 Psychology 2014. Vol.5, No.1, 47-52 Published Online January 2014 in SciRes (http://www.scirp.org/journal/psych) http://dx.doi.org/10.4236/psych.2014.51009 Dysexecutive Syndrome in a Patient with Wilson’s Disease Nataly Gutiérrez-Ávila1, Jimmy Zúñiga-Márquez1, Natalia Burgos-Torres1, John Aria s -Valencia1, Patricia Quintero-Cusguen2, Rocio Acosta-Barreto 1 1Faculty of Psychology, San Buenaventura Bogotá University, Neuropsychology Master and Specialization Program, Bogotá, Colombia 2Universitary Hospital La Samaritana, Bogotá, Colombia Email: rocioacosta93@yahoo.com, psnatalygutierrez@hotmail.com Received August 30th, 2013; revised September 28th, 2013; accepted October 25th, 2013 Copyright © 2014 Nataly Gutiérrez-Ávila et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved f or SCIRP and the owner of the intellectual property Nataly Gutié rrez-Ávila et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian. Clinical Case Report: This paper presents the alterations and deficits in executive functions of a 33 years old man with Wilson’s disease, patient at the Hospital Universitario La Samaritana in Bogotá, who in 2011 was diagnosed with cerebellar ataxia, and beginning to show a clinical picture of dysarthria and generalized motor difficulties. The presence of neuropsychological disorders, as well as the results ob- tained by Magnetic Resonance Imaging (MRI) of the brain and optical exam (Kayser-Fleischer rings) confirmed the diagnosis of Wilson’s disease. Results and Conclusion: The neuropsychological profile of the patient showed alterations of attention and mnemonic processes associated with frontal functioning, as well as slowing-down of motor activities and low speed in processing information. The assessment of ex- ecutive functions revealed impairment in cognitive flexibility, impulsivity and disinhibition, as well as difficulties in the process of planning, organizing and monitoring. All of these features indicated the presence of a dysexecutive syndrome in the patient and correlated with the results obtained by MRI. Keywords: Wilson’s Disease; Executive Functions; Neuropsychological Profile; Dysexecutive Syndrome Introduction Wilson disease (WD), also known as hepatolenticular dege- neration, is an autosomal recessive hereditary disorder that af- fects copper metabolism causing an excessive accumulation of this chemical in the body (Litwin et al., 2013; Hewlett, 2012; Teleive et al., 2012; Pfeiffer, 2011; Taly, Prashanth, & Sinha, 2009), primarily in the liver, kidney and brain, producing alte- rations and liver damage, neurological and psychiatric disorders. It has been reported that the incidence of the disease is one in every 30,000 live births (Moller et al., 2011) and affects both men and women, although the neurological manifestations are more common in male patients. Regarding the symptoms produced by excess metal in the Central Nervous System (CNS), the most affected areas that show degeneration are the lenticular nucleus, putamen, caudate head, globus pallidus, ventral thalamus, dentate nucleus, mid- brain (except for the red nuclei), pons and in some cases there is cerebellar atrophy. It is commonly observed that on magnetic resonance imaging (MRI) these areas are hyperintense on T2, and with the transcranial sonography tecnique, hyperechoic lesions are found (Svetel, Mijajlovi, Tomiet et al., 2012; Huster et al., 2012; Krysiak, Handzlik-Orlik, & Okopien, 2012; Nagel & Miralles, 2007; Hitoshi, Iwat, & Yoshikawa, 1991). WD presents a wide spectrum of manifestations depending on the areas affected by the deposition of metal which are: the liver form, the neurological and psychiatric forms and the form with predominance of other organs (Roberts, 2011; Xu et al., 2010; López & Serrano, 2007; Wilson, Alderman, Burgess, Emslie, & Evans, 2003). Litwin, Gromadzka, Członkowska, et al. (2012) conducted a