Paper Menu >>
Journal Menu >>
 Vol.3, No.3, 172-178 (2011) Health doi:10.4236/health.2011.33033 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Immunophenotyping of peripheral blood mononuclear cells and intracellular detection of IL-2, IFNγ and IL-4 by flow cytometry in patients with actinomycetoma by Nocardia brasiliensis and Actinomadura madurae. Findings in six patients Heuze de Icaza Ivonne1,6, Castrillón Rivera Laura Estela1,7, Garibay-Escobar Adriana2,3, Sandoval Trujillo Horacio1,7, Padilla Desgarennes Carmen4, Palma Ramos Alejandro1, Santos-Argumedo Leopoldo2 1Universidad Autónoma Metropolitana, Campus Xochimilco. Calzada del Hueso 1100, Villa Quietud, Coyoacán, México D. F. CP 04960. México; 2Department of Molecular Biomedicine, Centro de Investigacion y Estudios Avanzados del IPN. Ave. Instituto Politécnico Nacional No. 2508, Col. Zacatenco, CP 07360, México City, México; 3Universidad de Sonora. Hermosillo, Sonora, México; 4Laboratory of Mycology. Centro Dermatológico “Dr. Ladislao de la Pascua”, Servicios de Salud Pública of Distrito Federal. México City, México; 5Departamento de Biolgía, Facultad de Química, UNAM. Ciudad Universitaria, México D.F., México; 6Heuze de Icaza Yvonne is in the Program of Doctorate in Biological Sciences. Universidad Autónoma Metropolitana, México City, México; 7Laura E. Castrillón R and Horacio Sandoval T. are part of Tutorial Committee of Yvonne Heuze in the Program of Doctorate in Biological Sciences. Universidad Autónoma Metropolitana, México City, México. Received 20 January 2011; revised 5 February 2011; accepted 18 February 2011. ABSTRACT Mycetoma is a chronic, granulomatous, progres- sive inflammatory disease; it usually involves the subcutaneous tissue after a traumatic inoculation of the causative organism. Mycetoma may be caused by true fungi or bacteria (actinomycetes) and hence it is classified as eumycetoma and actinomycetoma respectively. Mycetoma immunological studies have fre- quently addressed humoral aspects. Few reports have addressed the role of cellular immunity in humans, for this reason, we were interested in finding differences in the circulating population of mononuclear cells or in the ability of this pathology to produce cytokines after mitogen stimulation. In this study, immunophenotyping of peripheral blood mononuclear cells (PBMN) and intracellular detection by flow cytometry of IL-2, IFNγ and IL-4 in patients with actino- mycetoma by Nocardia brasiliensis and Actino- madura madurae were evaluated. We investi- gated the expression of T-cells (CD3, CD4 and CD8), B-cells (CD19), monocytes (CD14), and natural killer cells (CD16 and CD56) markers. CD69 and CD25 were used to monitor individual activated cell subsets. The percentage values of the cells were calculated. Our results indicated that PBMC from patients with mycetoma show similar percentages of circulating cells when compared with healthy donors. The expression of IL-2 receptor (IL-2R) by mitogen activation was similar in these two groups. These findings suggest that circulating lymphocytes are not affected by this pathology. Intracellular IL-4 was increased only in patients with mycetoma by N. brasiliensis, suggesting a TH2 profile; this ob- servation has been reported by other authors. Keywords: Actinomycetoma; Nocardia brasili ensis; Actinomadura madurae; Cytokines; CD4+; CD8+; CD56+ 1. INTRODUCTION Mycetoma is a chronic, granulomatous, progressive inflammatory disease; it usually involves the subcuta- neous tissue after a traumatic inoculation of the causa-  H. de I. Yvonne et al. / Health 3 (2011) 172-178 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 173 tive organism. Mycetoma may be caused by true fungi or