Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2011, 209-214 doi:10.4236/msa.2011.23026 Published Online March 2011 (http://www.scirp.org/journal/msa) Copyright © 2011 SciRes. MSA Synthesis, Acid and Base Resistance of Various Copper Phosphate Pigments by the Substitution with Lanthanum Hiroaki Onoda1, Kenichi Okumoto2 1Department of Informatics and Environmental Sciences, Faculty of Life and Environmental Sciences, Kyoto Prefectural University, Kyoto, Japan; 2Taihei Chemical Industrial Co., Ltd., Nara, Japan E-mail: onoda@kpu.ac.jp Received November 30th, 2010; revised February 16th, 2011; accepted February 25th, 2011. ABSTRACT Transition metal phosphates are used as inorganic pigments, however these materials have a weak point for acid or base resistance. Because lanthanum phosphate is insoluble in acidic or basic solution, the addition of lanthanum was tried to improve the acid or base resistance of copper phosphate pigment. Various cooper – lanthanum phosphates were synthesized in wet (H3PO4, Cu(NO3)2, La(NO 3)3) or dry (H3PO4, CuCO3Cu(OH)2H2O, La2O3) processes. The additional effects of lantha num were studied on the chemical co mposition, particle shape and size distribution, sp ecific surface area, color, acid and base resistance of the precipitates and their thermal products. Keywords: Copper Phosphate, Pigment, Powder Properties, Acid and Base Resistance 1. Introduction Phosphates have been used for ceramic materials, cata- lysts, fluorescent materials, dielectric substances, metal surface treatment, detergent, food additives, fuel cells, pigments, etc [1-3]. In these applications, transition met- al phosphate is suitable as a pigment because of high anticorrosion properties [4-7]. However, there is a weak point that is a certain degree of solubility for acidic and basic solution. It is well known that rare earth phosphates are insolu- ble for acidic and basic solution in the groups of phos- phate materials. In general, the addition of rare earth elements gives higher functional properties to the mate- rial [8]. Consequently, the addition of rare earth cation had the anticipation to improve the acid and base resis- tance of inorganic phosphate pi gments. In p revious wo r k , the addition of rare earth cation was studied in so lid state syntheses and powder properties, acid and base resis- tance of cobalt orthophosphate, pyrophosphate, and cyc- lo-tetraphosphate [3]. The chemical composition and powder properties of thermal products were changed by the addition of rare