retrospective study of 204 cases with WD and found that the most common type is the neuropsy- chiatric with 105 cases, followed by the liver type with 67, and by the type with predominance of other organs, with 32 of the 204 cases studied. The neurological form of the disease, is particularly charac- terized by abnormal movements, postural and intentional tre- mor, chorea, athetosis, myoclonus, seizures, ataxia, dysarthria, hypophagia, hypersalivation, pyramidal signs and abnormalities of eye movement (European Association for the Study of the Liver-EASL, 2012; Svetel, Mijajlovi, Tomiet, et al., 2012). As the disease progresses it is common to find a more complex clinical picture (Lorincz, 2010). With respect to the WD neuropsychological profile there is little information at present. The majority of articles, research and scientific texts make a description of the general aspects of the neurocognitive profile (Carta et al., 2012; Birk et al., 2011; Jacob & Srivatsa, 2011; Levy & Dubois, 2006). In a study by Hegde, Sinha, Taly & Vasudev (2010), where he presented the cognitive profile of twelve patients contrasting it with MRI diagnostic studies and the signs and symptoms reported, a greater impairment was found in motor speed (73%), verbal working memory and focused and sustained attention (50%), verbal learning (42%), processing speed (33% - 34%), visual memory and verbal fluency (25% - 27%) and verbal recognition (17%). OPEN ACCESS 47 N. GUTIÉRREZ-ÁVILA ET AL. Schmitt et al. (2011), in a 40 years follow-up study with 36 patients diagnosed with WD reported that 25% of the patients had neuropsychiatric manifestations where the common symp- toms were attention deficit, changes in personality, irritability and hypersomnia. Other studies attribute the changes in mood and personality to a dopamine deficit present in the disease (Litwin, Gromadzka, Członkowska, Poniatowska, & Poniato- wska, 2013; Günther, Villmann & Hermann, 2011; Schmitt et al., 2011; Popević, Kisić, Đukić, & Bulat, 2011; Hegde, Sinha, Taly, & Vasudev, 2010). With respect to executive functions (EF) and Wilson’s di- sease, there is no conclusive research but there is an evidence that regarding the relationship between frontal lobes and basal ganglia, a relationship can be established with the presence of alterations in the executive functioning (Pladdy, 2007). The EF is dynamic processes which have a high degree of dependence and is performed by different domains or neural network interconnections that work as a unit involving a set of cognitive skills related to processes of programming, regulation and planning of the necessary behavior to achieve an adequate level of adjustment and problem solving related to various cor- tical-sub cortical areas of the Central Nervous System for its correct functioning. Alterations due to damage or injury of its structures and networks result in the presence of low capacity for self-control or self-direction, emotional lability, increased tendency to irri- tability and excitability, impulsivity, erratic behavior, stiffness and deterioration in self-care and grooming (Lezak, 1995; Ver- dejo-García y Tirapu-Ustárroz, 2012). Several studies have shown that executive functions are not totally dependent on the prefrontal cortex, but there is a net- work of neural interconnections that interact simultaneously involving other areas of the CNS, especially connections with subcortical structures such as basal ganglia (Suchy, 2009; Elliot, 2013). Additionally, it has been established that there is an interac- tion between the basal ganglia and other subcortical structures such as the thalamus, subthalamic nucleus and globus pallidus in the conformation and configuration of circuits that interact with the frontal lobe for generating a regulated, controlled and supervised behavior of executive functions (Verma, Patil, & Lalla, 2012; Verdejo & Bechara, 2010; Suchy, 2009). Thus, it has currently been established that the Executive Functions depend on the integration of network circuits be- tween the prefrontal cortex and the cortical subcortical struc- tures and not just on one specified region. Lesions in these net- works, even without impairment of the frontal lobe, can gener- ate dysexecutive clinical pictures characteristic of prefrontal syndromes as evidenced in progressive supranuclear palsy, multiple system atrophy and Huntington’s disease. Therefore, frontostriatal circuits are important in mediating executive function (Elliot, 2013). This case study aims to show the main changes in cognitive processes presented in a patient with WD, highlighting the al- terations or deficits in executive functions and their correlation with neuroanatomical findings obtained by brain MRI. The case study allows us to observe that the impairment in executive functions presented in the WD is not due to injuries in frontal regions as commonly occurs, but to lesions in the intercommu- nication Prefrontal Cortex-Basal Ganglia—Prefrontal Cortex. Method Desing A single case study was carried out, using a descriptive de- sign and following an analytical e mpirical methodology. This methodology was applied to a participant to assess his cognitive functions, focusing the process on his executive functions (Hernández, Fernández, & Baptista, 2006). Participant Male patient, aged 33, right laterality, with technical profes- sional education level, currently unemployed. He has a history of psychoactive substances consumption, specifically ethanol, with an intake frequency of 3 to 4 times per week which per- sisted for about 10 years. Consumption is terminated due to the initiation of the major symptoms of the disease. Similarly, there is history of non-abusive social use of cocaine for a year. In 2011 is diagnosed with cerebellar ataxia, initiating a clinical picture of dysarthria and progressive motor impairment. The patient is admitted to a hospital with a clinical picture of ataxia, dysarthria, hypersalivation, hypophagia and constant mood change. He goes through a multidisciplinary assessment process which found, from the dermatology area, copper accu- mulation after performing a hyperpigmentation biopsy in lower limbs. In the different diagnostic tests performed by the internal medicine staff, accumulation of this metal is found. The gas- troenterology examination gives evidence of liver damage. From the area of ophthalmology, the Kayser-Fleischer rings are found (See Figure 1). From the neurology area, a brain MRI was performed to the patient. Findings show hyperintense lesions in the basal ganglia (Figure 2) and midbrain (Figure 3), characteristic signs of the disease. The frontal lobes were found intact. Procedure This research was conducted in three phases. In the initial phase, an identification of the case was made at medical and neurological level. Intake interview and signature of the in- formed consent were carried out. In the second phase, the neu- ropsychological assessment took place. And in the final phase, the analysis of results and preparation of the final document were done. Instruments Brief Neuropsychological Battery in Spanish, Neuropsi: This is a screening test. In order to carry out its standardization, it was applied to a sample of 800 neurologically intact subjects aged between 16 and 85 years. The sample was divided into four groups according to age and educational level. Reliability was assessed by the test-retest method, with an interval of three months between each application and marking was done by dif- ferent assessors. Reliability between examiners was 0.89 to 0.95. This instrument allows assessing a wide range of cogni- tive functions in patients with psychiatric, geriatric, neurologi- cal and various medical problems (Ardila & Ostrosky, 2012). Trail Making