by bacteria (actinomycetes) and hence it is classified as eumycetoma and actinomycetoma respectively [1,2]. Tumefaction and formation of sinus tracts characterize mycetoma. The sinuses usually discharge purulent and seropurulent exudate containing microcolonies called grains. It may spread involving skin and the deep struc- tures, resulting in destruction, deformity, and loss of function. In rare occassions it may be fatal [3,4]. The clinical presentation of mycetoma is almost identical, regardless of the causal organism. However, the progress is faster in actinomycetoma comparatively with eumy- cetoma. In eumycetoma, the lesion grows slowly, with clear defined margins and it remains encapsulated for a long period, whereas, in actinomycetoma the lesion is more inflammatory, more destructive, and it invades the bone at an earlier period [1,4]. Mycetoma lesion has a distinct appearance in a cytology smear which is charac- terized by the presence of inflammatory cells consisting of a mixture of neutrophils, lymphocytes, plasma cells, histiocytes, macrophages, and foreign body giant cells in grains [5]. In tissue sections, three types of tissue reac- tions have been described (I, II and III). Type I is char- acterized by actinomycetoma lesion [6,7] where the grain is closely surrounded by polymorphonuclear leu- kocytes that are the predominant cell type in the in- flammatory infiltrate in the skin lesions and occasionally infiltrated by neutrophils, causing its fragmentation. Outside the neutrophil zone, monocytic cells and giant cells are observed. The lymphocytes are scant where the main population is TCD4+ [8]. Cenci et al. [9] demonstrated the cellular elements in the inflammatory infiltrate in skin lesions of actinomy- cetoma and eumycetoma and suggested that cellular me- diated immunity may play a role in mycetoma patho- genesis, in which a marked reduction of Langerhans cells may reflect a depressed cell immune response, par- tially explaining a chronic condition and unresponsive- ness to the treatment. Studies on the immunopathologic aspects of tissue reaction are much less frequent, immunological studies of mycetoma have frequently addressed humoral aspects [10-13]. Few reports have addressed the role of cellular immunity in humans. The main studies regarding cellu- lar immune response by delayed hypersensitivity to an- tigens from Nocardia was first describe in 1972 [14] and protective effect after spleen cell transfer from guinea pigs infected with Nocardia asteroids was demonstrated five years later [15]. A defective T-cell mediated re- sponse in eumycetoma patients was suggested as an im- portant element in this pathology when the lymphocyte proliferation stimulated with phytohemagglutinin af- fected skin reactivity to dinitrochlorobenzene in these patients [16]; in contrast, infections by Nocardia aster- oides did not show these efects [17]. Cell-mediated immunity plays a major protection role against intracellular microbes. Nocardia brasiliensis is a facultative intracellular pathogen that grows in macro- phages but not in PMN leukocytes [18]. The mecha- nisms that allow explaining the evasion, resistance, or neutralization of bactericidal action of macrophages and neutrophils are: 1) production of high levels of catalase and superoxide dismutase that reduce the oxygen toxic products generated by phagocyte, 2) reduction of ly- sosome enzymatic activity of some macrophage popula- tions, 3) blockade of phagosome acidification, and 4) inhibition of phagosome-lysosome fusion [19]. Regarding Actinomadura madurae infections, the ability of this bacteria to persist inside murine macro- phages in experimental in vivo and in vitro infections has