earth cation. Furthermore, this addi- tion improved the acid and base resistance of phosphate materials synthesized in solid state reaction. For the syntheses of inorganic phosphates, there are some methods, one is based on the solid state reaction, another one is on the cation exchange reaction in aque- ous solution. The method by the solid state reaction had some merits to be easy to form condensed phosphate and to control the molar ratio of cation/phosphorus, on the other hand, had a demerit to be difficult to keep the ho- mogeneity of materials. The preparation of transition metal phosphate in aqueous solution had an advantage to obtain the homogenized materials and various kinds of metal phosphates. However, it had a weak point to be difficult to control the molar ratio of cation/phosphorus. The synthetic method had much influence on the proper- ties of phosphate materials. There are some cases that the phosphate prepared in aqueous solution has the different properties with the phosphate synthesized in solid state reaction. It is important to clear the additional effects of rare earth cation on syntheses of inorganic phosphate materials prepared in wet process and their properties. The addition of rare earth cation causes the change of chemical composition, powder, and functional proper- ties. The substitution with lanthanum in nickel and cobalt phosphate materials prepared in wet process was studied on the chemical composition, powder condition, color, acid and base resistance [9,10]. Specific surface area of  Synthesis, Acid and Base Resistance of Various Copper Phosphate Pigments by the Substitution with Lanthanum Copyright © 2011 SciRes. MSA 210 phosphates increased and particle size became larger by the substitution with lanthanum. The substitution with lanthanum on acid and base resistance was effective for design of inorganic phosphate pigment. In this work, various copper – lanthanum phosphates were synthesized in aqueous solution or by solid state reaction. The obtained products were evaluated by their particle shape and size distribution, specific surface area, color, acid and base resistance. 