Test—TMT—Parts A and B: It is considered that Part A measures motor, viso-spatial skills of visual search and sustained attention, while Part B, additionally involves me- ntal flexibility and divided attention. The test-retest reliability varies greatly according to the specific study (0.60 to 0.90), OPEN ACCESS 48 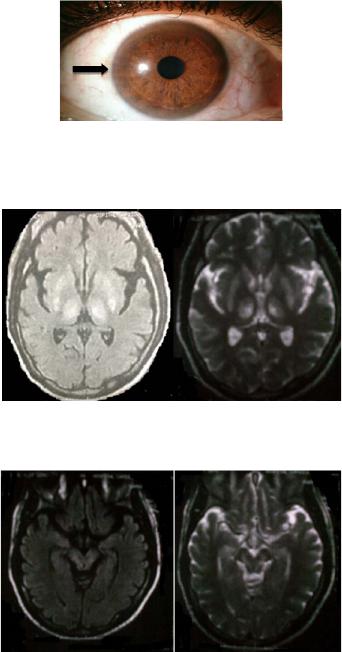 N. GUTIÉRREZ-ÁVILA ET AL. Figure 1. Kayser Fleischer Ring in the pa- tient. Figure 2. MRI, hyperintense lesions in basal ganglia. Figure 3. MRI hyperintense lesion s on T2 at midb rain level in patient with WD. depending on the part or applied version and the pathology (greater variability in schizophrenics, more stability in patients with vascular disease). The temporal stability of the B-A dif- ference is 0.71. The educational level has a significant influ- ence on the scores, especially in Part B. No gender differences were found. According to studies conducted, there is a signifi- cant correlation between the test score (both parts) and the de- gree of atrophy of the caudate nucleus (Ardila & Ostrosky, 2012). Stroop D-Kefs: It is used to assess inhibition in executive functions. The reliability of the test is carried out by the test- retest method, being 0.84. Likewise, it is marked by different assessors and obtains significant correlations (Lopez, Serrano, Llano, Mateos , López, & Sánchez, 2010). Wisconsin Card Sorting Test, WCST: neuropsychological test used to assess cognitive flexibility. It is especially sensitive to lesions involving the frontal lobes. The reliability was con- ducted from a test-retest with a correlation of 0.93 for perse- verant responses, 0.92 for perseverant errors, 0.88 for non- perseverant errors, and concurrent validity of 70% (Scheres et al., 2004). Visual-verbal Memory Curve by Ardila: A list of twelve words (animals, fruits, and body parts) is presented in three trials. After each trial the subject is asked to tell all the words he can remember. Also, intrusions and perseverations are re- corded, as well as primacy and recency effects (Ardila & Os- trosky, 2012). Chip Test: the purpose of this test is to assess the under- standing of verbal instructions of increasing complexity. The application consists of presenting the chips to the patient and asking him the respective questions which must be made clearly and slowly. Words must not be emphasized. The ad- ministration consists of 5 parts, only in Part A and B instruction can be repeated once (Ardila & Ostrosky, 2012). Wechsler Adult Intelligence Scale, WAIS III: The Wechsler Intelligence Scale for adults consists of a Verbal Scale and a Performance Scale, so that the application provides three scores: a Verbal IQ, Performance IQ or Manual IQ, and a Full Scale IQ. The subtests used were: block design which measures vis- ual-motor coordination, perception, capacity for analysis, syn- thesis, logic and reasoning. And the matrices subtest, that was used specifically to determine the attention and planning capac- ity. Test of emotion recognition in faces by Baron Cohen (Faces Test) and moral dilemmas of the theory of the mind. Results It was found that the patient showed slowness in the speed of information processing, disinhibition, hypersexuality, perse- vering behaviors, cognitive rigidity, difficulty to establish or follow sequences, and failure in the development of strategies for achieving goals. Additionally, he presents emotional lability, low frustration tolerance and impairment in the abstraction capacity, in encoding and recalling both verbal and visual stim- uli mediated by his executive functioning. On the contrary, his long term memory both episodic and semantic is preserved (Table 1). These findings account for a clinical picture of dysexecutive syndrome in the patient, which was not the by-product of the damage in the frontal structures, but in the cortico-subcortical