been reported [20]; as with nocardia infections, this could explain the tendency to chronicity by actinomy- cetales due their replicative capacity inside the phago- cytes, probably this fact is related to blood dissemination as it was demonstrated in experimental mycetoma by Nocardia brasiliensis [21]. In mycetoma, the local host response is characterized by neutrophil chemotaxis and small vessel congestion. Initially, the response is nonspecific, later, macrophages and monocytes present at the infection site are activated by interferon gamma and tumor necrosis factor-alpha; these cells have enhanced microbicidal activity [22]. For these reasons, the production of pro-inflammatory and anti-inflammatory cytokines and the expression of their receptors, after stimulation are important indicators of mononuclear cell activation in this pathology. 2. MATERIAL AND METHODS 2.1. Patients Mononuclear cells from four patients with mycetoma by Nocardia brasiliensis, two patients with mycetoma by Actinomadura madurae, and five healthy subjects were evaluated. These patients were diagnosed and treated by the Mycology Service of the Dermatologic Center “Ladislao de la Pascua” in Mexico City. This study was carried out with the written consent of all pa- tients and it was approved by the Ethics Committees of each participating institution. 2.2. Samples Peripheral blood samples from healthy volunteers and patients were collected in sodium heparin VACU- TAINERR tubes (Becton Dickinson, San Jose, CA). Per- centages and absolute counts of mature human lym-  H. de I. Yvonne et al. / Health 3 (2011) 172-178 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 174 phocytes subsets were evaluated from PBMC as follows: T lymphocytes (CD3+), B lymphocytes (CD19+), helper /inducer T lymphocytes (CD3+CD4+), cytotoxic T lym- phocytes (CD3+CD8+), and natural killer (NK) lym- phocytes (CD3-CD16 + CD56+). CD69 is expressed on all activated lymphocytes, thus it represents a generic marker to monitor individual subset responses to differ- ent stimuli [23]. 2.3. CD25 (IL-2αR) Expression PBMC were stimulated with 20 µg/mL phytohae- maglutinin (PHA Sigma) in RPMI, in 24 well flat bot- tom plates (Corning Glass Works, Corning, NY) for 24 h at 37˚C and 5% CO2. After incubation, PBMC were harvested by centrifugation at 500 × g for 5 min and then stained with a three-color combination using the follow- ing mAbs: anti-CD25PE (IL-2R) (Phar Mingen), anti- CD4FITC (Phar Mingen) and anti-CD3PerCP (Immmu- notech, Marseille, France). Percenteges of T lympho- cytes (gated on CD3+) expressing CD25 were measured by flow cytometry. 2.4. Intracellular Cytokine Detection: IFN-γ, IL-2 and IL-4 [24] The production of cytokines was measured through intracellular staining. Briefly, capped polystyrene Falcon tubes (Becton Dickinson) were used to incubate whole blood samples with 25 ng/ml phorbol myristate acetate (PMA) (Sigma), 1 µg/ml ionomycin (Sigma), and 10 µg/ml brefeldin A (BFA) (Sigma) or BFA only (control), for 5 h at 37˚C and 5% CO2. Anti-CD3 peridinin chlo- rophyll protein (PerCP) (Becton Dickinson) was added to aliquots of the stimulated and non-stimulated blood and then incubated for 15 min at room temperature in the dark. After incubation, erythrocytes were lysed with FACS lysing solution (Becton Dickinson) and the sam- ples were incubated for another 10 min at room tem- perature in the dark. The cells were then centrifuged for 5 min at 500 × g and supernatants were aspirated with- out disturbing the pellets. FACS permeabilizing solution (Becton Dickinson) was added to the pellets and incu- bated for 10 min at room temperature in the dark; cell suspensions were then washed with phosphate buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) (Research Organics, Cleveland, OH) and 0.01% sodium azide (PBA), and centrifuged for 5 min at 500 × G, the supernatants were removed. For intracellular stain- ing, anti-cytokine monoclonal antibodies (mAbs) were added to the pellets and cell suspensions were incubated at room temperature for 30 min in the dark, ac- cording to the following protocol: FastImmuneR INF-γ fluo- rescein isothiocyanate (FITC)/IL-4 phycoerythrin (PE) (Becton Dickinson) or FastImmuneR anti-HuIL-2 FITC (Becton Dickinson), and anti-CD69PE (Becton Dickin- son) were added to the pellets previously stained with anti-CD3PerCP. Simultest γ2a FITC/γ1PE with anti-CD3 PerCP was used as isotype control. For all conditions, the expression of CD69 was meas- ured as an activation control. The samples were washed with PBA and fixed with PBS containing 1% parafor- maldehyde (PFA). A fluorescent activated cell sorter FACSCaliburR (Becton Dickinson) equipped with a 15 mW argon ion laser and filter settings for FITC (530 nm), PE (585 nm) and PerCP(Becton Dickinson), emitting in the deep red (> 650 nm) was used. Cells (10,000) were computed in list mode and analyzed gating on CD3 (lymphocytes) using the CellQuestR software. 3. Results 3.1. Mononuclear Cell Populations The analysis of mononuclear cell populations in nor- mal subjects and from patients with mycetoma by No- cardia brasiliensis and Actinomadura madurae is pre- sented in Table 1. The results from healthy volunteers showed: CD3+T cells (84%), in which the percentages for the subpopulations were TCD4+ (56.6%), TCD8+ (28.2%); B cells (13.2%); NK cells and monocytes both were at 1%. In mycetoma patients, the percentages were as follows: T lymphocytes (CD3+) were 84.7%, in which 58.1% and 26.6% corresponded to CD4+ and CD8+ lymphocytes, respectively. B lymphocytes were 12.3%, NK cells 1% and monocytes 0.6%. 3.2. IL-2ΑR Expression Analysis A kinetic analysis for the expression of CD25+ (IL-2αR) was made. PBMC from healthy subjects were stimulated with 20 µg/mL PHA for 0, 24, 48 and 72 h and stained for CD25 (IL-2αR). Percentages of lympho- cytes positive for CD25 were mesured at each time by Table 1. Percentage of monocytes (CD14+), NK (CD16 + CD56+), TCD8 + (CD3+) and B (CD19+) lymphocytes from total PBMC in healthy and actinomycetoma patients. Monocytes % (CD14+) CD3+ CD4+ % CD3+ CD8+ % NK % (CD16+ CD56+) B % (CD19+) Patients with mycetoma by Nocardia brasiliensis 0.15 ± 0.0858.4 ± 13.3 25 ± 12.0 2.0 ± 1.2 14.5 ± 10.4 A ctinomadura madurae 1.1 ± 1.657.8 ± 11.9 28.3 ± 14.9 2.4 ± 1.810.2 ± 10.1 Healthy donors 1.0 ± 1.956.6 ± 13.7 28.2 ± 1.9 1.0 ± 1.213.2 ± 6.7 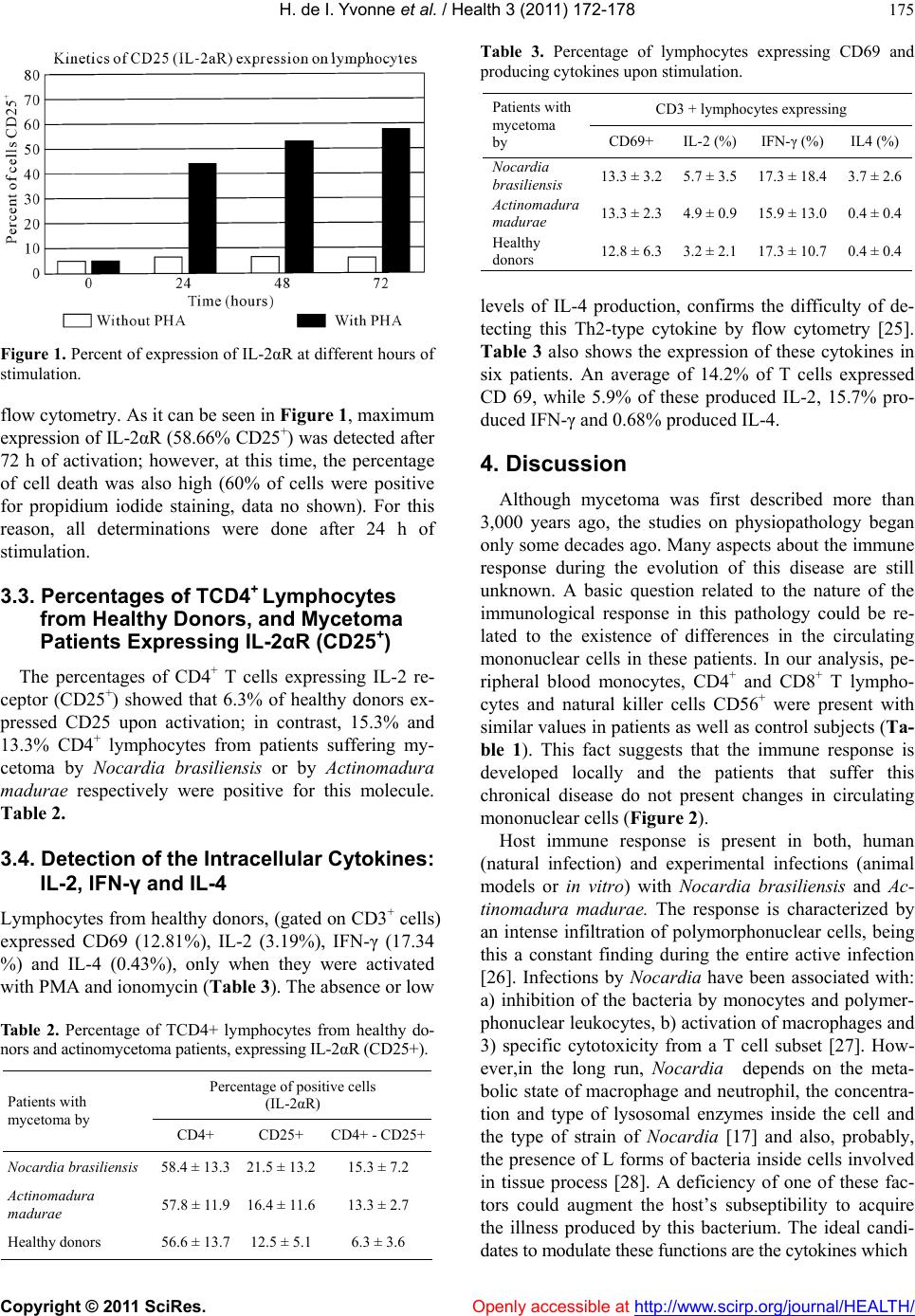 H. de I. Yvonne et al. / Health 3 (2011) 172-178 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 175 Figure 1. Percent of expression of IL-2αR at different hours of stimulation. flow cytometry. As it can be seen in Figure 1, maximum expression of IL-2αR (58.66% CD25+) was detected after 72 h of activation; however, at this time, the percentage of cell death was also high (60% of cells were positive for propidium iodide staining, data no shown). For this reason, all determinations were done after 24 h of stimulation. 3.3. Percentages of TCD4+ Lymphocytes from Healthy Donors, and Mycetoma Patients Expressing IL-2αR (CD25+) The percentages of CD4+ T cells expressing IL-2 re- ceptor (CD25+) showed that 6.3% of healthy donors ex- pressed CD25 upon activation; in contrast, 15.3% and 13.3% CD4+ lymphocytes from patients suffering my- cetoma by Nocardia brasiliensis or by Actinomadura madurae respectively were positive for this molecule. Table 2. 