2. Experimental 2.1. Synthesis of Various Copper—Lanthanum Phosphates The 0.1 mol/l of copper nitrate solution was mixed with 0.1 mol/l of phosphoric acid solution in the molar ratio of Cu/P = 1/1 (wet process). This ratio is settled from the chemical composition of copper orthophosphate, Cu- HPO4. A part of copper nitrate was substituted with lan- thanum nitrate in the molar ratio of La/Cu = 0/10 and 2/8. The solutions were mixed in the molar ratio of P/(Cu + La) = 1/1. Then, the mixed solution was adju sted to pH 7 by ammonia solution. The precipitate was filtered off and heated at 700˚C for 1 hour. These preparation ratios are shown in Table 1. Basic copper carbonate (CuCO3Cu(OH)2H2O) was mixed with 85 wt% phosphoric acid (H3PO4) at a mole ratios of P/Cu = 2/3 and 1/1 (dry process). Copper or- thophosphate, Cu3(PO4)2, and pyrophosphate, Cu2P2O7, were expected by heating the mixture at 700˚C for 1 hour via the following reaction. 3CuCO3Cu(OH)2H2O + 4H3PO4→ 2Cu3(PO4)2 + 3CO2 + 12H2O (1) CuCO3Cu(OH)2H2O + 2H3PO4→ Cu2P2O7 + CO2 + 5H2O (2) At the same time, basic copper carbonate was mixed with 85 wt% phosphoric acid in a mole ratios of P/Cu = 2/1. Copper cyclo-tetraphosphate, Cu2P4O12, was obtain- ed by heating the mixture at 420˚C for 1 hour via the following reaction. CuCO3Cu(OH)2H2O + 4H3PO4→ Cu2P4O12 + CO2 + 8H2O (3) A part of basic copper carbonate was substituted with lanthanum oxide, La2O3, in the molar ratio of La/Cu = 0/10 and 2/8. These synthetic conditions are also sum- marized in Table 1. 2.2. Evaluation of Phosphate Materials A part of the precipitates was dissolute in hydrochloric acid solution. The ratios of copper, lanthanum, and phosphorus in the precipitates were calculated from In- ductivity Coupled Plasma – Atomic Emission Spec- trometry (ICP) results of these solutions. The ICP esti- mation was measured with Shimadzu ICPS-8000. The chemical composition of these phosphates was analyzed by X-ray diffraction (XRD) and Fourier trans- form infrared spectroscopy (FT-IR). X-ray diffraction patterns were recorded on a Rigaku Denki RINT2000M X-Ray diffractometer using monochromated CuKα ra- diation. The IR spectra were recorded on a Shimadzu FT-IR spectrometer FT-IR8600 with a KBr disk method. The powder properties of thermal products were char- acterized by particle shape, particle size distribution, specific surface area, and their color. Particle shapes were observed by scanning electron micrographs (SEM) using VE8800 from Keyence Co. Ltd. Particle size dis- tribution was measured with laser diffraction/scattering particle size distribution HORIBA LA-910, which can measure samples in the range of 0.02 to 1000 µm at one time. Specific surface areas of phosphates were calcu- lated from the amount of nitrogen gas adsorbed at the temperature of liqu id nitrogen by BET method with Bel- sorp mini from BEL JAPAN, INC. The color of phos- phate