connections between basal ganglia and frontal lobes. Discussion The alterations found in the patient’s cognitive functions, which correspond to a dysexecutive syndrome, may be associ- ated with possible failure in the connections between basal ganglia and frontal lobes. That is why dysexecutive syndrome is a trigger for alterations involving different cognitive areas (Ardila, 2008). Results of this study are similar to those reported by Clark, Collazo, Ruenes & García (2011) who showed that patients with a neurological form of WD had no major impairment in basal ganglia, but they did in their interconnections, showing poor operating performance on executive functioning, memory, visuospatial processing and verbal and abstract reasoning. Many years ago, the research by Posner & Raichle (1994), identified the existence of interconnected neural networks to describe attention processes. They suggested that the central control mechanism of the alert system, the orientation system and the executive system forms a dependent circuit, where each one of them is responsible for a specific task in the attention process, but interacting dynamically and integrating into the executive system the interconnected networks of the frontal lobe, the anterior cingulate and the basal ganglia, which makes cognitive functions also interdependent in this process. In summary, the present case describes a patient who shows neurological involvement both in memory and attention medi- ated by the executive dysfunction which decreases the possibil- OPEN ACCESS 49  N. GUTIÉRREZ-ÁVILA ET AL. Table 1. Results of neuropsychological test. Neuropsy Score Score Normal 89 112 - 102 Trail Making Test A Time Score Normal 164 seconds 27 - 32 seconds Trail Making Test B Time Score Normal 677 seconds 56 - 69 seconds WAIS III Direct Score Normal Score Cubes 4 - 8 3 Matrices 11 - 12 8 Wisconsin Card Sorting Test Score Normal Score Trials 128 72 Categories 1 5 Corrects 62 67 Perseverative Errors 41 (32%) (5%) Conceptual level 40 67 Ardila`s Verbal Memory C urve Score Normal Score Initial Volume 5 7 ± 2 Maximum Volume 9 10 Trials 10 4 Evocation 3 ( 20) minutes 9 (5) 10 (8) Ardila`s V i sual Mem ory Curve Score Normal Score Initial Volume 5 7 ± 2 Maximum Volume 10 10 Trials 3 4 Evocation 3 ( 20) minutes 9 (8) 10 (8) Test of emotion recognition in faces Corrects Errors 17/20 Guilt—Anger/Boredom—Fear ity of making new effective learning, develops appropriate en- coding and recalling strategies, inhibits irrelevant information effectively, holds his attention on the tasks and foster flexibility. All of these impairments can be attributed more to a dysfunc- tion in the cortical-subcortical neuronal interconnection which interferes with the proper functioning of cognitive processes. The research that gives account of affectations on cognitive functions in WD suggests a deficit in different areas of these operations. However, considering the role of basal ganglia— executive functions, it is proposed that the main neuropsy- chological deficit caused in WD lies in executive functions. In similar profiles to the one reported in this study the other cog- nitive impairments evidenced are secondary to such a deficit. Although the description of executive functions and neuro- psychological involvement made in this study is similar to the descriptions made by other researchers, it diffe rs that it empha- sizes the relationship between cortical and subcortical struc- tures in the conformation of the dysexecutive clinical picture present in WD and points out that the impairments in other domains are secondary to this one. On the other hand, it is important to note that although the patient has a history of chronic alcohol consumption, and that this condition has some effects on the Central Nervous System —CNS—which, in turn, affects cognitive functions, several studies have found a phenomenon that