3.4. Detection of the Intracellular Cytokines: IL-2, IFN-γ and IL-4 Lymphocytes from healthy donors, (gated on CD3+ cells) expressed CD69 (12.81%), IL-2 (3.19%), IFN-γ (17.34 %) and IL-4 (0.43%), only when they were activated with PMA and ionomycin (Table 3). The absence or low Table 2. Percentage of TCD4+ lymphocytes from healthy do- nors and actinomycetoma patients, expressing IL-2αR (CD25+). Percentage of positive cells (IL-2αR) Patients with mycetoma by CD4+ CD25+ CD4+ - CD25+ Nocardia brasiliensis 58.4 ± 13.321.5 ± 13.2 15.3 ± 7.2 Actinomadura madurae 57.8 ± 11.916.4 ± 11.6 13.3 ± 2.7 Healthy donors 56.6 ± 13.712.5 ± 5.1 6.3 ± 3.6 Table 3. Percentage of lymphocytes expressing CD69 and producing cytokines upon stimulation. CD3 + lymphocytes expressing Patients with mycetoma by CD69+ IL-2 (%) IFN-γ (%) IL4 (%) Nocardia brasiliensis 13.3 ± 3.25.7 ± 3.5 17.3 ± 18.43.7 ± 2.6 Actinomadura madurae 13.3 ± 2.34.9 ± 0.9 15.9 ± 13.00.4 ± 0.4 Healthy donors 12.8 ± 6.33.2 ± 2.1 17.3 ± 10.70.4 ± 0.4 levels of IL-4 production, confirms the difficulty of de- tecting this Th2-type cytokine by flow cytometry [25]. Table 3 also shows the expression of these cytokines in six patients. An average of 14.2% of T cells expressed CD 69, while 5.9% of these produced IL-2, 15.7% pro- duced IFN-γ and 0.68% produced IL-4. 4. Discussion Although mycetoma was first described more than 3,000 years ago, the studies on physiopathology began only some decades ago. Many aspects about the immune response during the evolution of this disease are still unknown. A basic question related to the nature of the immunological response in this pathology could be re- lated to the existence of differences in the circulating mononuclear cells in these patients. In our analysis, pe- ripheral blood monocytes, CD4+ and CD8+ T lympho- cytes and natural killer cells CD56+ were present with similar values in patients as well as control subjects (Ta- ble 1). This fact suggests that the immune response is developed locally and the patients that suffer this chronical disease do not present changes in circulating mononuclear cells (Figure 2). Host immune response is present in both, human (natural infection) and experimental infections (animal models or in vitro) with Nocardia brasiliensis and Ac- tinomadura madurae. The response is characterized by an intense infiltration of polymorphonuclear cells, being this a constant finding during the entire active infection [26]. Infections by Noca rdia have been associated with: a) inhibition of the bacteria by monocytes and polymer- phonuclear leukocytes, b) activation of macrophages and 3) specific cytotoxicity from a T cell subset [27]. How- ever,in the long run, Nocardia depends on the meta- bolic state of macrophage and neutrophil, the concentra- tion and type of lysosomal enzymes inside the cell and the type of strain of Nocardia [17] and also, probably, the presence of L forms of bacteria inside cells involved in tissue process [28]. A deficiency of one of these fac- tors could augment the host’s subseptibility to acquire the illness produced by this bacterium. The ideal candi- dates to modulate these functions are the cytokines which 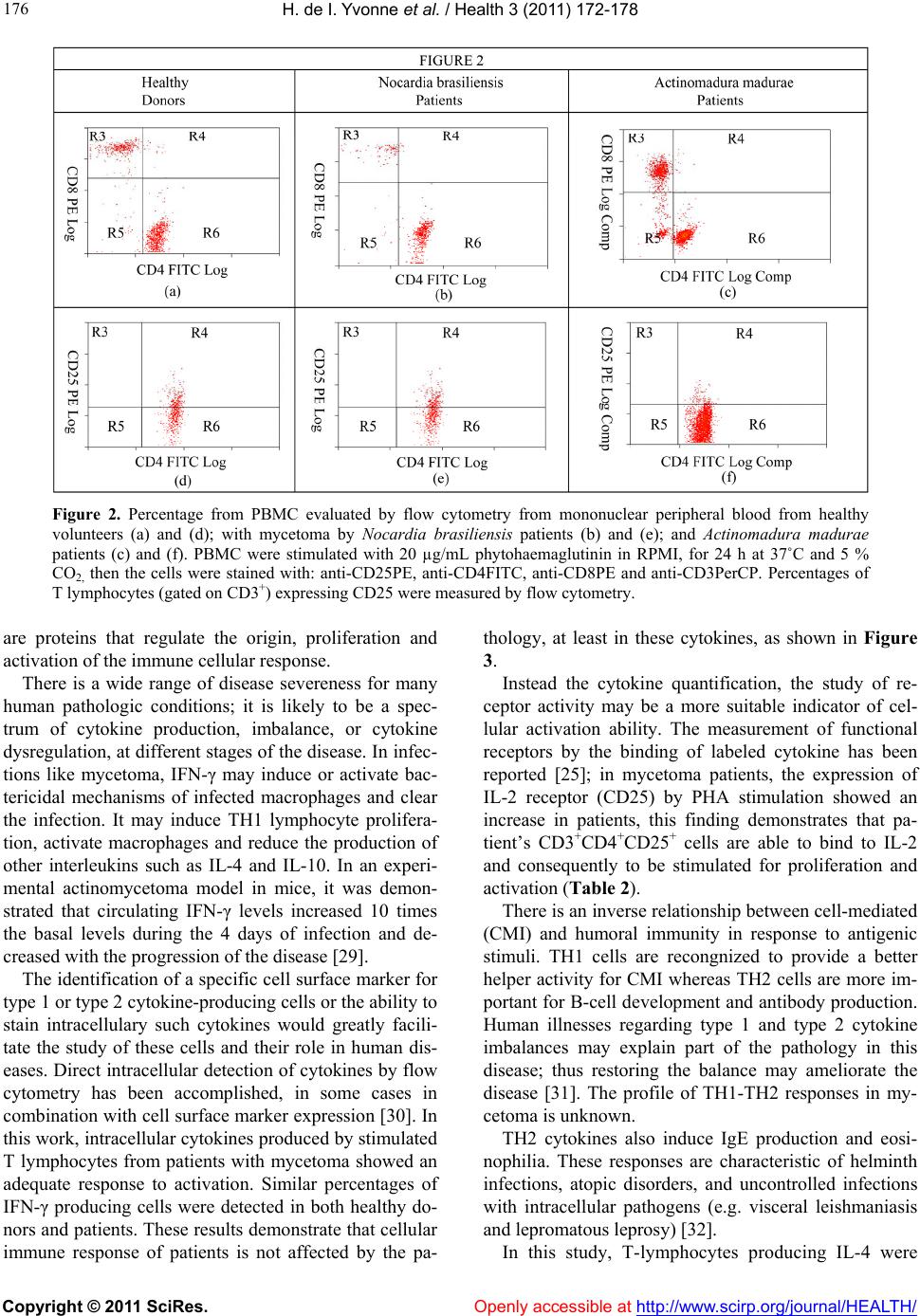 H. de I. Yvonne et al. / Health 3 (2011) 172-178 Copyright © 2011 SciRes. Openl y accessible at http://www. scirp.org/journal/HEALTH/ 176 Figure 2. Percentage from PBMC evaluated by flow cytometry from mononuclear peripheral blood from healthy volunteers (a) and (d); with mycetoma by Nocardia brasiliensis patients (b) and (e); and Actinomadura madurae patients (c) and (f). PBMC were stimulated with 20 µg/mL phytohaemaglutinin in RPMI, for 24 h at 37˚C and 5 % CO2, then the cells were stained with: anti-CD25PE, anti-CD4FITC, anti-CD8PE