pigments was estimated by ultraviolet-visible (UV-Vis) reflectance spectra with a Shimadzu UV365. Furthermore, the acid and base resistance of materials was estimated in following method. The 0.1 g of thermal products was allowed to stand in 100 ml of 0.1 wt% sul- phuric acid or 0.1 wt% sodium hydroxide solution for 1 day. Then, solid was removed off by filtration. The fil- tered solution was diluted with nitric acid. The concen- trations of phosphorus, copper, lanthanum cation were calculated by ICP results. As a resistance estimation, the solubility (%) of target elements was calculated for the concentration that thermal products were completely dissolved by warming hydrochloric acid. 3. Results and Discussion 3.1. Chemical Composition of Copper — Lanthanum Phosphates Table 1 shows ICP results of samples synthesized in various conditions. The preparation sections were the expected ratios calculated from experimental conditions. Samples prepared in wet process had lower ratio of P/(2Cu + 3La) than the expected ratios (Table 1(a,b)). The obtained precipitates were considered to be phos- phorus-poor compounds. Because copper and lanthanum cations were easy to react with phosphoric acid in these ratios, a certain degree of phosphate anion was filtered off [11]. In dry process, the ratio of P/(2Cu + 3La) in precipitates was generally a little lower than those in preparation process (Table 1(c-h)). Small amount of phosphorus was considered to volatilize in heating proc- ess. Samples substituted with lanthanum indicated wide range of La/(Cu + La) ratio (Table 1(b,d,f,h)), however the average of these ratios was near with the preparation ratio. 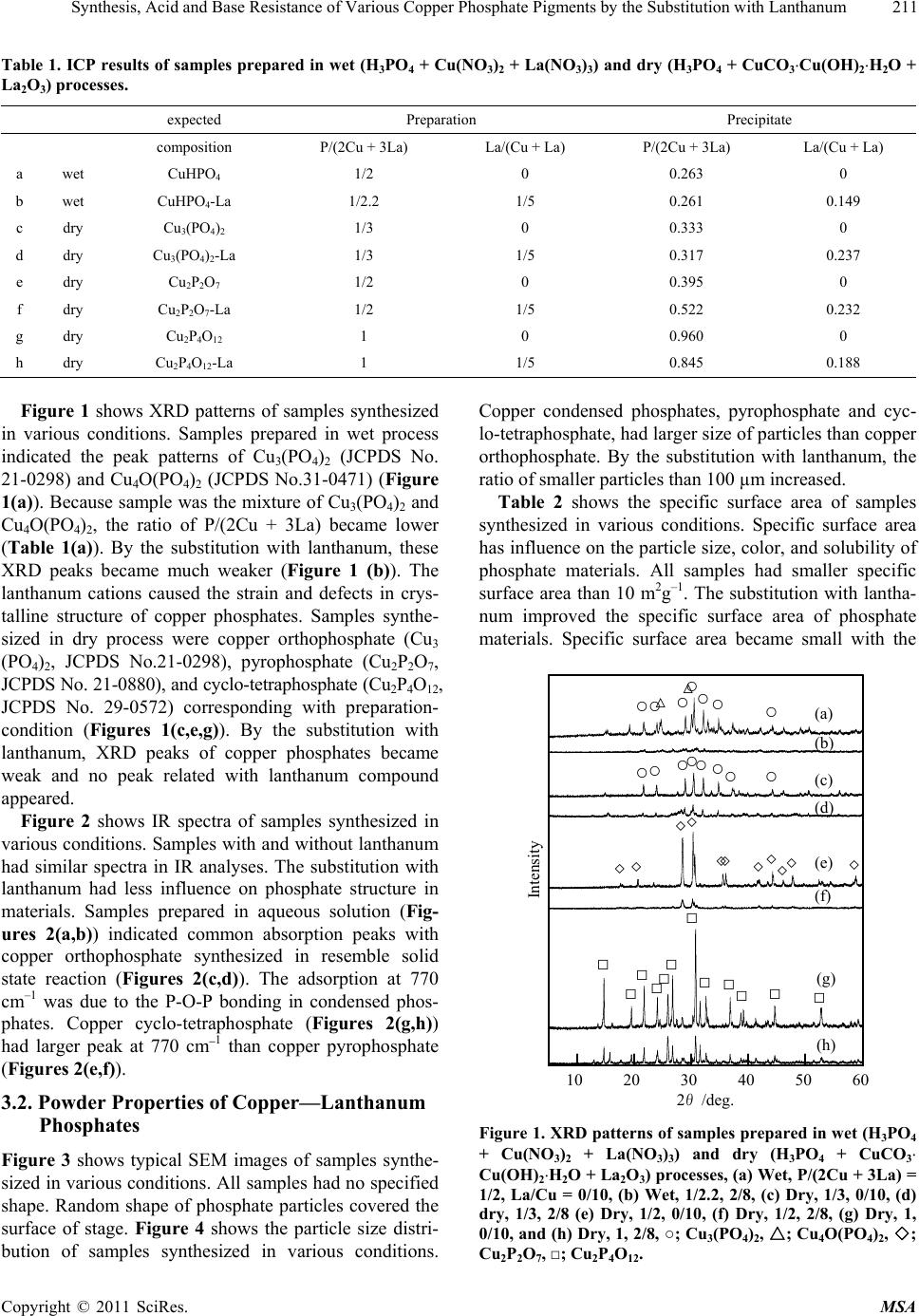 Synthesis, Acid and Base Resistance of Various Copper Phosphate Pigments by the Substitution with Lanthanum Copyright © 2011 SciRes. MSA 211 Table 1. ICP results of samples prepared in wet (H3PO4 + Cu(NO3)2 + La(NO3)3) and dry (H3PO4 + CuCO3Cu(OH)2H2O + La2O3) processes. expected Preparation Precipitate composition P/(2Cu + 3La) La/(Cu + La) P/(2Cu + 3La) La/(Cu + La) a wet CuHPO4 1/2 0 0.263 0 b wet CuHPO4-La 1/2.2 1/5 0.261 0.149 c dry Cu3(PO4)2 1/3 0 0.333 0 d dry Cu3(PO4)2-La 1/3 1/5 0.317 0.237 e dry Cu2P2O7 1/2 0 0.395 0 f dry Cu2P2O7-La 1/2 1/5 0.522 0.232 g dry Cu2P4O12 1 0 0.960 0 h dry Cu2P4O12-La 1 1/5 0.845 0.188 Figure 1 shows XRD patterns of samples synthesized in various conditions. Samples prepared in wet process indicated the peak patterns of Cu3(PO4)2 (JCPDS No. 21-0298) and Cu4O(PO4)2 (JCPDS