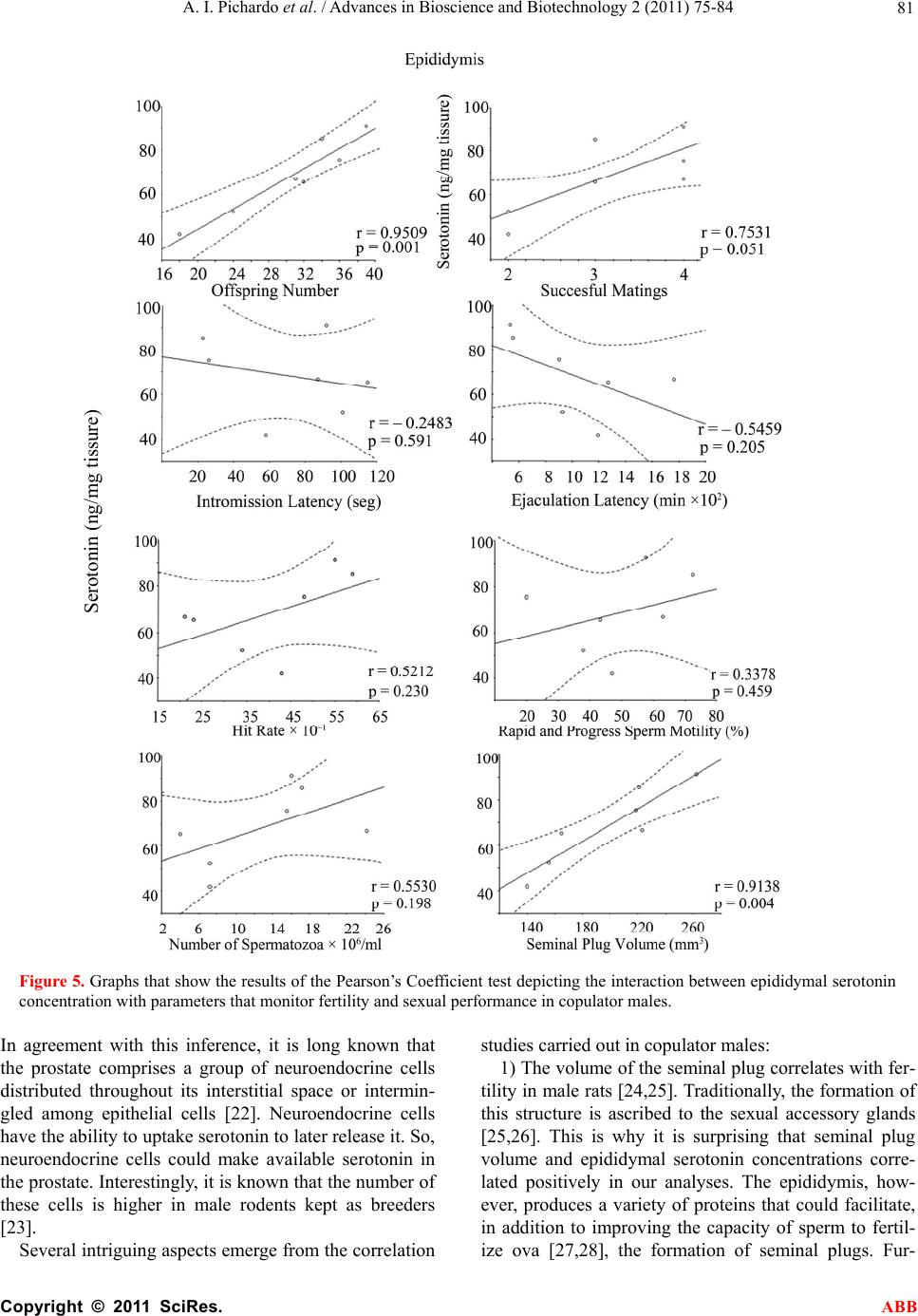 A. I. Pichardo et al. / Advances in Bioscience and Biotechnology 2 (2011) 75-84 Copyright © 2011 SciRes. ABB 81 Figure 5. Graphs that show the results of the Pearson’s Coefficient test depicting the interaction between epididymal serotonin concentration with parameters that monitor fertility and sexual performance in copulator males. In agreement with this inference, it is long known that the prostate comprises a group of neuroendocrine cells distributed throughout its interstitial space or intermin- gled among epithelial cells [22]. Neuroendocrine cells have the ability to uptake serotonin to later release it. So, neuroendocrine cells could make available serotonin in the prostate. Interestingly, it is known that the number of these cells is higher in male rodents kept as breeders [23]. Several intriguing aspects emerge from the correlation studies carried out in copulator males: 1) The volume of the seminal plug correlates with fer- tility in male rats [24,25]. Traditionally, the formation of this structure is ascribed to the sexual accessory glands [25,26]. This is why it is surprising that seminal plug volume and epididymal serotonin concentrations corre- lated positively in our analyses. The epididymis, how- ever, produces a variety of proteins that could facilitate, in addition to improving the capacity of sperm to fertil- ize ova [27,28], the formation of seminal plugs. Fur- Se r otonin (ng/mg tissure)  A. I. Pichardo et al. / Advances in Bioscience and Biotechnology 2 (2011) 75-84 Copyright © 2011 SciRes. ABB 82 thermore, the release of epididymal secretory products is modulated by serotonin [29]. Hence, increments in epididymal serotonin availability could be associated with factors that control seminal plug volume. 2) Epididymal serotonin concentration did not corre- late with sperm motility or sperm concentration. This is intriguing because serotonin increases sperm motility and concentration [30,31] and the epididymis is the or- gan from which sperms are ejaculated. We believe the discrepancy may reflect technical limitations. For in- stance, although the chromatographic analyses carried out in our work clearly portrait serotonin availability in the organ as a whole, they do not allow estimations of the amount released into the ductal system. In addition, female factors could modulate sperm parameters [32-34] regardless of the availability of serotonin in the epidi- dymis; sperm samples were taken from the uterine horns. 3) Lastly, the lack of a positive or negative correlation between testicular serotonin and fertility/reproductive fitness in copulator males was also unexpected. It is known that increased production of spermatozoa pro- vides reproductive advantages to males [19], that tes- ticular serotonin concentration is increased in breeder rats [7] and that serotonin modulates spermatogenesis [35] and testosterone production and release from Ley- dig cells [14,36,37], a hormone long known to promote spermatogenesis [38] and sexual behavior [39]. Up to now, we have no explanation for this counterintuitive outcome. We believe, however, that female factors may also explain these observations. In spite of the unexpected results, we believe that our observations support that copulator and non-copulator male rats feature a distinct serotoninergic tone in the epididymis, testicle and ventral prostate gland, and that the increment of this trait in copulator males probably is associated with greater sexual experience. In the future it would be fundamental to evaluate whether non-copulator males lack or have a diminished ability to display adap- tive plasticity when sexually trained for longer times. Finally, the observation documenting that epididymal serotonin concentration correlated with parameters that monitor male fertility and reproductive fitness in copu- lator males predicts that epididymal factors increase their chances of parenting offspring. 