and anti-CD3PerCP. Percentages of T lymphocytes (gated on CD3+) expressing CD25 were measured by flow cytometry. are proteins that regulate the origin, proliferation and activation of the immune cellular response. There is a wide range of disease severeness for many human pathologic conditions; it is likely to be a spec- trum of cytokine production, imbalance, or cytokine dysregulation, at different stages of the disease. In infec- tions like mycetoma, IFN-γ may induce or activate bac- tericidal mechanisms of infected macrophages and clear the infection. It may induce TH1 lymphocyte prolifera- tion, activate macrophages and reduce the production of other interleukins such as IL-4 and IL-10. In an experi- mental actinomycetoma model in mice, it was demon- strated that circulating IFN-γ levels increased 10 times the basal levels during the 4 days of infection and de- creased with the progression of the disease [29]. The identification of a specific cell surface marker for type 1 or type 2 cytokine-producing cells or the ability to stain intracellulary such cytokines would greatly facili- tate the study of these cells and their role in human dis- eases. Direct intracellular detection of cytokines by flow cytometry has been accomplished, in some cases in combination with cell surface marker expression [30]. In this work, intracellular cytokines produced by stimulated T lymphocytes from patients with mycetoma showed an adequate response to activation. Similar percentages of IFN-γ producing cells were detected in both healthy do- nors and patients. These results demonstrate that cellular immune response of patients is not affected by the pa- thology, at least in these cytokines, as shown in Figure 3. Instead the cytokine quantification, the study of re- ceptor activity may be a more suitable indicator of cel- lular activation ability. The measurement of functional receptors by the binding of labeled cytokine has been reported [25]; in mycetoma patients, the expression of IL-2 receptor (CD25) by PHA stimulation showed an increase in patients, this finding demonstrates that pa- tient’s CD3+CD4 +CD2 5+ cells are able to bind to IL-2 and consequently to be stimulated for proliferation and activation (Table 2). There is an inverse relationship between cell-mediated (CMI) and humoral immunity in response to antigenic stimuli. TH1 cells are recongnized to provide a better helper activity for CMI whereas TH2 cells are more im- portant for B-cell development and antibody production. Human illnesses regarding type 1 and type 2 cytokine imbalances may explain part of the pathology in this disease; thus restoring the balance may ameliorate the disease [31]. The profile of TH1-TH2 responses in my- cetoma is unknown. TH2 cytokines also induce IgE production and eosi- nophilia. These responses are characteristic of helminth infections, atopic disorders, and uncontrolled infections with intracellular pathogens (e.g. visceral leishmaniasis and lepromatous leprosy) [32]. In this study, T-lymphocytes producing IL-4 were 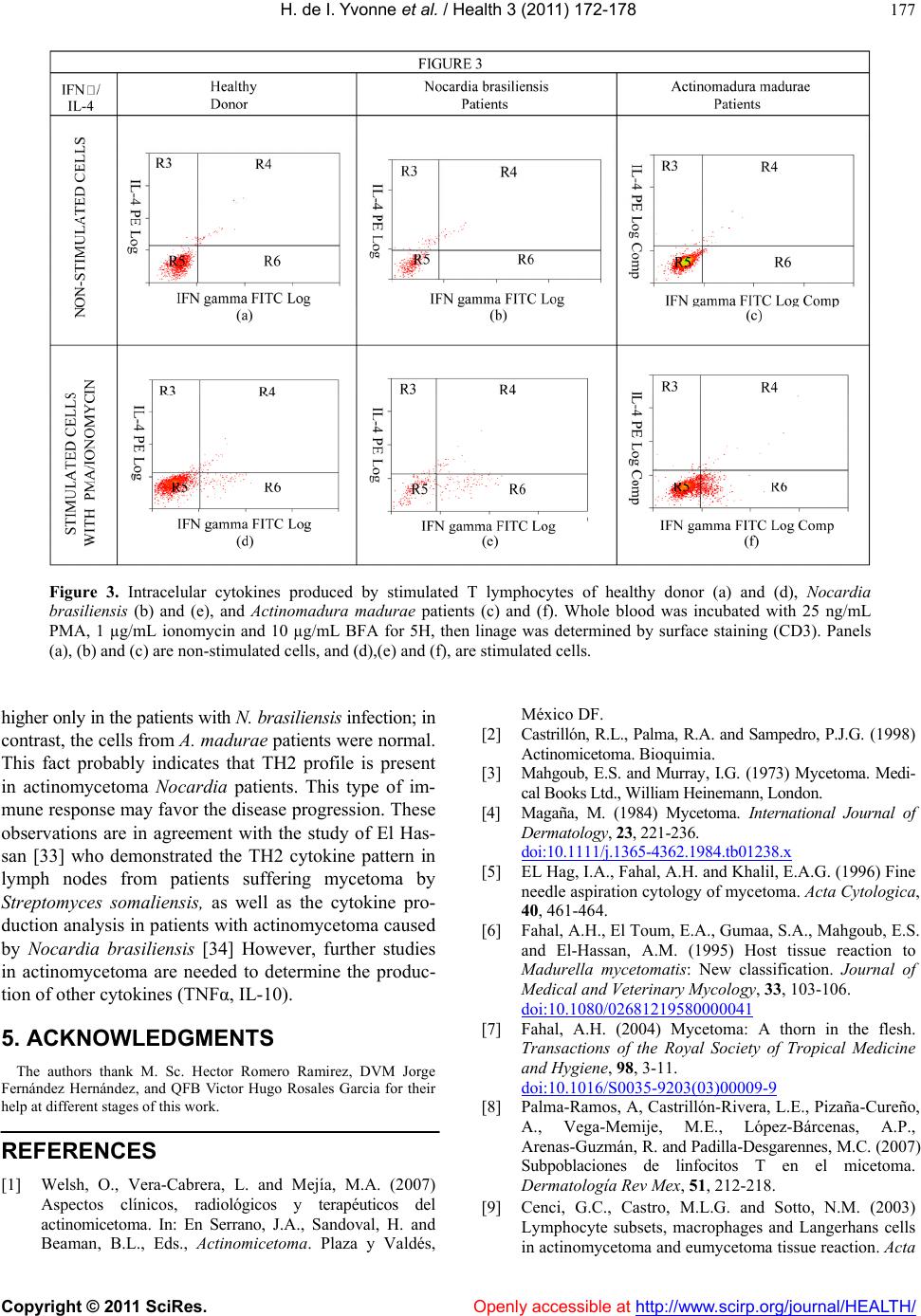 H. de I. Yvonne et al. / Health 3 (2011) 172-178 Copyright © 2011 SciRes. Openl y accessible at http://www. scirp.org/journal/HEALTH/ 177 Figure 3. Intracelular cytokines produced by stimulated T lymphocytes of healthy donor (a) and (d), Nocardia brasiliensis (b) and (e), and Actinomadura madurae patients (c) and (f). Whole blood was incubated with 25 ng/mL PMA, 1 µg/mL ionomycin and 10 µg/mL BFA for 5H, then linage was determined by surface staining (CD3). Panels (a), (b) and (c) are non-stimulated cells, and (d),(e) and (f), are stimulated cells. higher only in the patients with N. brasiliensis infection; in contrast, the