has been called “deficit recovery following abandonment” (Garrido & Fernandez, 2004), which refers to a decline in cognitive functions immedi- ately to cessation of the substance. But after overcoming the withdrawal symptoms and having abandoned consumption completely, patients are able to recover their functions within approximately one month. However, at present, the patient in this case study has been in abstinence for more than two years which leads to relating his clinical dysexecutive syndrome di- rectly with WD and copper accumulation in the basal ganglia (Bidaki et al., 2012; Carta, Mura, Sorbello, Farina, & Demelia, 2012; Di Stefano, Lionetti, Rotolo, La Rosa, & Leonardi, 2012; Castañeda, Ubilluz, Ávalos, Escalante, & Nicoll, 2002). Finally, it is important to note that the projections and cir- cuits between and from the basal ganglia to the cortex have been extensively described in the literature. It is now known that in addition to their circuits and projections with the frontal lobes there are also connections with other cortical areas. Tar- get projections of the thalamic nuclei, superior colliculus, re- ticular formation and pedunculopontine nucleus are located toward the temporal and parietal areas. Furthermore, it has been reported that such nuclei have a strong participation in the modulation of behavior (Peng, Lutsenko, Sun, & Muzik, 2012; Cheon et al., 2010; Stocco, Lebiere & Anderson, 2010; Bernal & Calle, 2007). This explains the wide variety of cognitive symptoms present in the clinical case shown in this article and the dynamism and complexity of executive processes in the CNS. Acknowledgemen ts First, we wish to express our gratitude and appreciation to the patient and his family for allowing documentation of the case, and thus being able to contribute to the scientific com- munity. Second, to the University Hospital La Samaritana, the MD Yamile Sierra, and to the neurologists Jose Hernandez and Pilar Guerrero. REFERENCES Ardila, A. (2008). On the evolutionary origins of executive functions. Brain and Cognition, 68, 92-99. http://dx.doi.org/10.1016/j.bandc.2008.03.003 Ardila, A., & Ostrosky, F. (2012). Guía para el Diagnóstico Neuro- psicológico. México D.F.: Universidad Nacional Autónoma de México. Bernal, D., & Calle, J. (2007). Aspectos neurop siquiátricos de la enfer- medad de Wilson y la esclerosis múltiple. Revista Colombiana de Psiquiatría, 36, 126-138. Bidaki, B., Zare, M., Mirhosseini, M., Moghadami, S., Hejrati, M., Kohnavard, M., & Shariati, B. (2012). El mal manejo de la enfer- medad de wilson como trastorno psicótico. Advanced Biomedical Research, 1. Birk, L., Horn, N., Dysgaard, T., Vissing, J., Wibrand, F., Jennum, P., & Ott, P. (2011). Clinical presentation and mutations in danish pa- tients with wilson disease. European Journal of Human Genetics, 19, OPEN ACCESS 50 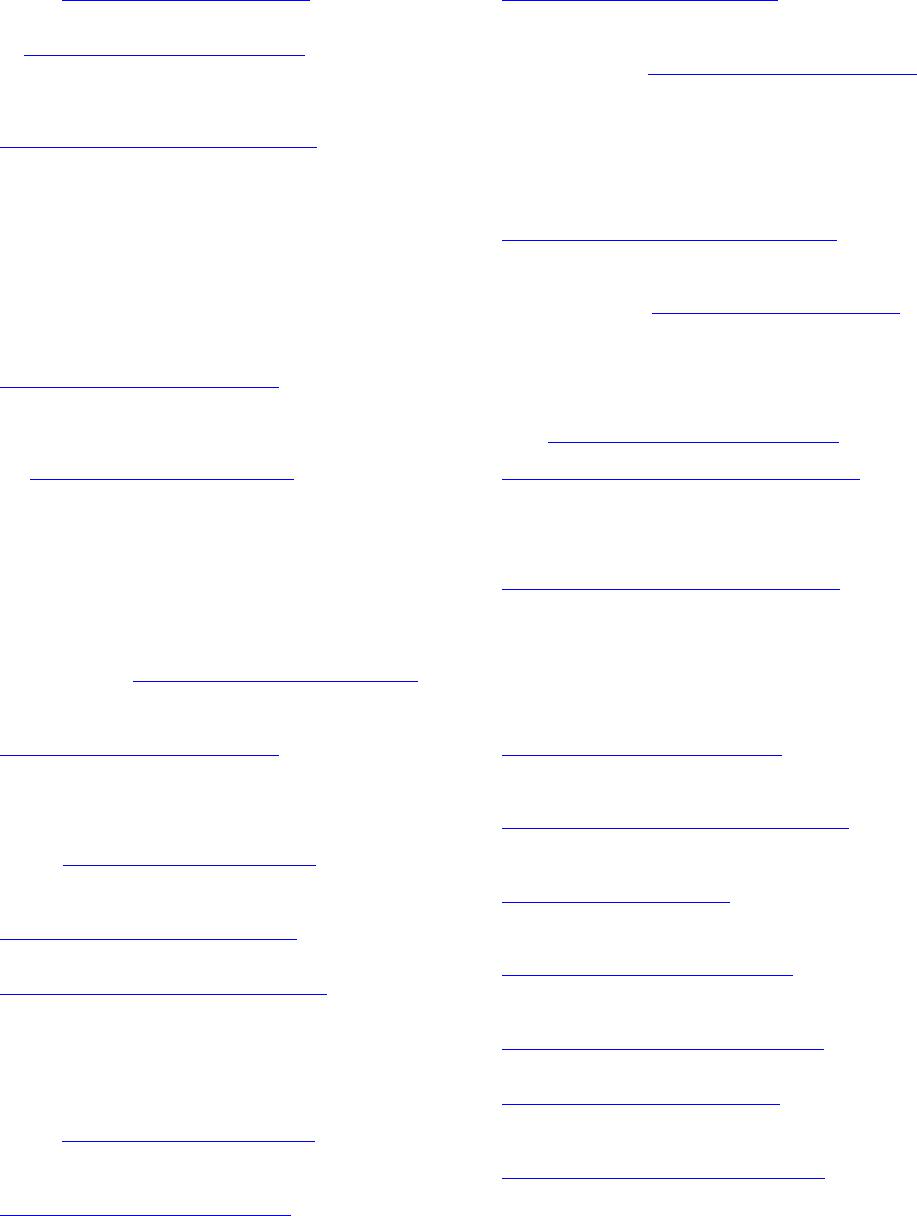 N. GUTIÉRREZ-ÁVILA ET AL. 935-941. http://dx.doi.org/10.1038/ejhg.2011.80 Carta, M., Sorbello, O., Moro, M., Bhat, K., Demelia, E., Serra, A., & Mura, G. (2012). Bipolar disorders and wilson. BMC Psychiatry, 12, 52. http://dx.doi.org/10.1186/1471-244X-12-52 Carta, M., Mura, G., Sorbello, O., Farina, G., & Demelia, L. (2012). Quality of life and psychiatric symptoms in wilson’s disease: The relevance of bipolar disorders. Clinical Practice & Epidemiology in Mental Health, 8, 102-109. http://dx.doi.org/10.2174/1745017901208010102 Castañeda, M., Ubilluz, R., Ávalos, C., Escalante, D., & Nicoll, J. (2002). Enfermedad de wilson: forma nueropsiquiátrica dominante presentación de un caso y su interpr etación fisiop atológica basada en resonancia magnética del encéfalo. Revista de Gas troenterología del Perú, 22, 74-80. Clark, Y., Collazo, T., Ruenes, C., & García, E. (2011). Detección de cambios conformacionales y mutaciones en el exón 8 del gen atp7b en pacientes cubanos con la enfermedad