No.31-0471) (Figure 1(a)). Because sample was the mixture of Cu3(PO4)2 and Cu4O(PO4)2, the ratio of P/(2Cu + 3La) became lower (Table 1(a)). By the substitution with lanthanum, these XRD peaks became much weaker (Figure 1 (b)). The lanthanum cations caused the strain and defects in crys- talline structure of copper phosphates. Samples synthe- sized in dry process were copper orthophosphate (Cu3 (PO4)2, JCPDS No.21-0298), pyrophosphate (Cu2P2O7, JCPDS No. 21-0880), and cyclo-tetraphosphate (Cu2P4O12, JCPDS No. 29-0572) corresponding with preparation- condition (Figures 1(c,e,g)). By the substitution with lanthanum, XRD peaks of copper phosphates became weak and no peak related with lanthanum compound appeared. Figure 2 shows IR spectra of samples synthesized in various conditions. Samples with and withou t lanthanum had similar spectra in IR analyses. The substitution with lanthanum had less influence on phosphate structure in materials. Samples prepared in aqueous solution (Fig- ures 2(a,b)) indicated common absorption peaks with copper orthophosphate synthesized in resemble solid state reaction (Figures 2(c,d)). The adsorption at 770 cm–1 was due to the P-O-P bonding in condensed phos- phates. Copper cyclo-tetraphosphate (Figures 2(g,h)) had larger peak at 770 cm–1 than copper pyrophosphate (Figures 2(e,f)). 3.2. Powder Properties of Copper—Lanthanum Phosphates Figure 3 shows typical SEM images of samples synthe- sized in various conditions. All sa mples had no specified shape. Random shape of phosphate particles covered the surface of stage. Figure 4 shows the particle size distri- bution of samples synthesized in various conditions. Copper condensed phosphates, pyrophosphate and cyc- lo-tetraphosphate, had larger size of particles than copper orthophosphate. By the substitution with lanthanum, the ratio of smaller particles than 100 µm increased. Table 2 shows the specific surface area of samples synthesized in various conditions. Specific surface area has influence on the particle size, color, and solub ility of phosphate materials. All samples had smaller specific surface area than 10 m2g–1. The substitution with lantha- num improved the specific surface area of phosphate materials. Specific surface area became small with the 10 20 30 40 50 60 Intensity 2 /deg. (a) (b) (c) (d) (e) (f) (g) (h) θ ○ ○ ○ ○ ○ ○ ○ ○○ ○○○ ○○ ○ △ △ ◇ ◇ ◇◇◇ ◇◇ ◇ ◇ ◇◇ □ □□ □□ □ □ □ □□ □ □ Figure 1. XRD patterns of samples prepared in wet (H3PO4 + Cu(NO3)2 + La(NO3)3) and dry (H3PO4 + CuCO3 Cu(OH)2H2O + La2O3) processes, (a) Wet, P/(2Cu + 3La) = 1/2, La/Cu = 0/10, (b) Wet, 1/2.2, 2/8, (c) Dry, 1/3, 0/10, (d) dry, 1/3, 2/8 (e) Dry, 1/2, 0/10, (f) Dry, 1/2, 2/8, (g) Dry, 1, 0/10, and (h) Dry, 1, 2/8, ○; Cu3(PO4)2, ; Cu△4O(PO4)2, ; ◇ Cu2P2O7, □; Cu2P4O12. 