cells from A. madurae patients were normal. This fact probably indicates that TH2 profile is present in actinomycetoma Nocardia patients. This type of im- mune response may favor the disease progression. These observations are in agreement with the study of El Has- san [33] who demonstrated the TH2 cytokine pattern in lymph nodes from patients suffering mycetoma by Streptomyces somaliensis, as well as the cytokine pro- duction analysis in patients with actinomycetoma caused by Nocardia brasiliensis [34] However, further studies in actinomycetoma are needed to determine the produc- tion of other cytokines (TNFα, IL-10). 5. ACKNOWLEDGMENTS The authors thank M. Sc. Hector Romero Ramirez, DVM Jorge Fernández Hernández, and QFB Victor Hugo Rosales Garcia for their help at different stages of this work. REFERENCES [1] Welsh, O., Vera-Cabrera, L. and Mejía, M.A. (2007) Aspectos clínicos, radiológicos y terapéuticos del actinomicetoma. In: En Serrano, J.A., Sandoval, H. and Beaman, B.L., Eds., Actinomicetoma. Plaza y Valdés, México DF. [2] Castrillón, R.L., Palma, R.A. and Sampedro, P.J.G. (1998) Actinomicetoma. Bioquimia. [3] Mahgoub, E.S. and Murray, I.G. (1973) Mycetoma. Medi- cal Books Ltd., William Heinemann, London. [4] Magaña, M. (1984) Mycetoma. International Journal of Dermatology , 23, 221-236. doi:10.1111 /j.13 65-43 62. 1984. tb 01238. x [5] EL Hag, I.A., Fahal, A.H. and Khalil, E.A.G. (1996) Fine needle aspiration cytology of mycetoma. Acta Cytologica, 40, 461-464. [6] Fahal, A.H., El Toum, E.A., Gumaa, S.A., Mahgoub, E.S. and El-Hassan, A.M. (1995) Host tissue reaction to Madurella mycetomatis: New classification. Journal of Medical and Veterinary Mycology, 33, 103-106. doi:10.1080/02681219580000041 [7] Fahal, A.H. (2004) Mycetoma: A thorn in the flesh. Transactions of the Royal Society of Tropical Medicine and Hygiene, 98, 3-11. doi:10.1016/S0035-9203(03)00009-9 [8] Palma-Ramos, A, Castrillón-Rivera, L.E., Pizaña-Cureño, A., Vega-Memije, M.E., López-Bárcenas, A.P., Arenas-Guzmán, R. and Padilla-Desgarennes, M.C. (2007) Subpoblaciones de linfocitos T en el micetoma. Dermatología Rev Mex, 51, 212-218. [9] Cenci, G.C., Castro, M.L.G. and Sotto, N.M. (2003) Lymphocyte subsets, macrophages and Langerhans cells in actinomycetoma and eumycetoma tissue reaction. Acta  H. de I. Yvonne et al. / Health 3 (2011) 172-178 Copyright © 2011 SciRes. Openl y accessible at http://www. scirp.org/journal/HEALTH/ 178 Tropica, 87, 377-384. doi:10.1016/S0001-706X(03)00139-6 [10] Humpreys, D.W., Crowder, J.G. and White, A. (1975) Serological reactions to Nocardia antigens. Ameri ca n Journal of Science, 269, 323-336. [11] Salinas-Carmona, M.C. (2001) Anticuerpos anti-Nocardia brasiliensis en pacientes con actinomicetoma y su utilidad clínica. Gac Med Mex, 137, 1-8. [12] Salinas-Carmona, M.C. and Pérez-Rivera, I. (2004) Hu- moral Immunity through IgM protects mice from an ex- perimental mycetoma infection by Nocardia brasiliensis. Infection and Immunity, 72, 5597-5604. doi:10.1128/IAI.72.10.5597-5604.2004 [13] Araujo, M.J. and Castaneda, E. (1997) Madurella myce- tomatis for the serodiagnosis of mycetoma. Revista Iberoamericana de Micología, 14, 31-35. [14] Ortíz-Ortíz, L. and Bojalil, L.F. (1972) Delayed skin reactions to cytoplasmic estracts of Nocardia organisms as a means of diagnosis and epidemiological study of Nocardia infections. Clinical & Experimental Immunol- ogy, 12, 225-229. [15] Sundararag, T. and Agarwal, S.C. (1977) Cell-mediated immunity in experimental Nocardia asteroides infection. Infection and Immunity, 15, 370-375. [16] Mahgoub, E.S., Gumaa, D.A. and El Hassan, A.M. (1977) Immunological status of mycetoma patients. Bull Soc Pathol Exot., 70, 48-54. [17] Beaman BL, Beaman L. Nocardia species: Host-parasite relationships. Clin Microbiol Rev. 1994; 7:48-54. [18] Beaman BL. Possible mechanisms of nocardial patho- genesis. In: Biology of the nocardiae pag 386-417. Ed Brownell G, Goodfellow M, Serrano JA. Academic Press London 1976. [19] Beaman BL, Black C. Interaction of Nocardia asteroides in BALB/c mice: Modulation of macrophage function, enzyme acvtivity and the induction of immunologically specific T-cell bactericidal activity. Curr Topics Microbiol. 