de wilson. Biotecnología Aplicada, 28, 83-86. Cheon, J., Kim, I., Seo , J. K., Ko, J. S ., Lee, J. M., Shin, C., Ki m, W. S ., & Yeon, K. M. (2010). Clinical application of liver MR imaging in wilson’s disease. Korean Journal of Radiology, 11, 665-672. http://dx.doi.org/10.3348/kjr.2010.11.6.665 Di Stefano, V., Lionetti, E., Rotolo, N., La Rosa, M., & Leonardi, S. (2012). Hypercalciuria and nephrocalcinosis as early feature of wil- son disease onset: Description of a pediatric case and literature re- view. International Monthly Journal in the field oh hepatology, 18, 1-4. http://dx.doi.org/10.5812/hepatmon.6233 Elliott, R. (2013). Executive functions and their disorders. British Me- dical Bulletin, 65. European Association for the Study of the Liver (2012). Easl clinical practice guidelines: Wilson’s disease. Journal of Hepatology, 56, 671-685. Garrido, M., & Fernández, S. (2004). Déficit neuropsicológicos en al- cohólicos: Implicaciones para la seguridad vial. Revista de Neu- rologia, 38, 277-283. Günther, P., Vill mann, T., & Hermann, W. (2 011). Even t related p oten- tials and cognitive evaluation in wilson. Journal of Neurological Sciences, 28, 79-85. http://www.jns.dergisi.org/text.php3?id=423 Hegde, S., Sinha, S., Taly, A., & Vasudev, M. (2010). Cognitive profile and structural findings in wilson disease: A neuropsychological and MRI-based study. Neurol I ndia, 58, 708-713. http://dx.doi.org/10.4103/0028-3886.72172 Hernández, R., Fer nánd ez, C., & Bap tist a, P . (2 008 ). Metodología de la investigación (4th ed.). México, México: Mc Graw Hill. Hewlett, A. (2012). Handbook of liver disease (3rd ed., pp. 230-244). Hitoshi, S., Iwata, M., & Yoshikawa, K. (1991). Mid-brain pathology of wilson. Journal of Neurology, Neurosurgery, and Psychiatry, 54, 624-626. http://dx.doi.org/10.1136/jnnp.54.7.624 Huster, D., Kühne, A., Bhattacharjee, A., Raines, L., Ja ntsch, V., Noe, J., Caca, K., & Lutsenko, S. (2012). Diverse functional properties of wilson disease atp7b variants. Gastroenterology, 142, 947-956. http://dx.doi.org/10.1053/j.gastro.2011.12.048 Jacob, R., & Srivatsa, V. (2011). Wilson’s disease presenting with psychosis. Asian Journal of Psychiatry, 4, 68-69. http://dx.doi.org/10.1016/S1876-2018(11)60261-X Krysiak, R., Handzlik-Orlik, G., & Okopien, B. (2012). Endocrine sym- ptoms as the initial manifestation of wilso n. The Yale Journal of Bio- logy and Medicine, 85, 249-254. Lezak, M. D. (1995) Neuropsychological assessment. Nueva York: Oxford University Press. Levy, R., & Dubois, B. (2006). Apathy and the functional anatomy of the prefrontal cortex—Basal ganglia circuits. Cerebral Cortex, 16, 916-928. http://dx.doi.org/10.1093/cercor/bhj043 Litwin, T., Gromadzka, G., Członkowska, A., Poniatowska, M., & Poniatowska, R. (2013). The effect of gender on brain MRI patho- logy in wilson’s disease. Springer US, 28, 69-75. http://dx.doi.org/10.1007/s11011-013-9378-2 Litwin, T., Gromadzka, G. , Szpak, G., Salach, J., Bul ska, E., & Człon- kowska, A. (2013). Brain metal accumulation in wilson. Journal of the Neurological Sciences, 329, 55-58. http://dx.doi.org/10.1016/j.jns.2013.03.021 Litwin, T., Gromadzka , G., Grzywacz, A. Członkowska, A., Członko- wska, A., Członkowski , J., & Samochowiec, J. (2012). Association of dopamine receptor gene p o lymorph isms with the clinical course of wilson disease, 73-80. http://dx.doi.org/10.1007/8904_2012_163 López, M., & Serrano, M. (2007). Enfermedad de wilson: Reporte de un caso y revisión de la literatura. Medicina Interna de México, 23, 458-463. López, J., Serrano, I., Llano, J., Mateos, J., López, S., & Sánchez, M. (2010) Utilidad del test de Stroop en el trastorno por d éficit de aten- ción/hiperactividad. Revista Española de Neurol ogía, 50, 333-340. Lorincz, M. T. (2010). Neurologic Wilson’s Ds, Lisease. Annals of New York Academic of Science, 1184, 173-187. http://dx.doi.org/10.1111/j.1749-6632.2009.05109.x