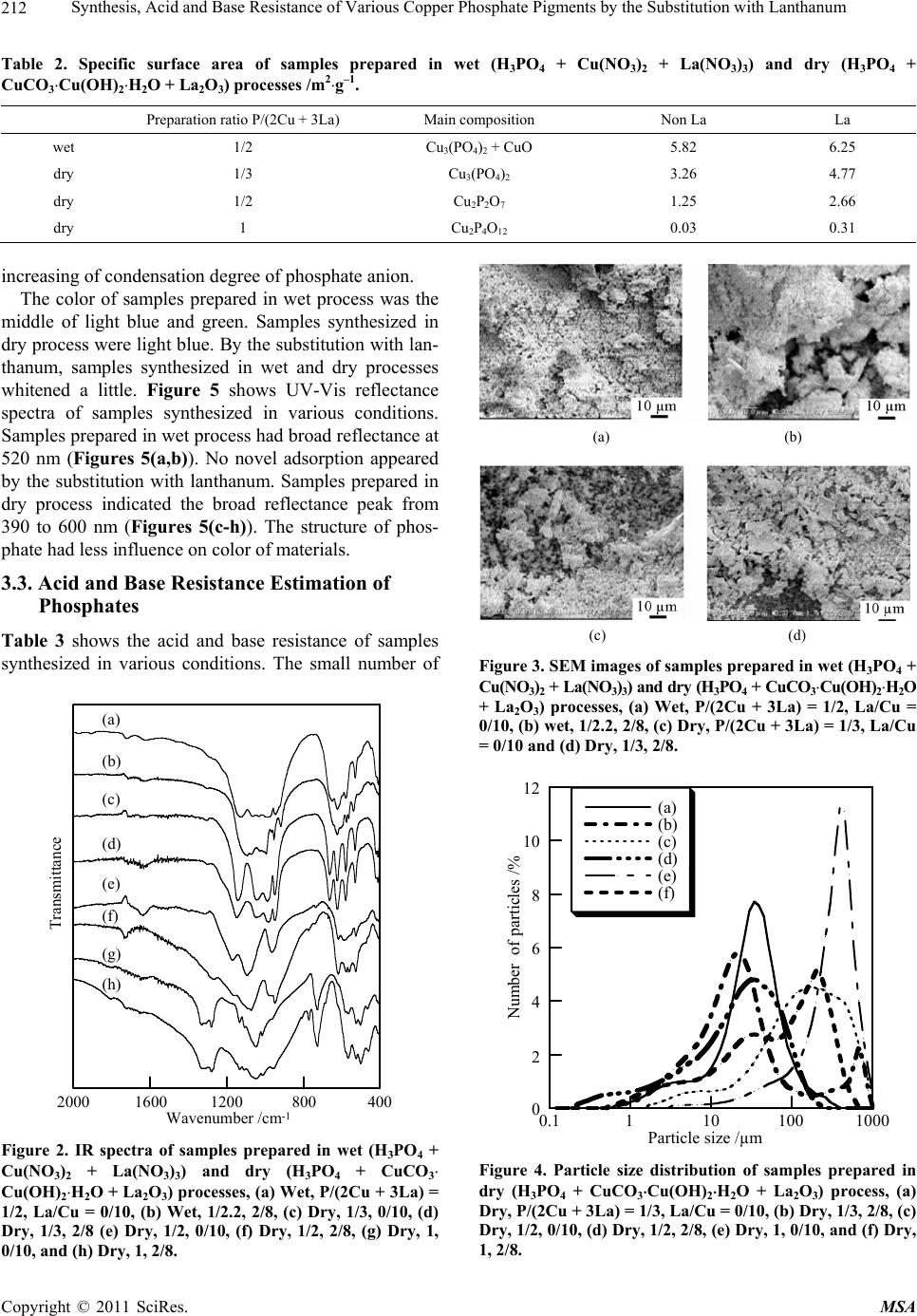 Synthesis, Acid and Base Resistance of Various Copper Phosphate Pigments by the Substitution with Lanthanum Copyright © 2011 SciRes. MSA 212 Table 2. Specific surface area of samples prepared in wet (H3PO4 + Cu(NO3)2 + La(NO3)3) and dry (H3PO4 + CuCO3Cu(OH)2H2O + La2O3) processes /m2g–1. Preparation ratio P/(2Cu + 3La) Main composition Non La La wet 1/2 Cu3(PO4)2 + CuO 5.82 6.25 dry 1/3 Cu3(PO4)2 3.26 4.77 dry 1/2 Cu2P2O7 1.25 2.66 dry 1 Cu2P4O12 0.03 0.31 increasing of conde nsat i o n de gree of phosph at e anion. The color of samples prepared in wet process was the middle of light blue and green. Samples synthesized in dry process were light blue. By the substitu tion with lan- thanum, samples synthesized in wet and dry processes whitened a little. Figure 5 shows UV-Vis reflectance spectra of samples synthesized in various conditions. Samples prepared in wet process had broad reflectance at 520 nm (Figures 5(a,b)). No novel adsorption appeared by the substitution with lanthanum. Samples prepared in dry process indicated the broad reflectance peak from 390 to 600 nm (Figures 5(c-h)). The structure of phos- phate had less influence on color of materials. 3.3. Acid and Base Resistance Estimation of Phosphates Table 3 shows the acid and base resistance of samples synthesized in various conditions. The small number of 400800120016002000 Transmittance Wavenumber /cm-1 (a) (b) (c) (d) (e) (f) (g) (h) Figure 2. IR spectra of samples prepared in wet (H3PO4 + Cu(NO3)2 + La(NO3)3) and dry (H3PO4 + CuCO3 Cu(OH)2H2O + La2O3) processes, (a) Wet, P/(2Cu + 3La) = 1/2, La/Cu = 0/10, (b) Wet, 1/2.2, 2/8, (c) Dry, 1/3, 0/10, (d) Dry, 1/3, 2/8 (e) Dry, 1/2, 0/10, (f) Dry, 1/2, 2/8, (g) Dry, 1, 0/10, and (h) Dry, 1, 2/8. (a) (b) (c) (d) Figure 3. SEM images of samples prepared in wet (H3PO4 + Cu(NO3)2 + La(NO3)3) and dry (H3PO4 + CuCO3Cu(OH)2H2O + La2O3) processes, (a) Wet, P/(2Cu + 3La) = 1/2, La/Cu = 0/10, (b) wet, 1/2.2, 2/8, (c) Dry, P/(2Cu + 3La) = 1/3, La/Cu = 0/10 and (d) Dry, 1/3, 2/8. 0 2 4 6 8 10 12 0.1110100 1000 (a) (b) (c) (d) (e) (f) Number of particles /% Particle size /µm Figure 4. Particle size distribution of samples prepared in dry (H3PO4 + CuCO3Cu(OH)2H2O + La2O3) process, (a) Dry, P/(2Cu + 3La) = 1/3, La/Cu = 0/10, (b) Dry, 1/3, 2/8, (c) Dry, 1/2, 0/10, (d) Dry, 1/2, 2/8, (e) Dry, 1, 0/10, and (f) Dry, 1, 2/8.  