1985; 122:138-147. [20] Castrillón RLE, Palma RA, Sampedro PGJ. Interacción de Actinomadura madurae con macrófagos peritoneales murinos. Rev Centro Dermatol Pascua. 1998; 7:37-43. [21] Palma RA, Cuevas MMV, Areanas GR, Vega MME, Castrillón RLE. Diseminación hematógena por Nocardia brasiliensis en tres ratones Balb/c a partir de un micetoma en cojinete plantar. Dermatol Rev Mex. 2008; 52:70-76. [22] López BAP, Arenas GA, Vega MME, Castrillón RLE, Palma RA. Identificación de células y mediadores in- flamatorios en lesiones de pacientes con diagnóstico de micetoma. Dermatol Rev Mex. 2008; 52:247-53. [23] Testi R, D’Ambrosio D, De Maria R, Santoni A. The CD69 receptor: a multipurpose cell-surface tigger for hematopoietic cells. Immunol Today. 1994; 15:479-483. doi:10.1016/0167-5699(94)90193-7 [24] Garibay-Escobar, A., Estrada-García, I., Estrada-Parra, S., Santos-Argumedo, L. Integrated measurements by flow cytometry of the cytokines IL-2, IFN-γ, IL-12, TNF-α and functional evaluation of their receptors in human blood. J Immunol. Methods. 2003; 280:73-88. doi:10.1016/S0022-1759(03)00249-7 [25] Collins, D. O. Cytokine and cytokine receptor expression as a biological indicator of immune activation: important considerations in the development of in vitro model sys- tems. J Immunol Methods. 2000; 243:125. doi:10.1016/S0022-1759(00)00218-0 [26] Salinas-Carmona MC. Nocardia brasiliensis: from mi- crobe to human and experimental infections. Microbes and Infect. 2000; 2:1373-1381. doi:10.1016/S1286-4579(00)01291-0 [27] Calcagno M. Status inmunológico del paciente con micetoma. Dermatol Ven 1989: 27:49-52. [28] Beaman BL, Scates SM. Role of L-forms of Nocardia caviae in the development of chronic mycetomas in nor- mal and immunodeficient murine models". Infect. Immun. 1981, 33 (3): 893-907. [29] Salinas-Carmona MC, Torres-López E, Ramos AI, Lincon-Trujillo González Spencer D. Immune response to Nocardia brasiliensis antigens in an experimental model in actinomicetoma in Balb/c mice. Infect Immun. 1999; 67:2428-2432. [30] Elson HL, Nutman BT, Metcalfe DDF, Prussin C. Flow cytometry analysis for cytokine production identifies T helper 1, T helper 2 and T helper 0 cells within the hu- man CD4 + CD27- lymnphocyte subpopulation. J Im- munol. 1995; 154:4294-4301. [31] Lucey RD, Clerici M,Shearer MG. Type 1 and Type 2 cytokine dysregulation in human infectious, neoplastic and inflammatory disease. Clin Microbiol Rev. 1996; 9:532-562. [32] Yamamura M, Uyemura K Deans RJ, Weinberg K, Rea TH, Bloom RB, Moldin RL. Defining protective reponses to pathogens: cytokine profiles in leprosy lesions. Sci- ence 1991; 254:277-279. doi:10.1126/science.1925582 [33] El Hasssan AM, Fahal AH, Ahmed AO, Ismail A, Veress B. The immunopathology of actinomycetoma lesions caused by Streptomyces somaliensis. Trans R Soc Trop Hyg 2001; 95:89-92. doi:10.1016/S0035-9203(01)90346-3 [34] Méndez-Tovar LJ, Mondragón-López R, Vega-López F, Dockrell MH, Hay R, López-Martínez R, Manzano- - Gayosso P, Hérnández-Hernández F, Padilla-Desgarennes C, Bonifaz A. Cytokine production and lymphocyte pro- liferation in patients with Nocardia brasiliensis actino- mycetoma. Mycopathologia 2004; 58:407-414. |