5. ACKNOWLEDGEMENTS Authors are grateful to Patricia Padilla Cortés, Jesús Ramírez Santos, Luz Lilia Jiménez, Rico, Georgina Díaz Herrera, Alfonso Malagón Mendiola, Raymundo Reyes and Marta Carrasco for their valuable technical, administrative assistance and animal care. We are indebted to Dr. John McHaffie for helpful criticisms and manuscript editing. This work was supported by a grant from the Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México (PAPIIT IN215208). AIPC, JLTL and MLMC are fellows from the Consejo Nacional de Ciencia y Tecnología. REFERENCES [1] Pfaus, J.G., Kippin, T.E. and Centeno, S. (2001) Condi- tioning and sexual behavior: A review. Hormones and Behavior, 40, 291-321. doi:10.1006/hbeh.2001.1686 [2] Whalen, R.E., Beach, F.A. and Kuehn, R.E. (1961) Ef- fects of exogenous androgens of sexually responsive and unresponsive male rats. Endocrinology, 69, 373-380. doi:10.1210/endo-69-2-373 [3] Portillo, W. and Paredes, R.G. (2003) Sexual and olfac- tory preference in non copulating male rats. Physiology and Behavior, 80, 155-162. doi:10.1016/S0031-9384(03)00231-2 [4] Portillo, W. and Paredes, R.G. (2004) Sexual incentive motivation, olfactory preference and activation of the vomeronasal projection pathway by sexually relevant cues in non-copulating and naive male rats. Hormones and Behavior, 46, 330-340. doi:10.1016/j.yhbeh.2004.03.001 [5] Portillo, W., Diaz, N.F., Cabrera, E.A., Fernandez-Guasti A. and Paredes, R.G. (2006) Comparative analysis of in- munoreactive cells for androgen receptors and oestrogen receptor α in copulating and non copulating male rats. Journal of Neuroendocrinology, 18, 168-176. doi:10.1111/j.1365-2826.2005.01401.x [6] Portillo, W., Castillo, C.G., Retana-Márquez, S., Roselli, C.E. and Paredes, R.G. (2007) Neuronal activity of aro- matase enzyme in non copulatory male rats. Journal of Neuroendocrinology, 19, 139-141. doi:10.1111/j.1365-2826.2006.01513.x [7] Jiménez-Trejo, F., Tapia-Rodríguez, M., Queiroz, D.B., Padilla, P., Avellar, M. C., Manzano, P.R., Manjarrez, G. and Gutiérrez-Ospina, G. (2007) Serotonin concentration synthesis cell origin and targets in the rat caput epidi- dymis during sexual maturation and variations associated whit adult mating status: Morphological and biochemical studies. Journal of Andro log y, 28, 136-149. [8] Frungeri, M.B., Gonzalez-Calvar, S.I., Rubio, M., Ozu, M., Lustig, L. and Calandras, R.S. (1999) Serotonin in golden hamster testes: Testicular levels, inmunolocaliza- tion and role during sexual development and photoperi- odic regression-recrudescence transition. Neuroendocri- nology, 69, 299-308. doi:10.1159/000054431 [9] Chaturvedi, C.M. and Singh, A.B. (1992) Suppression of annual testicular development in Indian palm squirrel, Funambulus pennati by 8 hrs temporal relationship of serotonin and dopamine precursor drugs. Journal of Neural Transmi ssion, 88, 53-60. doi:10.1007/BF01245036 [10] Kim, S.W. and Paick, J.S. (2004) Peripheral effects of serotonin on the contractile responses