Synthesis, Acid and Base Resistance of Various Copper Phosphate Pigments by the Substitution with Lanthanum Copyright © 2011 SciRes. MSA 213 Table 3. Acid and base resistance of samples prepared in wet (H3PO4 + Cu(NO3)2 + La(NO3)3) and dry (H3PO4 + Cu- CO3Cu(OH)2H2O + La2O3) processes. dry/ Preparation ratio Acid (solubility) Base (solubility) wet P/(2Cu + 3La) La/(Cu + La) P /% Cu /% La /% P /% Cu /% La /% a wet 1/2 0 100 100 - 11.7 0.6 - b wet 1/2.2 1/5 100 100 0.3 7.9 0 0 c dry 1/3 0 100 100 - 3.7 0 - d dry 1/3 1/5 98.0 100 34.9 6.9 0 0 e dry 1/2 0 100 100 - 4.5 0.2 - f dry 1/2 1/5 63.8 79.0 9.2 1.7 0 0.2 g dry 1 0 32.9 33.9 - 9.3 0 - h dry 1 1/5 16.3 22.2 3.5 8.1 0.9 0 400 500600 700 800 Reflectance Wavelength /nm (a) (b) (c) (d) (e) (f) (g) (h) Figure 5. UV-Vis reflectance spectra of samples prepared in wet (H3PO4 + Cu(NO3)2 + La(NO3)3) and dry (H3PO4 + CuCO3Cu(OH)2H2O + La2O3) processes, (a) Wet, P/(2Cu + 3La) = 1/2, La/Cu = 0/10, (b) Wet, 1/2.2, 2/8, (c) Dry, 1/3, 0/10, (d) Dry, 1/3, 2/8 (e) dry, 1/2, 0/10, (f) dry, 1/2, 2/8, (g) Dry, 1, 0/10, and (h) Dry, 1, 2/8. solubility means high acid and base resistance. Samples prepared in wet process with and without lanthanum (Table 3(a,b)) solved perfectly in acid solution. The solubility of samples prepared in dry process became small by the substitution with lanthanum. The solubility of phosphorus became lower than those of copper. In base resistance, some samples indicate higher solubility b y th e substitution with lanthanum. However, generally, the sub stitution with lanthanum improved the base resistance of copper phosph at es. 4. Conclusions The chemical composition of samples prepared in wet process was the mixture of Cu3(PO4)2 and Cu4O(PO4)2. On the other hand, in dry process, copp er orthop hosphate, pyrophosphate, and cyclo-tetraphosphate were obtained corresponding to the synthetic ratio of phosphorus and copper. Specific surface area of phosphate materials be- came larger by the substitution with lanthanum. The color of samples whitened a little. Acid and base resis- tance of copper phosphates improved by the substitution with lanthanum. REFERENCES [1] H. Onoda, H. Nariai, A. Moriwaki, H. Maki and I. Mo- tooka, “Formation and Catalytic Characterization of Var- ious Rare Earth Phosphates,” Journal of Materials Che- mistry, Vol. 12, No. 6, 2002, pp. 1754-1760. doi:10.1039/b110121h [2] H. Onoda, T. Ohta, J. Tamaki