Moller, L. B., Horn, N., Jop pesen, T. D., Vissing, J., Wibrand, F., Jen- num, P., & Ott, P. (2011). Clinical presentation and mutations in Danish patients with Wilson Disease. European Journal of Human Genetics, 19, 935-941. http://dx.doi.org/10.1038/ejhg.2011.80 Nagel, J., & Miralles, S . (2007 ). Enfermedad de Wilson: Comienzo con síntomas psiquiátricos. Hallazgos en reso nancia magnética encefálica. Revista Ar gentina de Radiologí a, 71, 45-49 Peng, F., Lutsenko, S., Sun, X., & Muzik, O. (2012). Positron emission tomography of copper metabolism in the atp7b−/− knock-out mouse model of wilson’s disease. Molecular Imaging and Biology, 14, 70-78. http://dx.doi.org/10.1007/s11307-011-0476-4 Pfeiffer, R. (2011). Handbook o f clinical neurology, 100, 681-709. http://dx.doi.org/10.1016/B978-0-444-52014-2.00049-5 Pladdy, H. (2007). Dysexecutive syndromes in neurologic disease. JNPT, 31. Popević, M., Kisić, G., Đukić, M., & Bulat, P. (2011). Work ability assessment in a patient with wilson’s disease. Archives of Industrial Hygiene and Toxicology, 62, 163-167. http://dx.doi.org/10.2478/10004-1254-62-2011-2102 Posner, M. I., & Raichle, M. E. (1994). Images of mind. New York: Scientific American Library. Roberts, E. (2011). Wilson’s disease. Metabolic Liver Disease, 39, 602- 604 Scheres, A., Oosterlaan, J., Geurts, H., Morein-Zamir, S., Meiran, N., Schut, H., Vlasveld, L., & Sergeant, J. (2004). Executive functioning in boys with ADHD: Primarily an inhibition deficit? Archives of Clinical Neuropsychology, 19, 569-594. http://dx.doi.org/10.1016/j.acn.2003.08.005 Schmitt de Bem, R., Araujo, D., Mitiko, M., Reis, E., Werneck, L., & Ghizoni, H. (2011). Wilson’s disease in southern Brazil: A 40-year follow-up study. Clinics, 66, 411-416. http://dx.doi.org/10.1590/S1807-59322011000300008 Stocco, A., Lebiere, C., & Anderson, J. (2010). Conditional routing of information to the cortex: A model of the b asal ganglia’ s role in cog- nitive coordination. P sychological Review, 117, 1-64. http://dx.doi.org/10.1037/a0019077 Suchy, Y. (2009). Executive functioning: Overview, assessment, and research issues for non-neuropsychologists. Annals of Behavioral Medicine, 37, 106-116. http://dx.doi.org/10.1007/s12160-009-9097-4 Svetel, M., Mijajlovi, M., Tomi, A., Kresojevi, N., Pekmezovi, T., & Kosti, V. (2012). Transcranial sonography in wilson’s disease. Par- kinsonism and Related Disorders, 18, 234-238. http://dx.doi.org/10.1016/j.parkreldis.2011.10.007 Taly, A. B., Prash an th, L. K., & Sinha, S . (2009). Wilson’s Disease: An Indian pespective. Neurology India, 57, 528-540. http://dx.doi.org/10.4103/0028-3886.57789 Teive, H., De Bem, R., Muzillo, D., Deguti, M., Munhoz, R., & Bar- bosa, E. (2012). Wilson’s disease in the south of brazil: A 40 years follow-up study. Parkinsonism and Related Disorders, 18, S66. http://dx.doi.org/10.1016/S1353-8020(11)70335-2 Verdejo-García, A., & Tirapu-Ustárroz, J. (2012). Neuropsicología clínica en perspectiva: Retos f uturos basados en desarrollos presentes. Revista Neur ología, 54, 180-186. Verdejo, A., & Bechara, A. (2010). Neuropsicologia de las funciones ejecutivas. Psicothetna, 22, 227-235. OPEN ACCESS 51  N. GUTIÉRREZ-ÁVILA ET AL. Verma, R., Patil, T., & Lalla, R. (2012). Acute extrapyramidal syn- drome and seizures as heralding manifestation of Wilson disease. Neurology India, 60, 363-364. http://dx.doi.org/10.4103/0028-3886.98547 Wilson, B., Alderman , N., Burgess, P., E mslie, H., & Evan s, J. (2003). Behavioural assessment of the dysexecutive syndrome (bads). Jour- nal of Occupational Psychology, Employment and Disabi lity, 5, 33-37. Xu, P., Lu, Z., Wang, X., Dosher, B ., Zhou, J., Zhang, D., & Zhou, Y. (2010). Category and perceptual learning in subjects with treated Wilson’s disease. PLoS ONE, 5, Article ID: e9635. http://dx.doi.org/10.1371/journal.pone.0009635 OPEN ACCESS 52
|