of rat seminal ve- sicles and vasa deferentia. Journal of Andrology, 25, 893- 899. [11] Piner, J., Sutherland, M., Millar, M., Turner, K., Newall, D. and Sharpe, R.M. (2002) Changes in vascular dynam- ics of the adult rat testis leading to transient accumulation  A. I. Pichardo et al. / Advances in Bioscience and Biotechnology 2 (2011) 75-84 Copyright © 2011 SciRes. ABB 83 of seminiferous tubule fluid after administration of a novel 5-hidroxycriptamine (5-HT) agonist. Reproductive Toxicology, 16, 141-150. doi:10.1016/S0890-6238(02)00008-4 [12] Leung, G.P., Dun, S.L., Dun, N.J. and Wong, P.Y. (1999) Serotonin via 5-HT1B and 5-HT2B receptors stimulates anion secretion in the rat epididimal epithelium. Journal of Physiology, 519, 657-667. doi:10.1111/j.1469-7793.1999.0657n.x [13] Killam, A.L., Watts, S.W. and Cohen, M.L. (1995) Role of the alpha 1-adrenoreceptors and 5-HT2 receptors in serotonin-induced contraction of rat prostate: Autoradio- graphical and functional studies. European Journal of Pharmacology, 273, 7-14. doi:10.1016/0014-2999(94)00613-C [14] Gerendai, I., Banzerowski, P., Csernus, V. and Halasz, B. (2007) Innervation and serotoninergic receptors of the testis interact with local action of interleukin-1beta on steroidogenesis. Autonomic Neuroscience, 131, 21-27. doi:10.1016/j.autneu.2006.06.002 [15] Das, T.K., Mazumder, R. and Biswas, N.M. (1982) Spermatogenesis in rat: Effect of L-typtophan loading. Andrologia, 14, 242-249. [16] Lucio, R.A., Tlachi, J.L., López, A.A., Zempoalteca, R. and Velázquez-Moctezuma, J. (2009) Analisis of the pa- rameters of the ejaculate in the laboratory wistar rat: Technical description. Veterinaria México, 40, 205-215. [17] Lucio, R.A., Manzo, J., Martínez-Gómez, M., Sachs, B.D. and Pacheco, P. (1994) Participation of pelvic nerve in male rat copulatory behavior. Physiology and Behavior, 55, 241-246. doi:10.1016/0031-9384(94)90129-5 [18] Portillo, W. and Paredes, R.G. (2009) Conditioned place preference induced by morphine in non-copulating male rats. Behavio ral B rain Research, 203, 308-311. [19] Ramm, S.A. and Stockley, P. (2009) Adaptative plasticity of mammalian spremproduction in response to social experience. Proceedings of the Royal Society B: Biologi- cal Sciences, 276, 741-755. doi:10.1016/j.bbr.2009.04.037 [20] Lemaitre, J.F., Ramm, S.A., Horst, J.L. and Stockley, P. (2010) Social cues of sperm competition influence ac- cessory reproductive gland size in a promiscuous mam- mal. Proceedings of the Royal Society B: Biological Sci- ences, Epub ahead of print. http://rspb.royalsocietypiblishing.org/conten/early/2010/0 9/24/rspb..1828.long [21] Jones, R.C. (1998) Evolution of the vertebrate epidi- dymis. Journal of Reproduction and Fertility Supplement, 53, 163-182. [22] Rodríguez, R., Pozuelo, J. M., Martin, R., Henriques-Gil, N., Haro, M., Arriazu, R. and Santamaria, L. (2003) Presence of neuroendocrine cells during postnatal devel- opment in rat prostate: Immunohistochemical, molecular and quantitative study. Prostate, 57, 176-185. [23] Di Sant’Agnese, P.A., Davis, N.S., Chen, M. and De Mesy Jensen, K.L. (1987) Age-related changes in the neuroendocrine (endocrine-paracrine) cell population and the serotonin content of the guinea pig prostate. Labora- tory Investigation, 57, 729-736. [24] Mathews, M. and Adler, N.T. (1977) Facilitative and inhibitory influences of reproductive behavior on sperm transport in rats. Journal of Comparative and Physio- logical Psychology, 91, 