and K. Kojima, “Decom- position of Trifluoromethane over Nickel Pyrophosphate Catalyst s Containing Metal Cation,” Applied Catalysis A; General, Vol. 288, No. 1-2, 2005, pp. 98-103. doi:10.1016/j.apcata.2005.04.028 [3] H. Onoda, K. Yokouchi, K. Kojima, and H. Nariai, “Ad- dition of Rare Earth Cation on Formation and Properties of Various Cobalt Phosphates,” Materials Science and Engineering B, Vol. 116, No. 2, 2005, pp. 189-195. doi:10.1016/j.mseb.2004.10.002 [4] D. M. Lenz, M. Delamar and C. A. Ferreira, “Improve- ment of the Anticorrosion Properties of Polypyrrole by Zinc Phosphate Pigment Incorporation,” Progress in Or- ganic Coatings, Vol. 58, No. 1, 2007, pp. 64-69. doi:10.1016/j.porgcoat.2006.12.002 [5] M. Mahdavian and M. M. Attar, “Investigation on Zinc Phosphate Effectiveness at Different Pigment Volume Concentrations via Electrochemical Impedance Spec- troscopy,” Eletrochimica Acta, Vol. 50, No. 24, 2005, pp. 4645-4648. doi:10.1016/j.electacta.2005.02.015  Synthesis, Acid and Base Resistance of Various Copper Phosphate Pigments by the Substitution with Lanthanum Copyright © 2011 SciRes. MSA 214 [6] M. A. Hernandez, F. Galliano and D. Landolt, “Mecha- nism of Cathodic Delamination Control of Zinc – Alu- minum Phosphate Pigment in Waterborne Coatings,” Corrosion Science, Vol. 46, No. 9, 2004, pp. 2281-2300. doi:10.1016/j.corsci.2004.01.009 [7] M. C. Deya, G. Blustein, R. Romagnoli and B. del Amo, “The Influence of the Anion Type on the Anticorrosive Behaviour of Inorganic Phosphates,” Surface and Coat- ing Technology, Vol. 150, No. 2-3, 2002, pp. 133-142. doi:10.1016/S0257-8972(01)01522-5 [8] N. E. Topp, “Chemistry of the Rare Earth Elements,” Kagakudojin, Kyoto, 1974. [9] H. Onoda, H. Matsui and I. Tanaka, “Improvement of Acid and Base Resistance of Nickel Phosphate Pigment by the Addition of Lanthanum Cation,” Materials Science and Engineering B, Vol. 141, No. 1-2, 2007, pp. 28-33. doi:10.1016/j.mseb.2007.05.009 [10] H. Onoda, K. Tange and I. Tanaka, “Influence of Lan- thanum Addition on Preparation and Powder Properties of Cobalt Phosphates,” Journal of Materials Science, Vol. 43, No. 16, 2008, pp. 5483-5488. doi:10.1007/s10853-008-2831-7 [11] J. Liu, F. Wang, K. Sun and X. Xu, “Creation of a Mo- nomeric Ruthenium Species on the Surface of Micro-Size Copper Hydrogen Phosphate: An Active Heterogeneous Catalyst for Selective Aerobic Oxidation of Alcohols,” Advanced Synthesis and Catalysis, Vol. 349, No. 16, 2007, pp. 2439-2444. doi:10.1002/adsc.200700187 |