727-741. doi:10.1037/h0077364 [25] Carballada, R. and Esponda, P. (1992) Role of fluid from seminal vesicles and coagulating glands in sperm trans- port into the uterus and fertility in rats. Journal of Re- production and Fertility, 95, 639-648. doi:10.1530/jrf.0.0950639 [26] Cukierski, M.A., Sina, J.L., Prahalada, S. and Robertson, R.T. (1991) Effects of seminal vesicle and coagulating gland ablation on fertility in rats. Reproductive Toxicol- ogy, 5, 347-352. doi:10.1016/0890-6238(91)90093-U [27] Jones, R.C., Dacheux, J.L., Nixon, B. and Ecroyd, H.W. (2007) Role of the epididymis in sperm competition. Asian Journal of Andrology, 9, 493-499. doi:10.1111/j.1745-7262.2007.00284.x [28] Zhou, Y., Zheng, M., Shi, Q., Zhang, L., Zhen, W., Chen, W. and Zhang, Y. (2008) An epididymis-specific secre- tory protein hongrES1 critically regulates sperm capaci- tation and male fertility. PLoS ONE, 3, 12. doi:10.1371/journal.pone.0004106 [29] Leung, G.P., Dun, S.L., Dun, N.J. and Wong, P.Y. (1999) Serotonin via 5-HT1B and 5-HT2B receptors stimulates anion secretion in the rat epididymal epithelium. The Journal of Physiology, 519, 657-670. doi:10.1111/j.1469-7793.1999.0657n.x [30] Parisi, E., De Prisco, P., Capasso, A. and Del Prete, M. (1984) Serotonin and sperm motility. Cell Biology Inter- national Reports, 8, 95. [31] Waldinger, M.D., Berendsen, H.H.G., Blok, B.F.M., Olivier, B. and Holstege, G. (1998) Premature ejaculation and serotonergic antidepressants-induced delayed ejacu- lation: the involvement of the serotonergic system. Be- havioral Brain Research, 92, 111-118. doi:10.1016/S0166-4328(97)00183-6 [32] Wilson, N., Tubman, S.C., Eady, P.E. and Robertson, G.W. (1997) Female genotype affects male success in sperm competition. Proceedings of the Royal Society B: Biological Sciences, 264, 1491-1495. doi:10.1098/rspb.1997.0206 [33] Ball, M.A. and Parker, G.A (2003) Sperm competition games: sperm selection by females. Journal of Theoreti- cal Biology , 224, 27-42. doi:10.1530/rep.1.00598 [34] Coria-Ávila, G.A., Jones, S.L., Solomon, C.E., Gavrila, A.M., Jordan, G.J. and Pfaus, J.G. (2006) Conditioned partner preference in female rats for strain of male. Physiology and Behavior, 88, 529-537. [35] Aragon, M.A., Ayala, M.E., Marin, M., Aviles, Da- mian-Matsumara, A.P. and Rodriguez, R. (2005) Sero- toninergic system blockage in the prepubertal rat inhibits spermatogenesis development. Reproduction, 129, 717- 727. [36] Csaba, C., Csernus, V. and Gerendai, I. (1998) Intrates- ticular serotonin affects stereidogenesis in the rat testis. Journal of Neuroendocrinology, 10, 371-376. [37] Frungieri, M.B., Zita, K., Pignataro, O.P., Gonzalez- Calvar, S.I. and Calandra, R.S. (2002) Interactions be- tween testicular serotoninergic, catecolaminergic and corticotropi-releasing hormone systems modulating cAMP and testosterone production in the Golden Hamster. Neu- roendocrinology, 76, 35-46. doi:10.1159/000063682 [38] Cheng, C.Y. and Mruk, D.D. (2010) A local autocrine  A. I. Pichardo et al. / Advances in Bioscience and Biotechnology 2 (2011) 75-84 Copyright © 2011 SciRes. ABB 84 axis in the testes that regulates spermatogenesis. Nature Reviews Endocrinology, 6, 380-395. doi:10.1038/nrendo.2010.71 [39] Buvat, J., Maggi, M., Gooren, L., Gudy, A.T., Kaufman, J., Morgentaler, A., Schulman, C., Tan, H.M., Torres, L.O., Yassin, A. and Zitzmann, M. (2010) Endocrine aspects of male sexual dysfunctions. Journal of Sex Medicine, 7, 1627-1656. doi:10.1111/j.1743-6109.2010.01780.x |

